Introduction
Biliary tract cancers (BTC) are a rare but
aggressive tumor entity. They comprise a heterogenous group of
individual malignancies arising from the biliary duct or
gallbladder. These tumors may be subdivided into intrahepatic,
perihilar and distal extrahepatic cholangiocarcinoma, as well as
gallbladder carcinoma. They currently account for <1% of
malignancies. At the same time, their rising incidence and
mortality are increasing the requirement for effective treatment
options (1-3).
Surgical resection is the only curative treatment
modality. The majority of BTCs are diagnosed at an advanced stage,
due to unspecific or complete lack of symptoms, leaving a minority
suitable for resection (4,5). Even after successful resection
relapse rates are high, resulting in 5-year overall survival rates
of only 10-35% (6,7).
Platinum-based systemic chemotherapy is the
established first-line treatment for advanced BTC based on the
phase-III ABC-02 trial. This study detected an improved overall
survival (OS) in patients treated with a combination chemotherapy
consisting of gemcitabine and cisplatin vs. gemcitabine alone (OS,
11.7 vs. 8.1 months) (8). However,
sustained response remains infrequent and progression rates are
high.
Of those patients who progressed under first-line
chemotherapy, 25-50% may be eligible for subsequent lines of
treatment (9). The ABC-06 trial
demonstrated the beneficial effects of second-line chemotherapy
(10). Fluorouracil-based
treatment regimens in combination with oxaliplatin or particularly
irinotecan are frequently used as second-line chemotherapy
(11-13).
Outcomes remain poor with a progression-free survival (PFS) and OS
between 2.2-3.6 and 6.7-11 months, respectively (13-15).
Nanoliposomal irinotecan (nal-IRI) is a
liposomal-encapsulated formulation of irinotecan. The tight
encapsulation of thousands of active irinotecan molecules within a
polyethylene-glycated lipid bilayer vesicle inhibits protein
adsorption and subsequent elimination. The liposomal nanoparticles
prolong systemic circulation with a slower release of irinotecan
molecules. This enhances the anti-tumor activity and prevents high
peak plasma levels, resulting in improved tolerability. In
addition, the lipophile formulation favors its accumulation in
tumor tissue, increasing exposure to the tumor cells. After
phagocytosis by tumor-associated macrophages, irinotecan is
converted to its active up to 1,000-fold more potent metabolite
SN-38. It induces double-strand DNA damage during DNA synthesis by
inhibiting topoisomerase-I (16-19).
A significant correlation between topoisomerase-I
activity and tumor cell sensitivity to SN-38 was previously
observed (20). Since
topoisomerase-I activity is enhanced in cisplatin-resistant cancer
cells, the use of irinotecan appears to be a reasonable choice in
subsequent therapy regimens (21).
The plasma concentration of unbound platinum is known to decline in
a biphasic manner with an initial and secondary half-life of 31.2
min and 20.1 h, respectively (22).
Nal-IRI combined with leucovorin and 5-fluorouracil
(FOLFnal-IRI) achieved notable results in patients with advanced
pancreatic cancer as a second-line treatment in the NAPOLI-1 trial
(23). These compelling results
led to the initiation of clinical studies to assess its performance
in first- and second-line treatments of advanced BTC.
At present, evidence for the efficacy of this
combination as a second-line treatment for advanced BTCs is scarce.
In the present study, the efficacy of a combined chemotherapy of
FOLFnal-IRI in advanced BTC previously treated with platinum-based
chemotherapy was assessed.
Materials and methods
Inclusion criteria
Patients were eligible for inclusion if they were 18
years or older with histologically confirmed cholangiocarcinoma or
gallbladder cancer and began treatment with nal-IRI in combination
with leucovorin and 5-fluorouracil between 01/08/2017 and
01/04/2020 after initial platinum-based chemotherapy at the
University Hospital of Cologne (Cologne, Germany). Patients with
ampullary tumors were excluded.
