Introduction
Positron emission tomography/computer tomography
(PET/CT) using fluorine-18-fluoro-2-deoxy-d-glucose
(18F-FDG PET/CT) is a hybrid imaging modality widely
used for staging, assessment of response to treatment and restaging
of lymphoma (1-4).
Because many lymphoma patients have significant immune suppression
from anti-lymphoma treatment, lymphoma patients were susceptible to
coronavirus disease 2019 (COVID-19) infection (5). It is important to recognize pulmonary
changes caused by COVID-19 infection in lymphoma patient without
clinical symptoms of COVID-19 pneumonia. There are different
patterns of pulmonary abnormalities of COVID-19 pneumonia on chest
CT (6-10).
The predominant pattern of early stage COVID-19 pneumonia on chest
CT is bilateral and multifocal ground-glass opacities. In contrast,
the predominant pattern of late stage COVID-19 pneumonia is mixed
pattern of consolidation and ground-glass opacities, and some
including a reticular pattern associated with bronchiolectasis and
irregular interlobular or septal thickening. Recognition of the
patterns of pulmonary abnormalities at different stages of COVID-19
pneumonia is important for incidental diagnosis of COVID-19
pneumonia in lymphoma patients who present for staging or restaging
of lymphoma with 18F-FDG PET/CT (11-13).
Because initiation of chemotherapy may cause undesirable
exacerbation of COVID-19 pneumonia, it is crucial to avoid
chemotherapy in lymphoma patients with active COVID-19 infection
(5).
Here, we report a case of a lymphoma patient with
incidental findings of COVID-19 pneumonia on restaging
18F-FDG PET/CT. Additional chemotherapy was delayed
based on the incidental findings of COVID-19 pneumonia in this
patient. Two weeks later, the patient received additional
chemotherapy when the patient tested negative for active COVID-19
infection and a complete metabolic response to lymphoma treatment
was confirmed by follow up 18F-FDG PET/CT. The findings
from this case report demonstrated the importance of recognizing
pulmonary abnormalities of COVID-19 pneumonia on 18F-FDG
PET/CT in clinical management of lymphoma patients during COVID-19
pandemic (5).
Case report
A 29-year-old man with no past medical history
presented with tender right supraclavicular and axillary masses
that had been enlarging over a month. The patient endorsed
subjective fevers, chills, and night sweats. There were no
overlying erythema or skin changers over the masses. The patient
had an elevated white blood cell count (26.48x109/l),
normal RBC (4.58x109/l), hemoglobin (11.9 g/dl), and
slightly elevated platelets (490x109/l). Lymphadenitis
of infectious etiology such as tuberculosis and lymphoma were among
differential diagnoses. The patient completed a one week course of
treatment with amoxicillin, without improvement of lymphadenopathy.
The patient underwent biopsy of a right supraclavicular lymph node
that confirmed the diagnosis of nodular sclerosis classical
Hodgkin's lymphoma.
18F-FDG PET/CT was performed in a
protocol similar to that previously described (14). Briefly, PET acquisition was
obtained from mid-thigh to base of skull (5 min/bed) using a
Siemens Biograph scanner, starting at 60 min post intravenous
injection of 397.75 MBq (10.75 mCi) of 18F-FDG. The
non-contrast CT scans (200 mAs, 120 kV, 0.5 sec rotation time, 5 mm
slice, in a caudal-to-cranial direction) were used for attenuation
correction and localization. Transaxial, coronal and sagittal PET
images were reviewed in conjunction with fused non-contrast CT.
Whole body biodistribution of radiotracer activity was assessed by
visual assessment and semi-quantitative analysis was performed to
determine radiotracer concentration (standardized uptake value,
SUV), in reference to blood pool radiotracer activity measured from
descending aorta and liver radiotracer activity measured from right
hepatic lobe.
