Introduction
Pulmonary malignant tumors are one of the frequently
occurring diseases worldwide; according to reports, the incidence
rate of lung cancer is 57.63/100,000, with an annual lung
cancer-related mortality rate of 48.8/100,000 (1,2).
With the continuous technical development of
low-dose spiral computed tomography (CT), the detection rate of
early lung cancer has greatly improved (3). Although surgical resection does
remain the primary and preferred approach for the treatment of lung
cancer, its extensive invasiveness, as well as lobectomy, can have
a profound effect on pulmonary function (4). When there are contraindications to
surgery (such as pulmonary dysfunction or comorbid medical
conditions) or patients refuse surgical procedures, minimally
invasive surgical techniques [such as radiofrequency ablation
(RFA), microwave ablation (MWA) and cryoablation] can be used,
which are effective, less invasive and less detrimental for
pulmonary function, particularly for patients with limited
pulmonary reserve (5). For early
lung cancer, the primary purpose of tumor ablation therapy is to
ensure eradication of all malignant cells, including a margin of
normal tissue; for advanced lung cancer, the main purpose is to
reduce tumor cell volume and minimize tumor burden (6). Among the ablation techniques, MWA has
been used with increasing frequency in the treatment of pulmonary
tumors. Percutaneous cryoablation, a relatively new ablation
technique, possesses several advantageous properties, such as good
visualization under CT or MRI guidance, minimal intra-procedural
pain and preservation of collagenous architecture, which are
conducive to application to the treatment of cancer in various
non-aerated organs, such as the liver, kidney and pancreas
(7,8). However, studies comparing the
performance of microwave ablation (MWA) vs. cryoablation in primary
or metastatic pulmonary malignant tumors remain scarce. The aim of
the present study was to compare the effectiveness and
complications associated with these two methods in the treatment of
pulmonary malignant tumors, and provide a basis for follow-up
research that guides clinical decision making in the treatment of
lung cancer.
Materials and methods
Patients and tumor criteria
In this retrospective study, the records of 48
consecutive patients (34 male patients and 14 female patients;
median age, 59 years; range, 45-73 years) who underwent MWA or
cryoablation procedures for primary or metastatic pulmonary
malignant tumors in The Third Hospital of Mianyang and The
Affiliated Hospital of North Sichuan Medical College between June
2014 and June 2018 were reviewed. Inclusion criteria for the
present study were as follows: i) Patients with a general condition
where they cannot tolerate thoracotomy, such as poor lung function
and elderly age; and ii) early lung cancer where there are
indications for surgical resection, but patients refused surgery.
The exclusion criteria were as follows: i) Tumor diameter >5 cm;
ii) severe pulmonary dysfunction, maximum ventilation volume
<39% or poor general condition; and iii) severe bleeding
diathesis. The final study group comprised of 29 patients in the
MWA group and 19 patients in the cryoablation group. The baseline
characteristics of the two groups prior to treatment are shown in
Table I. The median preoperative
Karnofsky Performance Status scale scores were >80. The
histological distribution and tumor location of primary and
secondary pulmonary malignancies are summarized in Table II. The present study was reviewed
and approved by the Ethics Committee of Affiliated Hospital of
North Sichuan Medical College. Written informed consent was
obtained from all the patients enrolled in the study.
 | Table IComparison of baseline characteristics
between the two groups of patients. |
Table I
Comparison of baseline characteristics
between the two groups of patients.
| Baseline
characteristic | Microwave
ablation | Cryoablation | P-value |
|---|
| Patients, n | 29 | 19 | |
| Age, years | 58.48±7.86 | 60.95±7.45 | 0.28 |
| Sex, n | | | 0.77 |
|
Male | 21 | 13 | |
|
Female | 8 | 6 | |
| KPS score | 87.35±4.09 | 87.42±3.31 | 0.94 |
| Tumor type, n | | | 0.50 |
|
Primary | 21 | 12 | |
|
Metastasis | 8 | 7 | |
| Tumor size, cm | 2.43±0.71 | 2.01±0.53 | 0.03 |
|
<3
cm | 2.11±0.37 | 1.88±0.39 | 0.72 |
|
≥3 cm | 3.46±0.52 | 3.1±0.14 | 0.39 |
| UICC stage, n | | | 0.97 |
|
I + II | 20 | 13 | |
|
III +
IV | 9 | 6 | |
| Ablation session,
n | | | 0.92 |
|
1 | 21 | 14 | |
|
≥2 | 8 | 5 | |
| Combined with
chemotherapy, n | | | 0.87 |
|
Yes | 21 | 15 | |
|
No | 8 | 4 | |
| Combined with
radiation therapy, n | | | 0.98 |
|
Yes | 6 | 4 | |
|
No | 23 | 15 | |
| Combined with
surgical resection, n | | | 0.65 |
|
Yes | 6 | 5 | |
|
No | 23 | 14 | |
 | Table IIHistological distribution and tumor
location of pulmonary malignant tumors. |
Table II
Histological distribution and tumor
location of pulmonary malignant tumors.