Data collection, end-points and
follow-up
The data collected were patient demographics,
subtype of BTC and stage at diagnosis in accordance with the ‘Union
Internationale Contre le Cancer’ guidelines (24), prior resection, and plasma levels
of carbohydrate antigen 19-9 (CA19-9) during treatment. In
addition, the type of chemotherapy prior to and after FOLFnal-IRI
therapy, as well as the start and end date of FOLFnal-IRI
treatment, were documented.
For the assessment of response, computed tomography
and magnetic resonance imaging performed during treatment with
FOLFnal-IRI were reviewed and response was classified as complete
or partial response, or stable or progressive disease according to
the radiologist's evaluation.
Toxicity was graded according to the ‘Common
Terminology Criteria for Adverse Events’ (version 5.0) published by
the US National Cancer Institute (25). All documented grade III or higher
adverse events, as well as alterations of laboratory parameters
leading to dose reduction, were analyzed.
Patients were followed up until death or 01/08/2020.
OS was defined as the period from the start of FOLFnal-IRI until
death from any cause. Data on patients who were alive at the end of
the follow-up (01/08/2020) were regarded censored to the date of
the last follow-up. PFS was defined as the period from the start of
FOLFnal-IRI to the date of a documented disease progression or
death.
In accordance with the regional law (paragraph 15,
sentence 1, North Rhine Medical Association's Professional Code of
Conduct from 14th November 1998, as amended on 16th November 2019,
and paragraph 6, sentence 1, Health Data Protection Act of North
Rhine Westphalia) (26), approval
by a local ethics committee and written informed consent from the
participants were not required due to the strictly retrospective
design of the study.
Treatment
FOLFnal-IRI was applied according to the NAPOLI-1
trial protocol: Liposomal irinotecan at 70 mg/m2 and
folinic acid at 400 mg/m2, followed by 5-fluorouracil
infusion at 2,400 mg/m2 over the course of 46 h every 2
weeks (23).
Statistical analysis
For descriptive analyses, SPSS version 26 (IBM
Corporation) was used. Categorical variables are presented as
absolute numbers and relative frequencies. Continuous variables are
expressed as the median (range). PFS and OS were estimated from
Kaplan-Meier curves.
Results
Patient characteristics
A total of 14 patients were treated with FOLFnal-IRI
during the period of interest. Of these, three patients were
excluded from analysis: One patient received only one cycle of
FOLFnal-IRI and wished to stop further treatment due to personal
reasons. One patient, already hospitalized and moribund due to
end-stage BTC and liver failure, received 50% FOLFnal-IRI off-label
as a last resort but died shortly after and one patient was lost to
follow-up. Finally, 11 patients were included in the present study.
The median age was 54 years (range, 41-69 years). A total of 4
patients were female (36.4%). Furthermore, 7 (63.6%) patients were
diagnosed with intrahepatic cholangiocarcinoma, 2 (18.2%) with
gallbladder cancer and 1 (9.1%) with extrahepatic
cholangiocarcinoma. Surgical resection was performed in 4 (36.4%)
patients prior to therapy with FOLFnal-IRI. The initial stage at
the start of FOLFnal-IRI administration according to the
classification of the ‘Union International Contre Le Cancer’ was IV
in the 11 (100%) patients (all previously resected patients had
progressed to stage IV) (Table
I).
 | Table IPatient characteristics (n=11). |
Table I
Patient characteristics (n=11).
