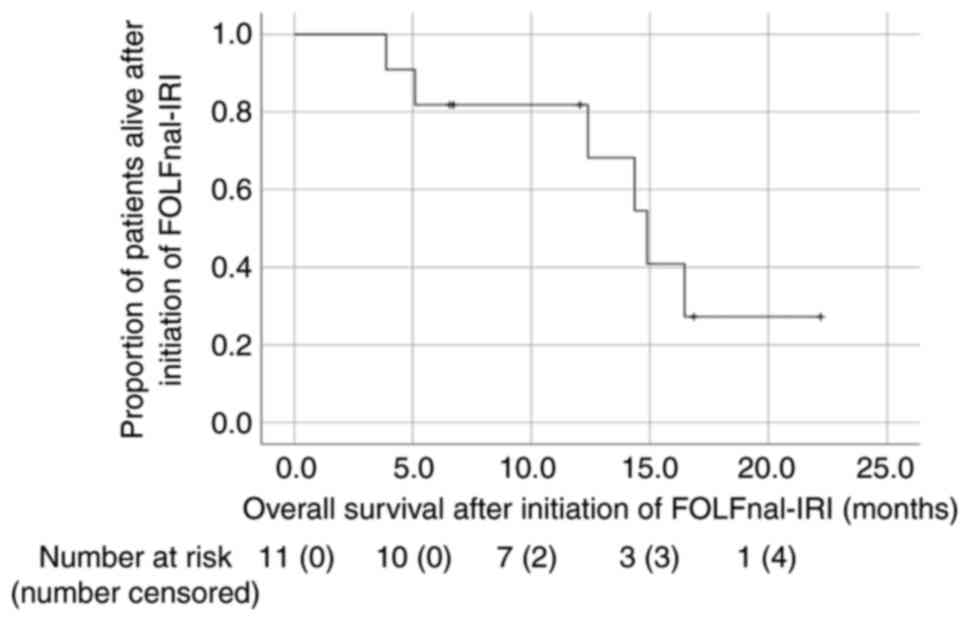
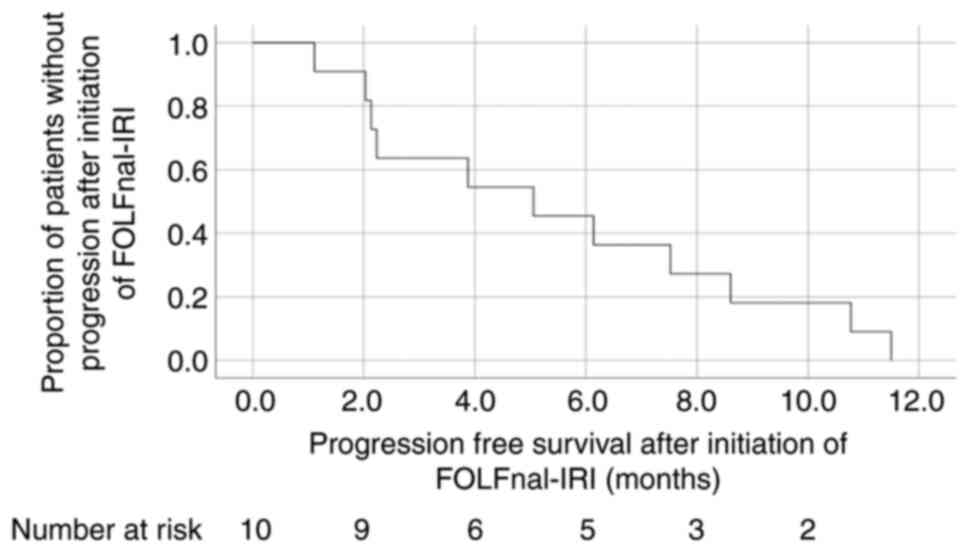
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
von Hahn T, Ciesek S, Wegener G, Plentz
RR, Weismüller TJ, Wedemeyer H, Manns MP, Greten TF and Malek NP:
Epidemiological trends in incidence and mortality of hepatobiliary
cancers in Germany. Scand J Gastroenterol. 46:1092–1098.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saha SK, Zhu AX, Fuchs CS and Brooks GA:
Forty-year trends in cholangiocarcinoma incidence in the U.S.:
Intrahepatic disease on the rise. Oncologist. 21:594–599.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matsuo K, Rocha FG, Ito K, D'Angelica MI,
Allen PJ, Fong Y, Dematteo RP, Gonen M, Endo I and Jarnagin WR: The
blumgart preoperative staging system for hilar cholangiocarcinoma:
Analysis of resectability and outcomes in 380 patients. J Am Coll
Surg. 215:343–355. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fong Y, Jarnagin W and Blumgart LH:
Gallbladder cancer: Comparison of patients presenting initially for
definitive operation with those presenting after prior noncurative
intervention. Ann Surg. 232:557–569. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tamandl D, Herberger B, Gruenberger B,
Puhalla H, Klinger M and Gruenberger T: Influence of hepatic
resection margin on recurrence and survival in intrahepatic
cholangiocarcinoma. Ann Surg Oncol. 15:2787–2794. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan
Z, Wan X, Liu G, Wu D, Shi L, et al: Prognostic nomogram for
intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin
Oncol. 31:1188–1195. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim
TY, Heo DS and Bang YJ: Gemcitabine-based
versusfluoropyrimidine-based chemotherapy with or without platinum
in unresectable biliary tract cancer: A retrospective study. BMC
Cancer. 8(374)2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lamarca A, Palmer DH, Wasan HS, Ross PH,
Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, et al:
ABC-06 | A randomised phase III, multi-centre, open-label study of
active symptom control (ASC) alone or ASC with oxaliplatin/5-FU
chemotherapy (ASC+mFOLFOX) for patients (pts) with locally
advanced/metastatic biliary tract cancers (ABC) previously-treated
with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol.
37(4003)2019.
|
|
11
|
Feisthammel J, Schoppmeyer K, Mössner J,
Schulze M, Caca K and Wiedmann M: Irinotecan With 5-FU/FA in