| Criterion | Microwave ablation,
n (%) | Cryoablation, n
(%) | P-value |
|---|
| Tumor type | | | 0.50 |
|
Primary
(NSCLC) | 21 (72.41) | 12 (63.16) | |
|
Secondary | 8 (27.59) | 7 (34.84) | |
| Primary tumor
(NSCLC) type | | | 0.69 |
|
Squamous
carcinoma | 4 (13.79) | 3 (15.79) | |
|
Adenocarcinoma | 17 (58.62) | 9 (47.36) | |
| Secondary
tumor | | | |
|
Colorectal | 3 (10.34) | 4 (21.05) | |
|
Hepatocellular | 2 (6.70) | 0 (0) | |
|
Breast | 2 (6.70) | 2 (10.53) | |
|
Renal cell
carcinoma | 1 (3.45) | 1 (5.26) | |
| Tumor location | | | 0.90 |
|
Central | 5 (17.24) | 3 (15.79) | |
|
Peripheral | 24 (82.76) | 16 (84.21) | |
Ablation technique
All of the lung MWA and cryoablation were performed
by using CT (Philips MX16; Koninklijke Philips N.V.) with the
following parameters: section thickness, 3-6 mm; 20-40 mAs; and
120-150 kV.
MWA procedure
MWA was performed with a KY-2000 microwave
multi-function therapeutic instrument (Jiangsu Kangyou Medical
Instrument Co., Ltd.), which can produce 10-100 W (continuously
adjustable) of power at a microwave frequency of 2,450 MHz. A
microwave antenna (14-20 gauge, depending on tumor size and
location) was inserted into the lesion.
Cryoablation procedure
A cryoablation therapeutic instrument [CryoHit
argon-helium cryoablation system; AccuTarget MediPharma (Shanghai)
Co., Ltd.] was used, which can reduce the needle temperature to
between -120 and -165˚C. The specifications of the argon-helium
puncture needle are 14G, 16G and 18G. Cryoablation was performed
using a three-cycle freeze-thaw phase protocol. The freezing
temperatures ranged from -140˚C to -165˚C, and 20-40˚C for thawing.
The times for each phase were recorded and varied depending on the
size of the tumor (target times: freeze, 3 min; thaw, 3 min;
freeze, 8 min; thaw, 5 min; freeze, 8 min; followed by active
thawing). For lesions <3.0 cm in diameter, one antenna or
cryoprobe was inserted, whereas two antennas or cryoprobes were
inserted for lesions >3.0 cm. Each procedure was monitored using
non-contrast CT imaging at intervals of 3-5 min to visualize the
growing ablation zone, with the goal of achieving a circumferential
margin of 0.5 cm beyond the tumor. If the tumor was not ablated in
one session, multiple sequential ablations based on tumor size,
location and geometry were performed to achieve complete
necrosis.
All treatments were performed by one board-certified
interventional radiologist with patients under local anesthesia
(subcutaneous injection of 2% lidocaine). The patients were
continuously monitored throughout the procedure with
electrocardiography and pulse oximetry. Blood pressure was measured
and recorded at 5-min intervals. At the end of every procedure, a
CT scan was performed to identify any complications, after which
the patients were transferred to the in-patient ward for 24-h
observation.
Measurement of intraprocedural
pain
Prior to the commencement of the procedure, the
visual analog scale (VAS) was introduced to patients as a
measurement of intraprocedural pain. The VAS consists of a 10-cm
line anchored at one end by a label ‘no pain, score 0’ and at the
other end by a label ‘pain as bad as can be, score 10’ (9). After the procedure, the patients were
instructed to report the severity of pain felt during the MWA or
cryoablation procedure using the VAS.
Complication, follow-up and
evaluation
Complications were recorded on a per-treatment basis
and classified in accordance with the Common Terminology Criteria
for Adverse Events (CTCAE) (10).
Patients were followed up at the outpatient department or by
contacting through telephone. Patients were reviewed by performing
contrast-enhanced CT or positron emission tomography (PET)/CT, in
addition to laboratory examination, to evaluate ablation efficacy.
In both the MWA and cryoablation groups, the initial follow-up
contrast-enhanced CT was generally performed monthly for the first
3 months, at 3-month intervals after that for the rest of the first
year, and then annually thereafter. PET/CT was generally carried
out when patients had severe iodine contrast agent allergy, or when
local control and/or systemic progression needed to be evaluated.
Irregular focal soft-tissue enhancement (>15 HU) or increased
uptake in the PET/CT were defined as a sign of residual cancer or
cancer recurrence (11,12). Outcomes were evaluated according to
Response Evaluation Criteria in Solid Tumors protocol (13): Lesion disappearance (scar) or
<5% of original size is defined as complete response (CR);
partial response (PR) is ≥30% decrease; stable disease (SD)
exhibits no change; and progressive disease (PD) is ≥20% increase
in the sum of the longest diameter of the target lesion.