| Item | Value |
|---|
| Female sex | 4 (36.4) |
| Age at initial
diagnosis, years | 54 (41-69) |
| Age at start of
FOLFnal-IRI, years | 56 (44-69) |
| Subtype of BTC | |
|
Intrahepatic
cholangiocarcinoma | 7 (63.6) |
|
Extrahepatic
cholangiocarcinoma | 1 (9.1) |
|
Gallbladder
carcinoma | 2 (18.2) |
| Prior resection of
primary tumor performed | 4 (36.4) |
| Stage IV at treatment
with FOLFnal-IRI | 11(100) |
| Initial
platinum-based chemotherapy | 11(100) |
| FOLFnal-IRI as n line
of therapy | |
|
Second | 7 (63.6) |
|
Third | 3 (27.3) |
|
Fourth | 1 (9.1) |
| Patients with
subsequent lines of therapy | 6 (54.6) |
Treatment
The first-line chemotherapy regimen was
platinum-based in all cases. Of the patients, 7 (63.6%) received
FOLFnal-IRI as their second-, 3 (27.3%) as third- and 1 (9.1%) as
their fourth-line chemotherapy. Furthermore, one patient received
FOLFnal-IRI in combination with trastuzumab. A total of 6 (54.6%)
patients received at least one subsequent chemotherapy after
FOLFnal-IRI (Table I).
The median duration of treatment with FOLFnal-IRI
was 8.7 months (range, 0.9-12 months) (Table II). FOLFnal-IRI was postponed at
least once in 5 (45.5%) patients and the dose of applied
chemotherapy was reduced at least once in 7 patients (63.7%). A
total of 3 grade III toxicities were recorded. In two patients,
grade III diarrhea led to termination of treatment (data not
shown).
 | Table IIOutcomes after initiation of
FOLFnal-IRI. |
Table II
Outcomes after initiation of
FOLFnal-IRI.
| Item | Value |
|---|
| Duration of
treatment, months | 8.7 (0.9-12) |
| Best radiological
response | |
|
Partial
response | 0 (0) |
|
Stable
disease | 6 (54.6) |
|
Progressive
disease | 5 (45.5) |
| Progression-free
survival, months | |
|
Total | 5.1 (1.1-11.5) |
|
Second-line | 6.1 (1.1-11.5) |
|
Third-line | 3.9 (2.1-10.8) |
|
Fourth-line | 5.1 (-) |
| OS after
initiation, months | |
|
Total | 12.4
(3.9-22.2) |
|
Second-line | 12.1
(5.1-22.2) |
|