advanced biliary tract adenocarcinomas. Am J Clin Oncol.
30:319–324. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guion-Dusserre JF, Lorgis V, Vincent J,
Bengrine L and Ghiringhelli F: FOLFIRI plus bevacizumab as a
second-line therapy for metastatic intrahepatic cholangiocarcinoma.
World J Gastroenterol. 21:2096–2101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brieau B, Dahan L, De Rycke Y, Boussaha T,
Vasseur P, Tougeron D, Lecomte T, Coriat R, Bachet JB, Claudez P,
et al: Second-line chemotherapy for advanced biliary tract cancer
after failure of the gemcitabine-platinum combination: A large
multicenter study by the association des gastro-entérologues
oncologues. Cancer. 121:3290–3297. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lowery MA, Goff LW, Keenan BP, Jordan E,
Wang R, Bocobo AG, Chou JF, O'Reilly EM, Harding JJ, Kemeny N, et
al: Second-line chemotherapy in advanced biliary cancers: A
retrospective, multicenter analysis of outcomes. Cancer.
125:4426–4434. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schweitzer N, Kirstein MM, Kratzel AM,
Mederacke YS, Fischer M, Manns MP and Vogel A: Second-line
chemotherapy in biliary tract cancer: Outcome and prognostic
factors. Liver Int. 39:914–923. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kalra AV, Kim J, Klinz SG, Paz N, Cain J,
Drummond DC, Nielsen UB and Fitzgerald JB: Preclinical activity of
nanoliposomal irinotecan is governed by tumor deposition and
intratumor prodrug conversion. Cancer Res. 74:7003–7013.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leonard SC, Lee H, Gaddy DF, Klinz SG, Paz
N, Kalra AV, Drummond DC, Chan DC, Bunn PA, Fitzgerald JB and
Hendriks BS: Extended topoisomerase 1 inhibition through liposomal
irinotecan results in improved efficacy over topotecan and
irinotecan in models of small-cell lung cancer. Anticancer Drugs.
28:1086–1096. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Drummond DC, Noble CO, Guo Z, Hong K, Park
JW and Kirpotin DB: Development of a highly active nanoliposomal
irinotecan using a novel intraliposomal stabilization strategy.
Cancer Res. 66:3271–3277. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawato Y, Aonuma M, Hirota Y, Kuga H and
Sato K: Intracellular roles of SN-38, a metabolite of the
camptothecin derivative CPT-11, in the antitumor effect of CPT-11.
Cancer Res. 51:4187–4191. 1991.PubMed/NCBI
|
|
20
|
Kigawa J: New strategy for overcoming
resistance to chemotherapy of ovarian cancer. Yonago Acta Med.