Postoperative complications were followed up by CT scan at 1 month
after the ablation. Telephone follow-up mainly enquired about
symptoms, quality of life and survival.
Statistical analysis
All of the data processing was performed with SPSS
statistical software 23.0 (IBM Corp.). Measurement and numeration
data were analyzed using the χ2 test and unpaired
t-test, respectively, to compare the two groups. VAS scores were
assessed using a Mann-Whitney test. Fisher's exact test was used
when expected number of cases was ≤5. The χ2 or Fisher's
exact test was used to analyze the CR, PR, SD and PD of the two
groups. Overall survival (OS) rates were estimated according to the
life-table method. Kaplan-Meier survival analyses was used to
calculate survival curves at 6, 12, 24 and 36 months after MWA and
cryoablation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Intraprocedural pain, and short- and
long-term efficacy evaluation
The patients in the MWA group reported more pain
than those in cryoablation group; the VAS scores in the MWA group
were significantly increased compared with those in the
cryoablation group (P<0.001; Table III). The short-term efficacy
rates (CR + PR) in the MWA and cryoablation groups were 72.41%
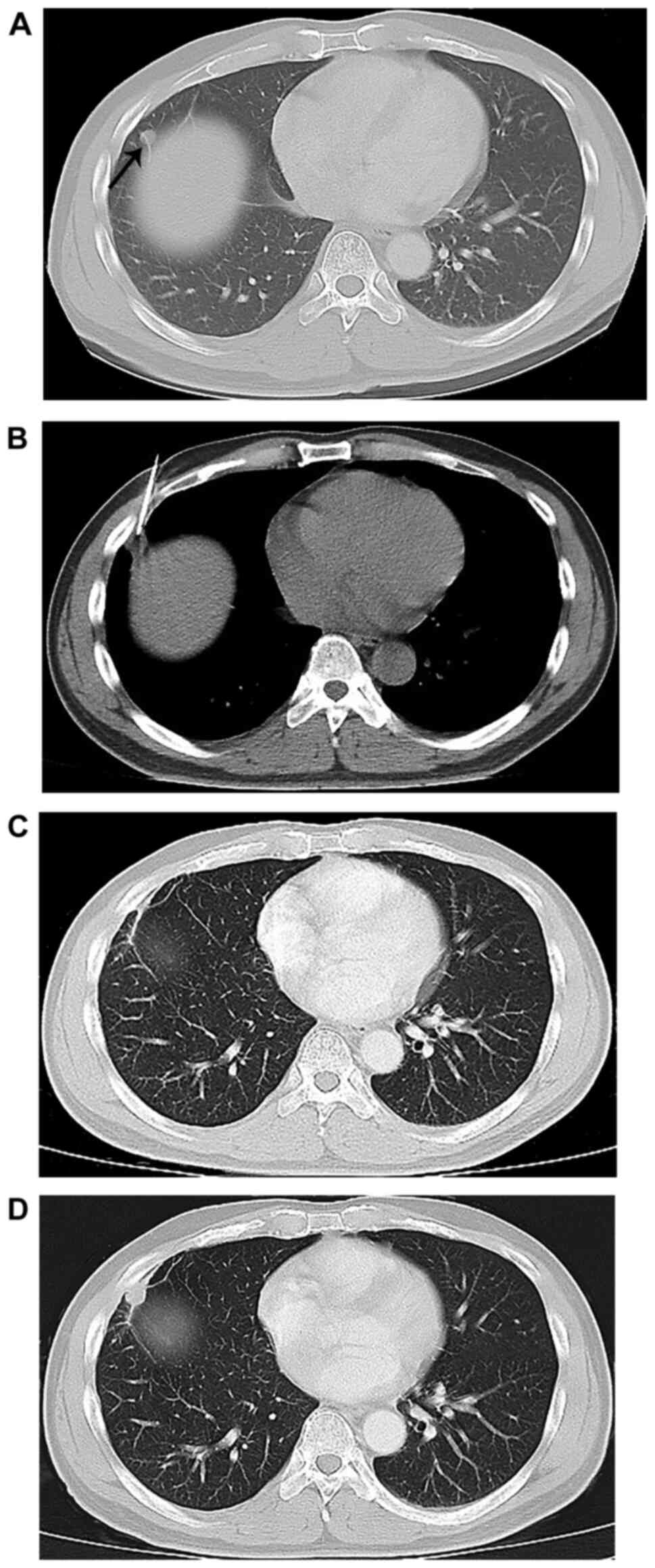
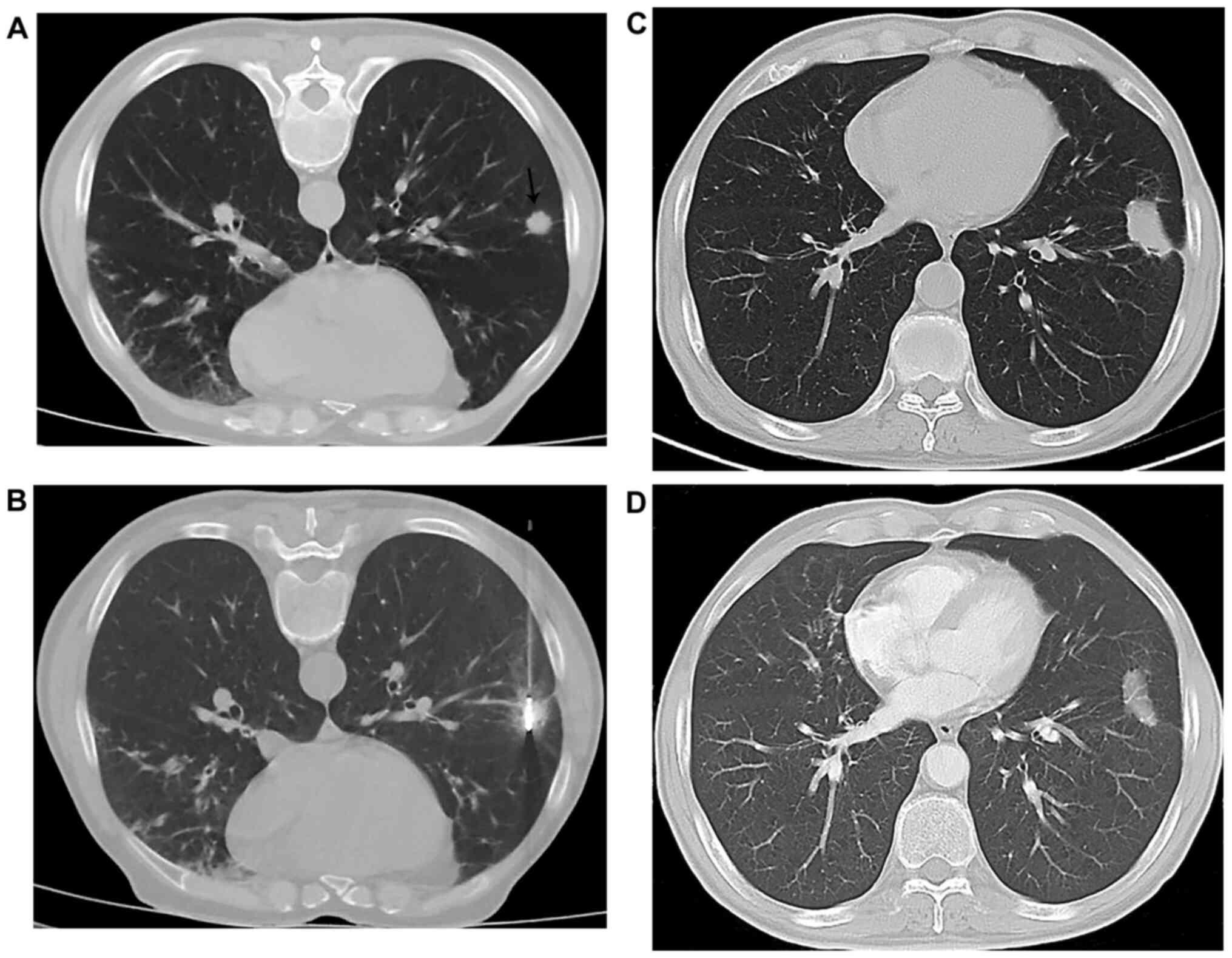
(21/29) and 73.68% (14/19), respectively (Figs. 1 and 2 and Table
IV); there was no statistically significant difference for the
short-term efficacy rates between the two groups (P=0.92). For
long-term evaluation, six patients the in MWA group and five
patients in the cryoablation group were lost to follow-up, leaving
23 patients in the MWA group and 14 patients in the cryoablation
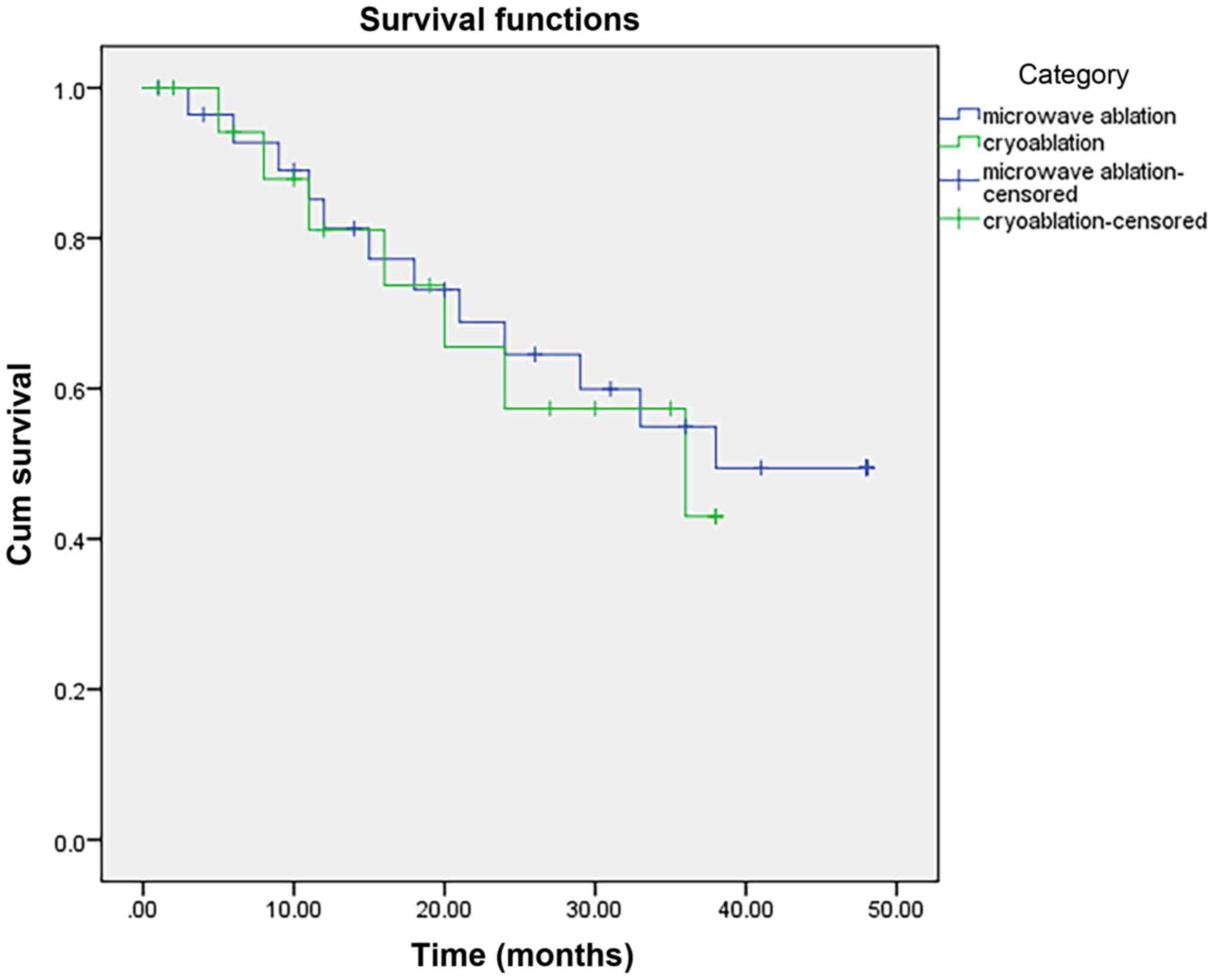
group available for long-term efficacy analysis. The 6-, 12-, 24-,
36-month OS rates in the MWA and cryoablation groups were 92.72,
81.28, 64.54 and 54.91%, and 94.07, 81.13, 57.33 and 43.04%,
respectively. No significant differences were observed for OS
between the two groups (P=0.79; Fig.
3). In addition, in the MWA group, one (3.45%) patient
exhibited disease progression at ablative sites, whereas in the
cryoablation group, one (5.26%) patient exhibited disease
progression at ablative sites, which was statistically
insignificant (P=0.64). Regarding disease progression distant from
the ablation site, in the MWA group, six (20.69%) patients
developed metastases in lobes other than at the ablative sites or