Third-line | 12.4
(3.9-14.9) |
|
Fourth-line | 16.5 (-) |
| OS after initial
diagnosis, months | 24.7
(10.1-65.2) |
The mean CA19-9 levels at the start and end of
FOLFnal-IRI were 309.6±449.1 and 708.9±1891.7 kU/l, respectively.
The mean maximum reduction of CA19.9 during treatment was
24.7±31.7% (data not shown).
Patient survival
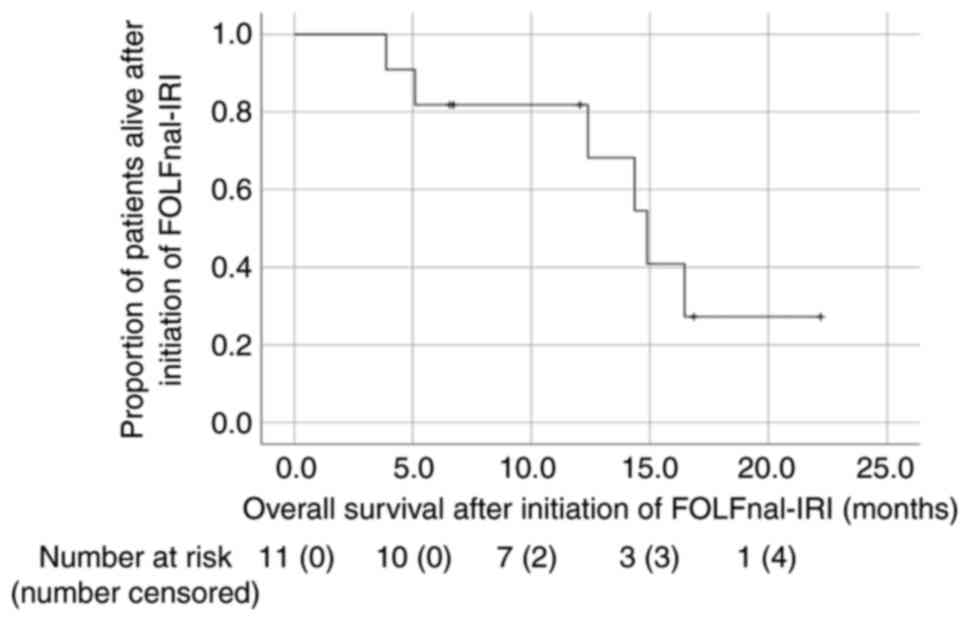
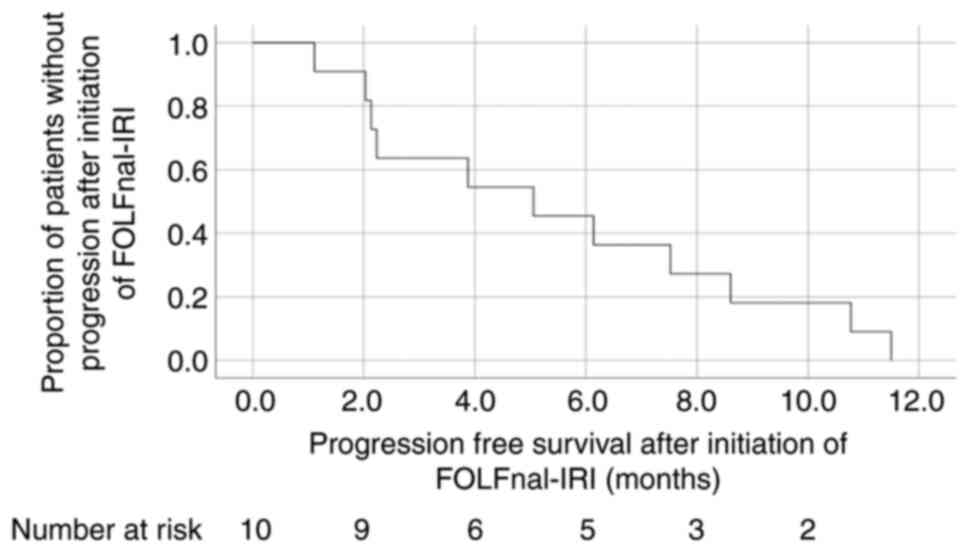
The median PFS and OS from the start of FOLFnal-IRI
and OS from initial diagnosis of stage IV BTC were 5.1, 12.4 and
24.7 months, respectively (Figs. 1
and 2; Table II). The median PFS of patients
treated with FOLFnal-IRI as second-, third- and fourth-line
chemotherapy was 6.1, 3.9 and 5.1 months, respectively. The median
OS of patients after the start of FOLFnal-IRI as their second-,
third- and fourth-line chemotherapy was 12.1, 12.4 and 16.5 months,
respectively. A total of 5 (45.5%) patients were still alive at the
date of censoring. Furthermore, 1 (9.1%) patient died prior to
undergoing their subsequent staging examination after the start of
treatment. The best documented radiological response was stable
disease, which was achieved in 6 patients, resulting in a disease
control rate of 54.6% (Table
II).
Discussion
Patients with advanced BTC only have a small number
of established therapeutic options in contrast to those with other
tumor entities, e.g. lung cancer, where established options for
further lines of treatment positively influence patient survival
(27).
In the present study, promising results for
FOLFnal-IRI in advanced BTC after failure of initial platinum-based
chemotherapy are provided. FOLFnal-IRI had remarkable efficiency
with a disease control rate of 54.5%, a median PFS of 5.1 months
and OS of 12.4 months. OS after initial diagnosis of stage IV BTC
was 24.7 months and toxicity remained modest. At present, only
limited data are available on the efficacy of FOLFnal-IRI in
advanced biliary tract malignancies.