56:43–50. 2013.PubMed/NCBI
|
|
21
|
Fukuda M, Nishio K, Kanzawa F, Ogasawara
H, Ishida T, Arioka H, Bojanowski K, Oka M and Saijo N: Synergism
between cisplatin and topoisomerase I inhibitors, NB-506 and SN-38,
in human small cell lung cancer cells. Cancer Res. 56:789–793.
1996.PubMed/NCBI
|
|
22
|
Kitajima K, Fukuoka M, Kobayashi S,
Kusunoki Y, Takada M, Negoro S, Matsui K, Sakai N, Ryu S and
Takifuji N: Studies on the appropriate administration of cisplatin
based on pharmacokinetics and toxicity. Gan To Kagaku Ryoho.
14:2517–2523. 1987.PubMed/NCBI(In Japanese).
|
|
23
|
Wang-Gillam A, Li CP, Bodoky G, Dean A,
Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et
al: Nanoliposomal irinotecan with fluorouracil and folinic acid in
metastatic pancreatic cancer after previous gemcitabine-based
therapy (NAPOLI-1): A global, randomised, open-label, phase 3
trial. Lancet. 387:545–557. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. Wiley Blackwell,
Oxford, 2017.
|
|
25
|
US Department of Health and Human
Services. National Institutes of Health NCI: Common Terminology
Criteria for Adverse Events (CTCAE) Common Terminology Criteria for
Adverse Events (CTCAE) v5.0. Accepted November 27, 2017.
|
|
26
|
Ärztekammer Nordrhein: Berufsordnung Für
Die Nordrheinischen Ärztinnen und Ärzte. Vom 14. November 1998 in
der Fassung vom 16. November 2019 (in Kraft getreten am 4. April
2020), 2019. Available from: https://www.aekno.de/aerzte/berufsordnung.
|
|
27
|
Nadler E, Espirito JL, Pavilack M, Boyd M,
Vergara-Silva A and Fernandes A: Treatment patterns and clinical
outcomes among metastatic non-small-cell lung cancer patients
treated in the community practice setting. Clin Lung Cancer.
19:360–370. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lamarca A, Hubner RA, David Ryder W and
Valle JW: Second-line chemotherapy in advanced biliary cancer: A
systematic review. Ann Oncol. 25:2328–2338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mizrahi JD, Gunchick V, Mody K, Xiao L,
Surapaneni P, Shroff RT and Sahai V: Multi-institutional
retrospective analysis of FOLFIRI in patients with advanced biliary
tract cancers. World J Gastrointest Oncol. 12:83–91.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moretto R, Raimondo L, De Stefano A, Cella
CA, Matano E, De Placido S and Carlomagno C: FOLFIRI in patients
with locally advanced or metastatic pancreatic or biliary tract
carcinoma. Anticancer Drugs. 24:980–985. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chibaudel B, Maindrault-Gœbel F, Bachet
JB, Louvet C, Khalil A, Dupuis O, Hammel P, Garcia ML, Bennamoun M,
Brusquant D, et al: PEPCOL: A GERCOR randomized phase II study of
nanoliposomal irinotecan PEP02 (MM-398) or irinotecan with
leucovorin/5-fluorouracil as second-line therapy in metastatic
colorectal cancer. Cancer Med. 5:676–683. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Taghizadeh H, Unseld M, Schmiderer A,
Djanani A, Wilthoner K, Buchinger D and Prager GW: First evidence
for the antitumor activity of nanoliposomal irinotecan with
5-fluorouracil and folinic acid in metastatic biliary tract cancer.
Cancer Chemother Pharmacol. 86:109–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Abou-Alfa GK, Macarulla T, Javle MM,
Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ,
Bridgewater J, et al: Ivosidenib in IDH1-mutant,
chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A
multicentre, randomised, double-blind, placebo-controlled, phase 3
study. Lancet Oncol. 21:796–807. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abou-Alfa GK, Sahai V, Hollebecque A,
Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson
D, Murphy AG, et al: Pemigatinib for previously treated, locally
advanced or metastatic cholangiocarcinoma: A multicentre,
open-label, phase 2 study. Lancet Oncol. 21:671–684.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Boscoe AN, Rolland C and Kelley RK:
Frequency and prognostic significance of isocitrate dehydrogenase 1
mutations in cholangiocarcinoma: A systematic literature review. J
Gastrointest Oncol. 10:751–765. 2019.PubMed/NCBI View Article : Google Scholar
|