distant sites after 3 years of follow-up, whereas in the
cryoablation group, 4 (21.05%) patients developed metastases in
lobes other than at the ablative sites or distant sites.
 | Table IIIResults of intraprocedural pain
evaluation. |
Table III
Results of intraprocedural pain
evaluation.
| Variable | Microwave ablation
(n=29) | Cryoablation
(n=19) | P-value |
|---|
| VAS, median
(interquartile range) | 5 (4,8) | 3 (1,5) | <0.001 |
 | Table IVResults of short-term efficacy
evaluation. |
Table IV
Results of short-term efficacy
evaluation.
| Outcome | Microwave ablation,
n (%) | Cryoablation, n
(%) |
|---|
| Complete
response | 10/29 (34.48) | 7/19 (36.84) |
| Partial
response | 11/29 (37.93) | 7/19 (36.84) |
| Stable disease | 7/29 (24.14) | 4/19 (21.05) |
| Progressive
disease | 1/29 (3.45) | 1/19 (5.26) |
| Overall
response | 21/29 (72.41) | 14/19 (73.68) |
Follow-up and postoperative
complications
In the two groups of patients, all ablation sessions
were successfully completed and all pulmonary malignant tumors were
ablated. Complications were recorded on a per-treatment basis and
classified in accordance with the Common Terminology Criteria for
Adverse Events (CTCAE) (10).