The analysis of the present study indicated
favorable results compared to previous studies evaluating the
benefit of second-line chemotherapy in advanced BTC. A large
multicenter retrospective study by Brieau et al (13) in 2015 involving patients treated
with different second-line regimens recorded a median PFS and OS of
3.2 and 6.7 months, respectively. A meta-analysis of 25 studies
provided comparable results for the mean PFS and OS (28). Of note, these studies were not able
to demonstrate any difference between regimens with regard to
fluorouracil- or gemcitabine-based chemotherapy nor single or
combination protocols.
The results of the present study likely reflect the
expected benefit of the novel liposomal formula of irinotecan
compared to published data from patients with advanced BTC
receiving conventional FOLFIRI as a second-line treatment. In these
studies, PFS and OS ranged from 2.4 to 3.5 months and from 5.5 to
6.6 months, respectively (11,29,30).
Superiority of liposomal irinotecan over standard irinotecan in
other malignancies has also been described in previous preclinical
and clinical studies (17,18,31).
To the best of our knowledge, only one small
retrospective analysis of FOLFnal-IRI in BTC was previously
performed (32). This study
included 14 patients from Austria and provided exceptional results.
The PFS of 10.6 months and OS of 24.1 months after initiation of
FOLFnal-IRI as a subsequent line of treatment appear
extraordinarily effective when comparing them to the OS of 11.7
months achieved by the combination therapy of gemcitabine and
cisplatin as first-line therapy in the ABC-O2-trial (8). The results of the present study
relativize the exceptional results from Austria; however,
FOLFnal-IRI remains a promising treatment option with regard to
limited results of other subsequent treatment regimens in advanced
BTC. Due to these nonetheless promising results, further
investigation of the value of FOLFnal-IRI as a treatment option in
BTC is warranted.
The actual PFS may have been even higher in the
present study if the radiologist's evaluation had been based on the
RESPONSE EVALUATION CRITERIA IN SOLID TUMORS (RECIST) (33), as it has been in several of the
above mentioned studies (29,30,32).
Any reported increase in size or quantity was classified as disease
progression, whereas progression according to the RECIST criteria
is classified as an increase in size of >20%. Since these
criteria are not applied in routine clinical practice at our
institution, much higher rates of progressive disease may have been
documented in this patient population.
Currently, numerous clinical studies are evaluating
FOLFnal-IRI in various disease entities. Of these prospective
trials, six investigate its performance in advanced BTC. The phase
II NAPOLI-2 trial (no. NCT04005339) evaluates the clinical activity
of FOLFnal-IRI in a single-arm setting following gemcitabine and
platinum chemotherapy. In 2017, the ‘Working Group for Internal
Oncology of the German Cancer Society’-associated randomized phase
II trial NALIRICC (no. NCT03043547), which compares FOLFnal-IRI to
fluorouracil/folinic acid monotherapy in patients who progressed
after first-line chemotherapy with gemcitabine-based regimens was
started. Since 2018, another randomized phase II trial (no.
NCT03524508) is comparing FOLFnal-IRI to fluorouracil/folinic acid
monotherapy as second-line therapy after failure of initial
combination therapy with gemcitabine and cisplatin. In addition,
the randomized multicenter phase II trial NIFE (no. NCT03044587) is
comparing FOLFnal-IRI with gemcitabine and cisplatin as a
first-line treatment in patients with locally advanced or
metastatic BTC.
In addition, further improvement of FOLFnal-IRI is
under investigation. The NAPOLI-3 trial examines the efficacy of
the addition of oxaliplatin to FOLFnal-IRI in pancreatic cancer and
potential positive results may translate to further investigation
in BTC. Furthermore, novel therapeutic agents are being
investigated in combination with FOLFnal-IRI. ACCRU-GI-1603 (no.