On the whole body PET/CT images (base of skull to
mid-thigh), there was FDG-vid lymphadenopathy with multiple
hypermetabolic nodal lesions in the right axilla, right subpectoral
region, left axilla, and mediastinum, along with possible pleural
metastases (Fig. 1A-C). The
patient underwent chemotherapy for treatment of lymphoma with ABVD
(a chemotherapy combination that includes Adriamycin or
doxorubicin, bleomycin, vinblastine and dacarbazine or DTIC). After
completion of two cycles of ABVD, the patient underwent interim
18F-FDG PET/CT for assessment of treatment response
(2,4). There was interval resolution of
multiple previously noted FDG avid mediastinal nodes and decrease
of FDG uptake by a few mediastinal lymph nodes (decrease of the
SUVmax of index left para-aortic lymph node from 9.0 to 4.0),
suggesting a good treatment response. However, there were
bilateral, multilobar, basal predominant peripheral FDG-avid
ground-glass and consolidative opacities, some with a rounded
morphology (Fig. 1D-F). Multiple
new FDG avid lymph nodes were detected in the right hilum and
mediastinum, including 1.5 cm subcarinal lymph node with SUVmax 9.4
and 1 cm right paratracheal lymph node with SUVmax 9.9. The
findings were highly suspicious for COVID-19 pneumonia. On clinical
evaluation, the patient had fever and fatigue. The patient tested
positive for COVID-19 coronavirus infection the next day by Roche
SARS-COV-2 polymerase chain reaction (PCR) test.
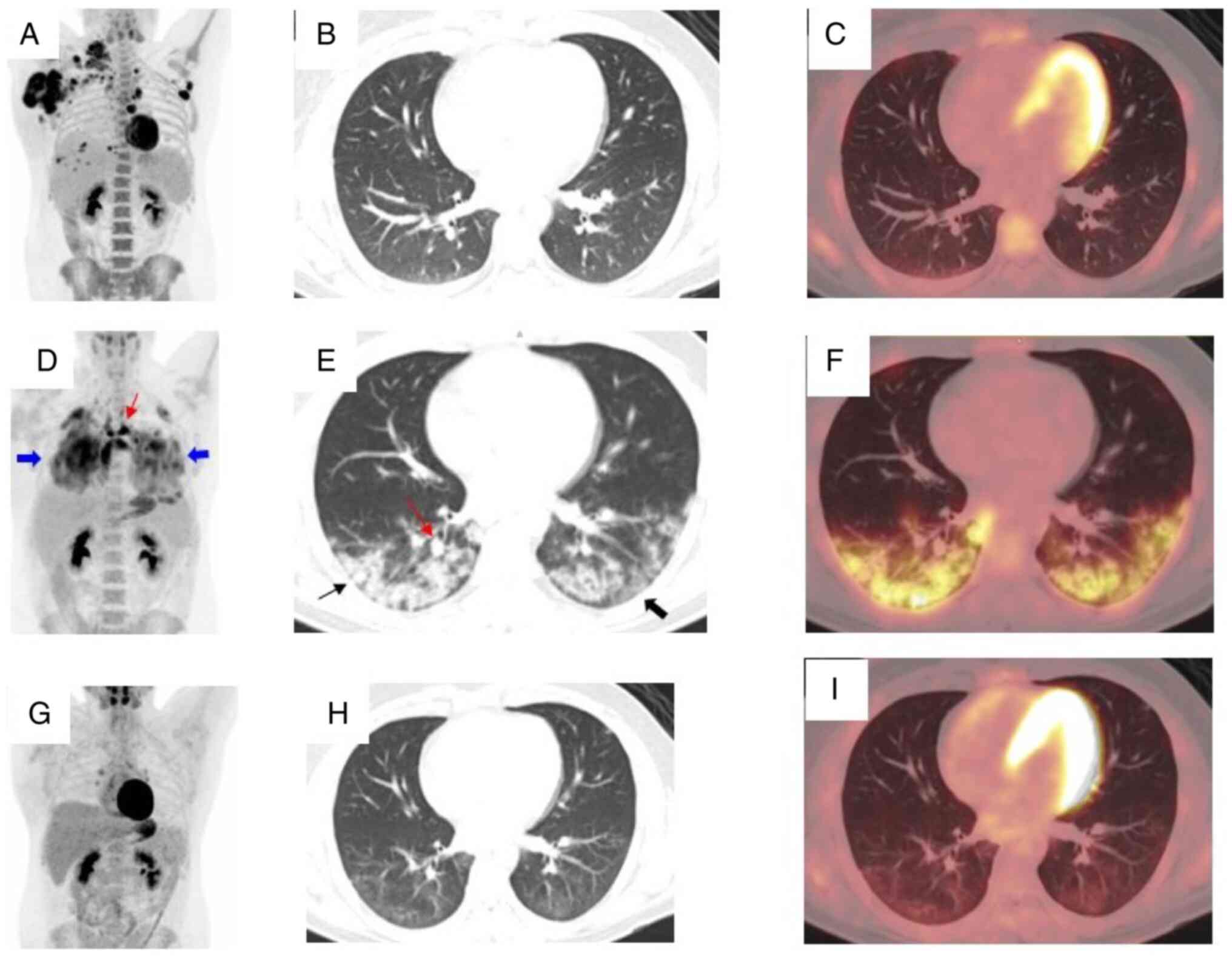 | Figure 1Incidental finding of COVID-19
pneumonia in a patient with lymphoma who presented for evaluation
of lymphoma treatment. (A) MIP image of the initial staging
18F-FDG PET/CT demonstrated multiple hypermetabolic
nodal lesions in the right axilla, right subpectoral region, left
axilla and mediastinum, along with possible pleural metastases. (B)
Axial CT and (C) hybrid PET/CT images were negative for pulmonary
air space opacities. (D) MIP image showing interval decrease of FDG
uptake in previously seen lymphadenopathy, suggesting good response
to chemotherapy. However, there were diffuse increased FDG uptake
in the bilateral lungs (thick blue arrow) and new foci of abnormal
increased FDG uptake in the mediastinum (thin red arrow). (E) Axial