There were no intraprocedural deaths. Out of 48 patients, 37 were
followed up until the completion of the study (23 cases from the
MWA group and 14 from the cryoablation group), with 11 patients
lost to follow-up. The follow-up period was 6-48 months, and the
mean follow-up period was 22.3 months. The frequency of
procedure-related complications after ablation is reported in
Table V.
 | Table VFrequency of procedure-related
complications after ablation. |
Table V
Frequency of procedure-related
complications after ablation.
| Procedure-related
complication | Microwave ablation,
n (%) | Cryoablation, n
(%) | P-value |
|---|
| Pneumothorax | 3/29 (10.34) | 2/19 (10.53) | |
| Pulmonary
hemorrhage | 4/29 (13.79) | 3/19 (15.79) | |
| Hemoptysis | 2/29 (6.90) | 1/19 (5.26) | |
| Pleural
effusion | 1/29 (3.45) | 1/19 (5.26) | |
| All
complications | 10/29 (34.48) | 7/19 (36.84) | 0.59 |
The mean operation time was 36 min (range, 30-63
min) in the MWA group and 53 min (range, 42-78 min) in the
cryoablation group, which was significantly different between the
two group (P<0.001). In the MWA group, the mean post-operative
hospital stay was 6.7 days (range, 1-22 days). In total, one case
was treated with closed drainage of pleural cavity, and sixteen
cases were treated with hemostatic drugs or similar conservative
treatments without surgical interference, which were recovered
within one month. The remaining four patients exhibited a longer
postoperative hospital stay (mean, 15.5 days; range, 11-22 days)
due to co-morbidities, such as chronic bronchitis, emphysema or
heart failure. In the cryoablation group, the mean postoperative
hospital stay was 7.1 days (range, 3-26 days). A total of 16 cases
treated with hemostatic drugs or morphine recovered within 3-8
days; the remaining three patients exhibited a longer postoperative
hospital stay (mean, 17 days; range, 10-26 days) due to
co-morbidities. However, there was no significant difference in
postoperative hospital stay.
In the MWA group, the total incidence of
pneumothorax was 10.3% (3/29); two cases (CTCAE grade 1) were
treated conservatively without interference, and one case (CTCAE
grade 2) was managed with closed drainage of the pleural cavity.
Intraparenchymal pulmonary hemorrhage (CTCAE grade 1) was detected
in 13.8% (4/29) of cases, which was self-limiting. Additionally,
two cases of hemoptysis and one case of pleural effusion were
detected, with complete spontaneous resolution within 1 month. In
the cryoablation group, the total incidence of pneumothorax was
10.5% (2/19), which was self-limiting. Intraparenchymal pulmonary
hemorrhage (CTCAE grade 1) developed in 15.6% (3/19) of cases, with
complete resolution within 1 month. Finally, one case of hemoptysis
and one case of pleural effusion were found and treated without
interference. There was no statistically significant difference in
the incidence of complications between the two groups (P=0.59).
Discussion
Image-guided percutaneous ablation in the treatment
of primary or metastatic malignant tumors has been increasingly
used, which has the advantages of reproducibility, good efficacy,
low cost and less trauma (14). At
present, percutaneous ablation under CT guidance has been
effectively implemented in patients with primary or metastatic
pulmonary malignant tumors who are medically inoperable or refuse
surgery (15).
MWA is increasingly used to treat stage I non-small
cell lung carcinoma (NSCLC), metastatic lung cancer and advanced
lung cancer combined with radiotherapy and chemotherapy. Yao et
al (16) compared results of
54 patients with stage I NSCLC undergoing MWA with that of 795
patients with stage I NSCLC undergoing lobectomy, and concluded MWA
has a similar therapeutic effect compared with lobectomy for stage
I NSCLC, but with fewer complications and less pain. Yang et
al (17) retrospectively
analyzed the local recurrence and repeatability of MWA in 104
patients with stage I NSCLC and concluded that the local recurrence
rate was lower in tumors ≤3.5 cm compared with tumors >3.5 cm.
The same study reported that compared with patients without local
recurrence, using MWA repeatedly can achieve a similar OS and
progression-free survival, but without additional complications. In
addition, the study also found that high-frequency ablations
exhibited larger ablation margins and reduced local progression
compared with low-frequency ablations (18). In conclusion, a number of studies
have shown that the use of MWA in the treatment of stage I/II NSCLC
and metastatic nodules is safe and effective.