NCT03337087) is a phase I/II trial testing FOLFnal-IRI in
combination with the PARP inhibitor rucaparib in BTC and other
gastrointestinal malignancies and simultaneous checkpoint
inhibition with nivolumab in combination with FOLFnal-IRI is being
investigated in a phase Ib/II trial (no. NCT03785873) as a
second-line treatment in advanced BTC. The results of these studies
are highly anticipated.
Recently, targeted therapy became an encouraging
option for subsequent therapy for BTC with fibroblast growth factor
receptor fusion or isocitrate dehydrogenase-1 mutations (34,35).
However, merely 10-15% of BTCs carry such targetable genetic
alterations, limiting the impact of these drugs in the overall
population of patients with advanced BTC (35,36).
While the results of the present analysis are
promising, its limitations should be mentioned. The sample size was
relatively small and the study was of a monocentric and
retrospective nature. The lack of a control group and proper
randomization may have led to survivorship bias, since healthier
patients are more likely to receive subsequent lines of
chemotherapy after failure of initial treatment regimens.
Considering the dismal results of second-line trials from the past
in this difficult-to-treat disease, further investigation of the
value of FOLFnal-IRI as a treatment option in BTC is
worthwhile.
In conclusion, FOLFnal-IRI demonstrated promising
antitumor potential with an acceptable safety profile as a
subsequent therapy regimen in advanced biliary tract malignancies.
Further results of the above-mentioned trials are highly
anticipated to determine its potential as a treatment option in
this difficult-to-treat entity.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GA, DW and FK designed the study and drafted the
manuscript. GA, ADC and NV acquired data and performed data
analysis. FK and DW checked and approved the authenticity of the
raw data. GA, DW and FK performed the statistical analysis. TG, RW,
FK and DW were involved in the conceptualization, methodology and
supervision of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
In accordance with the regional law (paragraph 15,
sentence 1, North Rhine Medical Association's professional code of
conduct from 14th November 1998 as amended on 16th November 2019,
and paragraph 6, sentence 1, Health Data Protection Act of North
Rhine-Westphalia) (26), approval
by a local ethics committee and written informed consent from the
participants were not required due to the strictly retrospective
design of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
von Hahn T, Ciesek S, Wegener G, Plentz
RR, Weismüller TJ, Wedemeyer H, Manns MP, Greten TF and Malek NP:
Epidemiological trends in incidence and mortality of hepatobiliary
cancers in Germany. Scand J Gastroenterol. 46:1092–1098.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saha SK, Zhu AX, Fuchs CS and Brooks GA:
Forty-year trends in cholangiocarcinoma incidence in the U.S.:
Intrahepatic disease on the rise. Oncologist. 21:594–599.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matsuo K, Rocha FG, Ito K, D'Angelica MI,
Allen PJ, Fong Y, Dematteo RP, Gonen M, Endo I and Jarnagin WR: The
blumgart preoperative staging system for hilar cholangiocarcinoma:
Analysis of resectability and outcomes in 380 patients. J Am Coll
Surg. 215:343–355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fong Y, Jarnagin W and Blumgart LH:
Gallbladder cancer: Comparison of patients presenting initially for
definitive operation with those presenting after prior noncurative
intervention. Ann Surg. 232:557–569. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tamandl D, Herberger B, Gruenberger B,
Puhalla H, Klinger M and Gruenberger T: Influence of hepatic
resection margin on recurrence and survival in intrahepatic
cholangiocarcinoma. Ann Surg Oncol. 15:2787–2794. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan
Z, Wan X, Liu G, Wu D, Shi L, et al: Prognostic nomogram for
intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin
Oncol. 31:1188–1195. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim
TY, Heo DS and Bang YJ: Gemcitabine-based
versusfluoropyrimidine-based chemotherapy with or without platinum
in unresectable biliary tract cancer: A retrospective study. BMC
Cancer. 8(374)2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lamarca A, Palmer DH, Wasan HS, Ross PH,
Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, et al:
ABC-06 | A randomised phase III, multi-centre, open-label study of
active symptom control (ASC) alone or ASC with oxaliplatin/5-FU
chemotherapy (ASC+mFOLFOX) for patients (pts) with locally
advanced/metastatic biliary tract cancers (ABC) previously-treated
with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol.