CT and (F) hybrid PET/CT images of the chest demonstrated abnormal
increased FDG uptake by numerous new pulmonary airspace opacities.
The pulmonary opacities were bilateral, peripheral, multilobar,
either ground-glass (thick black arrow) or consolidative (thin
black arrow), and some with a rounded morphology (thin red arrow),
which are all classic CT manifestations of COVID-19 pneumonia. (G)
MIP image of follow-up FDG PET demonstrated interval decrease of
diffuse abnormal FDG uptake in the bilateral lungs and foci of FDG
uptake in the mediastinum. (H) Axial CT and (I) hybrid PET/CT
images demonstrated interval resolution of residual FDG avid
mediastinal lymphadenopathy and near complete resolution of
previously seen FDG-avid peripheral pulmonary opacities, with very
faint FDG uptake by the small residual ground glass opacities. (G,
H and I) These images were acquired 2 month after the patient
tested negative for COVID-19 and 1 month after two cycles of AVD.
18F-FDG PET/CT, positron emission tomography/computed
tomography using fluorine-18-fluoro-2-deoxy-d-glucose; AVD, a
chemotherapy combination that includes Adriamycin or doxorubicin,
vinblastine, and dacarbazine or DTIC; MIP, maximum intensity
projection. |
Additional cycles of chemotherapy were postponed due
to COVID-19 pneumonia. One month after diagnosis of COVID-19
pneumonia, the patient received 4 cycles of chemotherapy with AVD
(a chemotherapy combination that includes Adriamycin or
doxorubicin, vinblastine, and dacarbazine or DTIC). Bleomycin was
discontinued per the Response Adapted Treatment in Hodgkin Lymphoma
trial (Rathl Trial) based on good response to initial two cycles of
ABVD demonstrated by FDG PET/CT imaging. On follow-up
18F-FDG PET/CT images obtained two months after
diagnosis of COVID-19 and 4 cycles of chemotherapy with AVD, there
was interval resolution of residual mediastinal nodes related to
lymphoma such as a residual left para-aortic lymph node and
decrease of focal FDG uptake by the lymph nodes in the mediastinum
related to COVID-19 pneumonia (decrease of the SUVmax of index
subcarinal lymph node from 9.4 to 3.8), along with decrease of
diffuse FDG uptake in the bilateral lungs, indicating complete
metabolic response of lymphoma to chemotherapy and improvement of
COVID-19 pneumonia (Fig.