Although numerous studies have investigated MWA,
there are only a small number of studies focusing on cryoablation
for lung malignant tumors. The mechanism of cryoablation includes
intracellular ice crystal formation, disruption of organelles and
cell membranes, vascular stasis and microvascular thrombosis, which
lead to cell death (19,20). Kawamura et al (21) reported the results of 22
cryoablation sessions in 20 patients with 35 pulmonary metastases
and found that there was local recurrence of seven (20%) tumors in
seven (35%) patients during a 9- to 28-month (median, 21 months)
follow-up period, with a 1-year survival rate of 89.4%. Another
study of 117 patients with 193 tumors treated with cryoablation
also suggested that percutaneous cryoablation could be performed
with minimal invasion and acceptable rates of complications
(22). Furthermore, in a
retrospective study of cryoablation using thin needles for 34
pulmonary tumors (11 NSCLC, 23 metastases), technical success
(complete lack of enhancement) was achieved in 82, 97 and 91% of
treated lesions at the 1-, 3- and 6-month CT follow-ups (23). These studies demonstrated that
percutaneous cryoablation of lung tumors treatment is effective,
minimally invasive and safe with satisfactory local control.
In addition, Li et al (24) suggested that cryoablation not only
leads to destruction of targeted cells directly, but also results
in reduced release of immunosuppressive factors from tumor cells
and enhanced antitumor immune response, which plays an important
role in eliminating the residual tumor cells and inhibiting the
growth of local tumors.
The present study indicated that the patients from
the MWA group experienced more pain compared with those in the
cryoablation group, with significantly higher VAS scores, which is
in line with the findings of Das et al (25). There were no statistically
significant differences between the two groups in terms of
short-term efficacy and OS rates. Das et al (25) previously demonstrated that MWA and
cryoablation procedures were comparably effective treatment
modalities with similar survival benefits in patients with advanced
NSCLC with small tumors.
At present, there are various comparative studies of
MWA and radiofrequency for the treatment of malignant tumors of the
lung, but there are few studies comparing MWA and cryoablation.
Compared with RFA, MWA is considered to achieve more homogenous
heating, higher tissue temperatures, larger ablation volumes and
less heat sink effect, resulting in reduced treatment time and an
improved convection profile (26,27).
However, initial microwave systems suffered from poor antenna
design, inability to create spherical ablation zones and concerns
regarding remote conduction of energy (28,29).
Additionally, the range of MWA is not easy to control, which can
inadvertently injure adjacent organs. Cryoablation has
certain advantages, including good visualization under CT or MRI
guidance, low intraprocedural pain and preservation of collagenous
architecture, which are conducive to application in the treatment
of cancer in various non-aerated organs (7,8).
However, in comparison with microwave probes, the cryoprobes are
larger and have a blunt tip, which leads to substantial
difficulties for percutaneous cryoablation of lung tumors.
Therefore, both MWA and cryoablation can be used to
treat the majority of primary or metastatic pulmonary malignant
tumors. MWA therapy could be a therapeutic option when the tumors
are relatively large and far away from the large blood vessels and
other important organs. For tumors that are relatively small (<3
cm) and adjacent to blood vessels or important organs, and in
patients who cannot endure pain, cryoablation is considered to be a
preferred approach (30). In
addition, for patients with non-resectable advanced malignant
tumors, both ablation therapies can substantially alleviate tumor
burden, reduce breathing impairments in patients with borderline
lung function and improve the effect of comprehensive treatment
(29,31).
The current study is limited by a number of factors,
including a non-randomized, non-controlled retrospective design,
relatively small sample size and the element of bias in the
selection of the modality used for ablation. Furthermore, the
analysis of this study did not consider effects before or after
radiotherapy, chemotherapy or other systemic treatment.
In conclusion, the present study reported a
comparison of two ablation modalities in the treatment of lung
malignant tumors. According to the study findings, cryoablation
exhibited a similar therapeutic efficacy compared with MWA in the
treatment of pulmonary malignant tumors, but with reduced pain.
Therefore, the preferred approach should be determined primarily
based on pre-ablation tumor size, location in relation to the
important organs and pain tolerance of the patients.
Acknowledgements
The authors would like to thank Dr Morgan A McClure
(Department of Radiology, Nanchong Central Hospital, Sichuan,
China) for helping to polish the grammar and structure of the
manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
HWL, YJL, YD and GWY contributed to the study
conception and design. LHZ, HCY, JZ, XXZ, PXH, HFY and AB
contributed to the literature search, data collection, statistical
analysis and data interpretation. YD and AB revised the manuscript.
HWL, YD and GWY confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of Affiliated Hospital of North Sichuan Medical
College (approval no. 202016). Written informed consent was
obtained from all the patients enrolled in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
HWL-ORCID ID: 0000-0003-0083-8279; YJL-ORCID ID:
0000-0003-4930-6716; LHZ-ORCID ID: 0000-0001-9986-9045; HCY-ORCID
ID: 0000-0001-9099-4886; JZ-ORCID ID: 0000-0002-9507-7013;
XXZ-ORCID ID: 0000-0002-9801-1909; PXH-ORCID ID:
0000-0003-1408-8114; AB-ORCID ID: 0000-0002-4782-3018; HFY-ORCID
ID: 0000-0001-5156-2984; YD-ORCID ID: 0000-0002-8119-3195.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oudkerk M, Liu S, Heuvelmans MA, Walter JE
and Field JK: Lung cancer LDCT screening and mortality
reduction-evidence, pitfalls and future perspectives. Nat Rev Clin
Oncol. 18:135–151. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nomori H, Yamazaki I, Machida Y, Otsuki A,
Cong Y, Sugimura H and Oyama Y: Lobectomy versus segmentectomy: A
propensity score-matched comparison of postoperative complications,
pulmonary function and prognosis. Interact Cardiovasc Thorac Surg.