37(4003)2019.
|
|
11
|
Feisthammel J, Schoppmeyer K, Mössner J,
Schulze M, Caca K and Wiedmann M: Irinotecan With 5-FU/FA in
advanced biliary tract adenocarcinomas. Am J Clin Oncol.
30:319–324. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guion-Dusserre JF, Lorgis V, Vincent J,
Bengrine L and Ghiringhelli F: FOLFIRI plus bevacizumab as a
second-line therapy for metastatic intrahepatic cholangiocarcinoma.
World J Gastroenterol. 21:2096–2101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brieau B, Dahan L, De Rycke Y, Boussaha T,
Vasseur P, Tougeron D, Lecomte T, Coriat R, Bachet JB, Claudez P,
et al: Second-line chemotherapy for advanced biliary tract cancer
after failure of the gemcitabine-platinum combination: A large
multicenter study by the association des gastro-entérologues
oncologues. Cancer. 121:3290–3297. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lowery MA, Goff LW, Keenan BP, Jordan E,
Wang R, Bocobo AG, Chou JF, O'Reilly EM, Harding JJ, Kemeny N, et
al: Second-line chemotherapy in advanced biliary cancers: A
retrospective, multicenter analysis of outcomes. Cancer.
125:4426–4434. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schweitzer N, Kirstein MM, Kratzel AM,
Mederacke YS, Fischer M, Manns MP and Vogel A: Second-line
chemotherapy in biliary tract cancer: Outcome and prognostic
factors. Liver Int. 39:914–923. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kalra AV, Kim J, Klinz SG, Paz N, Cain J,
Drummond DC, Nielsen UB and Fitzgerald JB: Preclinical activity of
nanoliposomal irinotecan is governed by tumor deposition and
intratumor prodrug conversion. Cancer Res. 74:7003–7013.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leonard SC, Lee H, Gaddy DF, Klinz SG, Paz
N, Kalra AV, Drummond DC, Chan DC, Bunn PA, Fitzgerald JB and
Hendriks BS: Extended topoisomerase 1 inhibition through liposomal
irinotecan results in improved efficacy over topotecan and
irinotecan in models of small-cell lung cancer. Anticancer Drugs.
28:1086–1096. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Drummond DC, Noble CO, Guo Z, Hong K, Park
JW and Kirpotin DB: Development of a highly active nanoliposomal
irinotecan using a novel intraliposomal stabilization strategy.
Cancer Res. 66:3271–3277. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawato Y, Aonuma M, Hirota Y, Kuga H and
Sato K: Intracellular roles of SN-38, a metabolite of the
camptothecin derivative CPT-11, in the antitumor effect of CPT-11.
Cancer Res. 51:4187–4191. 1991.PubMed/NCBI
|
|
20
|
Kigawa J: New strategy for overcoming
resistance to chemotherapy of ovarian cancer. Yonago Acta Med.
56:43–50. 2013.PubMed/NCBI
|
|
21
|
Fukuda M, Nishio K, Kanzawa F, Ogasawara
H, Ishida T, Arioka H, Bojanowski K, Oka M and Saijo N: Synergism
between cisplatin and topoisomerase I inhibitors, NB-506 and SN-38,
in human small cell lung cancer cells. Cancer Res. 56:789–793.
1996.PubMed/NCBI
|
|
22
|
Kitajima K, Fukuoka M, Kobayashi S,
Kusunoki Y, Takada M, Negoro S, Matsui K, Sakai N, Ryu S and
Takifuji N: Studies on the appropriate administration of cisplatin
based on pharmacokinetics and toxicity. Gan To Kagaku Ryoho.