1G-I).
Discussion
Typical CT findings of COVID-19 pneumonia include
bilateral ground-glass opacities with or without consolidation or
intralobular lines (‘crazy-paving’) in a peripheral, and lower lung
zone distribution. FDG avid multifocal ground-glass opacities with
rounded morphology or FDG-avid focal consolidations with central
ground-glass attenuation (reverse halo sign) are also typical
manifestations on FDG PET/CT imaging. Non-peripheral, non-rounded
groundglass opacities with diffuse, unilateral, multifocal or
perihilar distribution is indeterminate for COVID-19 pneumonia and
can also be seen with other infectious or non-infectious processes.
Lobar consolidation and discrete centrilobular nodules are atypical
for COVID-19 pneumonia (10).
In this patient, the findings of bilateral,
multilobar, basal predominant peripheral FDG-avid ground-glass and
consolidative opacities were similar to the findings on the case
report by Playe et al (11)
and different from the findings of bilateral tree-in-bud opacities
and several peripheral and subpleural ground-glass opacities with
mild FDG activity on the case report by Boulvard Chollet et
al (12). Additionally, there
were new subcentimeter FDG-avid lymph nodes in the right hilum and
mediastinum, in addition to multiple peripheral, multilobar,
FDG-avid ground-glass and consolidative opacities in bilateral
lungs. On the follow-up 18F-FDG PET/CT images, there
were interval resolution of residual FDG avid mediastinal
lymphadenopathy and near complete resolution of previously seen
FDG-avid peripheral pulmonary opacities, with very faint FDG uptake
by the small residual ground glass opacities. To the best of our
knowledge this is the first case report regarding changes of
pulmonary and mediastinal abnormalities in lymphoma patient with
COVID-19 pneumonia prior to and after additional chemotherapy. It
is imperative to consider both inflammatory lymphadenopathy
associated with COVID-19 pneumonia and residual or recurrent
lymphoma in patients with incidental findings of COVID-19
pneumonia.
Early diagnosis of COVID-19 pneumonia through
recognition of pulmonary abnormalities on FDG PET/CT is important
for the treatment of lymphoma patients who are often
immunocompromised and might be more vulnerable to complications
caused by COVID-19 pneumonia (5),
particularly for the treatment of the lymphoma patients with
unexpected SARS-Cov-2 co-infection despite double reverse
transcription-PCR negativity (12). In addition to challenges in the
timely completion of the staging and restaging studies, clinicians
might encounter the challenges of making complex treatment
decisions in management of patients with hematologic malignancies
during the COVID-19 pandemic (5).
It was recommended to hold treatment of COVID-19 positive multiple
myeloma patients for at least 2 to 3 weeks and antineoplastic
therapy should be reintroduced only after complete convalescence,
ensuring safety (5). It might be
also appropriate to hold chemotherapy for 2 to 3 weeks in lymphoma
patients with incidental CT findings of pulmonary abnormalities
associated with COVID-19 pneumonia, in order to ensure safety. As
illustrated in this case report, incidental diagnosis of COVID-19
pneumonia in this patient helped oncologists to decide to postpone
chemotherapy in order to avoid exacerbation of COVID-19 pneumonia.
The patients tolerated chemotherapy well that was received one
month after incidental diagnosis of COVID-19 pneumonia, and a
complete metabolic response to chemotherapy that was confirmed by
follow up 18F-FDG PET/CT imaging. Moreover, incidental
diagnosis of COVID-19 pneumonia facilitated accurate interpretation
of restaging 18F-FDG PET/CT imaging by distinguishing
FDG avid nodal disease of lymphoma from FDG avid lymphadenopathy
related to COVID-19 pneumonia.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM, AK and FP contributed to conception and design,
and acquisition, analysis and interpretation of data. JM and FP
contributed to drafting, and revision of the manuscript. AK
contributed to revising the manuscript critically for important
intellectual content. JM, AK and FP have confirmed the authenticity
of all the raw data, and agreed to be accountable for all aspects
of the work in ensuring that questions related to the accuracy or
integrity of any part of the work were appropriately investigated
and resolved. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the study involving
human participants were performed in accordance with the ethical
standards of the institutional and/or national research committee
and with the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. The case report protocol was approved
by the Institutional Review Board (IRB# STU 102014-055) of the
University of Texas Southwestern Medical Center, Dallas, TX, USA.