14:2517–2523. 1987.PubMed/NCBI(In Japanese).
|
|
23
|
Wang-Gillam A, Li CP, Bodoky G, Dean A,
Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et
al: Nanoliposomal irinotecan with fluorouracil and folinic acid in
metastatic pancreatic cancer after previous gemcitabine-based
therapy (NAPOLI-1): A global, randomised, open-label, phase 3
trial. Lancet. 387:545–557. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. Wiley Blackwell,
Oxford, 2017.
|
|
25
|
US Department of Health and Human
Services. National Institutes of Health NCI: Common Terminology
Criteria for Adverse Events (CTCAE) Common Terminology Criteria for
Adverse Events (CTCAE) v5.0. Accepted November 27, 2017.
|
|
26
|
Ärztekammer Nordrhein: Berufsordnung Für
Die Nordrheinischen Ärztinnen und Ärzte. Vom 14. November 1998 in
der Fassung vom 16. November 2019 (in Kraft getreten am 4. April
2020), 2019. Available from: https://www.aekno.de/aerzte/berufsordnung.
|
|
27
|
Nadler E, Espirito JL, Pavilack M, Boyd M,
Vergara-Silva A and Fernandes A: Treatment patterns and clinical
outcomes among metastatic non-small-cell lung cancer patients
treated in the community practice setting. Clin Lung Cancer.
19:360–370. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lamarca A, Hubner RA, David Ryder W and
Valle JW: Second-line chemotherapy in advanced biliary cancer: A
systematic review. Ann Oncol. 25:2328–2338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mizrahi JD, Gunchick V, Mody K, Xiao L,
Surapaneni P, Shroff RT and Sahai V: Multi-institutional
retrospective analysis of FOLFIRI in patients with advanced biliary
tract cancers. World J Gastrointest Oncol. 12:83–91.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moretto R, Raimondo L, De Stefano A, Cella
CA, Matano E, De Placido S and Carlomagno C: FOLFIRI in patients
with locally advanced or metastatic pancreatic or biliary tract
carcinoma. Anticancer Drugs. 24:980–985. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chibaudel B, Maindrault-Gœbel F, Bachet
JB, Louvet C, Khalil A, Dupuis O, Hammel P, Garcia ML, Bennamoun M,
Brusquant D, et al: PEPCOL: A GERCOR randomized phase II study of
nanoliposomal irinotecan PEP02 (MM-398) or irinotecan with
leucovorin/5-fluorouracil as second-line therapy in metastatic
colorectal cancer. Cancer Med. 5:676–683. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Taghizadeh H, Unseld M, Schmiderer A,
Djanani A, Wilthoner K, Buchinger D and Prager GW: First evidence
for the antitumor activity of nanoliposomal irinotecan with
5-fluorouracil and folinic acid in metastatic biliary tract cancer.
Cancer Chemother Pharmacol. 86:109–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Abou-Alfa GK, Macarulla T, Javle MM,
Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ,
Bridgewater J, et al: Ivosidenib in IDH1-mutant,
chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A
multicentre, randomised, double-blind, placebo-controlled, phase 3
study. Lancet Oncol. 21:796–807. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abou-Alfa GK, Sahai V, Hollebecque A,
Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson
D, Murphy AG, et al: Pemigatinib for previously treated, locally
advanced or metastatic cholangiocarcinoma: A multicentre,
open-label, phase 2 study. Lancet Oncol. 21:671–684.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Boscoe AN, Rolland C and Kelley RK:
Frequency and prognostic significance of isocitrate dehydrogenase 1
mutations in cholangiocarcinoma: A systematic literature review. J
Gastrointest Oncol. 10:751–765. 2019.PubMed/NCBI View Article : Google Scholar
|