The requirement for informed consent to participate was waived
based on the approved protocol of the retrospective case
report.
Patient consent for publication
The requirement for patient consent for publication
was waived based on the protocol of the retrospective case report
approved by the Institutional Review Board (IRB# STU 102014-055) of
the University of Texas Southwestern Medical Center (Dallas, TX,
USA).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weihrauch MR, Re D, Bischoff S, Dietlein
M, Scheidhauer K, Krug B, Textoris F, Ansén S, Franklin J, Bohlen
H, et al: Whole-body positron emission tomography using
18F-fluorodeoxyglucose for initial staging of patients with
Hodgkin's disease. Ann Hematol. 81:20–25. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheson BD: Role of functional imaging in
the management of lymphoma. J Clin Oncol. 29:1844–1854.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Alliance
Australasian Leukaemia and Lymphoma Group; Eastern Cooperative
Oncology Group et al. Recommendations for initial
evaluation, staging, and response assessment of hodgkin and
non-hodgkin lymphoma: The lugano classification. J Clin Oncol.
32:3059–3068. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gallamini A and Zwarthoed C: Interim
FDG-PET imaging in lymphoma. Semin Nucl Med. 48:17–27.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Isidori A, de Leval L, Gergis U, Musto P
and Porcu P: Management of patients with hematologic malignancies
during the COVID-19 pandemic: Practical considerations and lessons
to be learned. Front Oncol. 10(1439)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zou S and Zhu X: FDG PET/CT of COVID-19.
Radiology. 296(E118)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Berhheim A, Mei X, Huang M, Yang Y, Fayad
ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, et al: Chest CT findings
in coronavirus disease 2019 (COVID-19): relationship to duration of
infection. Radiology. 295:685–691. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dane B, Brusca-Augello G, Kim D and Katz
DS: Unexpected findings of coronavirus disease (COVID-19) at the
lung based on abdominopelvic CT. AJR Am J Roentgenol. 215:603–605.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shi H, Han X, Jiang N, Cao Y, Alwalid O,
Gu J, Fan Y and Zheng C: Radiological findings from 81 patients
with COVID-19 pneumonia in Wuhan, China: A descriptive study.
Lancet Infect Dis. 20:425–434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Simpson S, Kay FU, Abbara S, Bhalla S,
Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP and Litt
H: Radiological society of North America expert consensus document
of reporting chest CT findings related to COVID-19 endorsed by the
society of thoracic radiology, the American college of radiology,
and RSNA. Radiol Cardiothorac Imaging. 2(e200152)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Playe M, Siavellis J, Braun T and Soussan
M: FDG PET/CT in a patient with mantle cell lymphoma and COVID-19:
Typical findings. Clin Nucl Med. 45:e305–e306. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chollet XLE, Robles LG, Garrastachu P,
Villegas AC, Almada MCA, Colletti PM, Rubello D, Lasanta RR and
Bolton RCD: 18F-FDG PET/CT in hodgkin lymphoma with
unsuspected COVID-19. Clin Nucl Med. 45:652–653. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zanoni L, Mosconi C, Cervati V, Diegoli M,
Monteduro F, Golfieri R and Fanti S: [18F]-FDG PET/CT
for suspected lymphoma relapse in a patient with concomitant
pneumococcal pneumonia during COVID-19 outbreak: Unexpected
SARS-Cov-2 co-infection despite double RT-PCR negativity. Eur J
Nucl Med Mol Imaging. 47:2038–2039. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Iyamu I, Wachsmann J, Truelson J, Mathews
D and Peng F: Detection of widespread metastasis in a case of
aggressive carcinoma showing thymuslike differentiation (CASTLE
Disease) Using 18F-FDG PET/CT. Clin Nucl Med. 40:689–691.
2015.PubMed/NCBI View Article : Google Scholar
|