25(ivab212)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Akalin A, Mu X, Kon MA, Ergin A,
Remiszewski SH, Thompson CM, Raz DJ, Diem M, Bird B and Miljković
M: Classification of malignant and benign tumors of the lung by
infrared spectral histopathology (SHP). Lab Invest.
95(697)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Du S, Qin D, Pang R, Zhang Y, Zhao S, Hu M
and Zhi X: Long-term efficacy of radiofrequency ablation combined
with chemotherapy in the treatment of patients with advanced
non-small cell lung cancer-a retrospective study. Zhongguo Fei Ai
Za Zhi. 20:675–682. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
7
|
Sonntag PD, Hinshaw JL, Lubner MG, Brace
CL and Lee FT Jr: Termal ablation of lung tumors. Surg Oncol Clin N
Am. 20:369–387. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Erinjeri JP and Clark TW: Cryoablation:
Mechanism of action and devices. J Vasc Interv Radiol. 21 (8
Suppl):S187–S191. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee S, Rhim H, Kim YS, Choi D, Lee WJ, Lim
HK and Shin B: Percutaneous radiofrequency ablation of
hepatocellular carcinomas: Factors related to intraprocedural and
postprocedural pain. AJR Am J Roentgenol. 192:1064–1070.
2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dueck AC, Mendoza TR, Mitchell SA, Reeve
BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM,
O'Mara AM, et al: National cancer institute PRO-CTCAE study group.
Validity and reliability of the US National cancer institute's
patient-reported outcomes version of the common terminology
criteria for adverse events (PRO-CTCAE). JAMA Oncol. 1:1051–1059.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ahmed M: Technology Assessment Committee
of the Society of Interventional Radiology. Image-guided tumor
ablation: Standardization of terminology and reporting criteria-a
10-year update: supplement to the consensus document. J Vasc Interv
Radiol. 25:1706–1708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bojarski JD, Dupuy DE and Mayo-Smith WW:
CT imaging findings of pulmonary neoplasms after treatment with
radiofrequency ablation: Results in 32 tumors. AJR Am J Roentgenol.
185:466–471. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tsuchida Y and Therasse P: Response
evaluation criteria in solid tumors (RECIST): New guidelines. Med
Pediatr Oncol. 37:1–3. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Dupuy DE: Image-guided thermal ablation of
lung malignancies. Radiology. 260:633–655. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Howington JA, Blum MG, Chang AC, Balekian
AA and Murthy SC: Treatment of stage I and II non-small cell lung
cancer: Diagnosis and management of lung cancer, 3rd ed: American
College of chest physicians evidence-based clinical practice
guidelines. Chest. 143 (5 Suppl):e278S–e313S. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yao W, Lu M, Fan W, Huang J, Gu Y, Gao F,
Wang Y, Li J and Zhu Z: Comparison between microwave ablation and
lobectomy for stage I non-small cell lung cancer: A propensity
score analysis. Int J Hyperthermia. 34:1329–1336. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang X, Ye X, Huang G, Han X, Wang J, Li
W, Wei Z and Meng M: Repeated percutaneous microwave ablation for
local recurrence of inoperable Stage I nonsmall cell lung cancer. J
Cancer Res Ther. 13:683–688. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vogl TJ, Roman A, Nour-Eldin NA,
Hohenforst-Schmidt W, Bednarova I and Kaltenbach B: A comparison
between 915 MHz and 2450 MHz microwave ablation systems for the
treatment of small diameter lung metastases. Diagn Interv Radiol.
24:31–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gage AA and Baust J: Mechanisms of tissue
injury in cryosurgery. Cryobiology. 37:171–186. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hoffmann NE and Bischof JC: The
cryobiology of cryosurgical injury. Urology. 60 (2 Suppl
1):S40–S49. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kawamura M, Izumi Y, Tsukada N, Asakura K,
Sugiura H, Yashiro H, Nakano K, Nakatsuka S, Kuribayashi S and
Kobayashi K: Percutaneous cryoablation of small pulmonary malignant
tumors under computed tomographic guidance with local anesthesia
for nonsurgical candidates. J Thorac Cardiovasc Surg.
131:1007–1013. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Inoue M, Nakatsuka S, Yashiro H, Ito N,
Izumi Y, Yamauchi Y, Hashimoto K, Asakura K, Tsukada N, Kawamura M,
et al: Percutaneous cryoablation of lung tumors: Feasibility and
safety. J Vasc Interv Radiol. 23:295–302. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pusceddu C, Sotgia B, Fele RM and Melis L:
CT-guided thin needles percutaneous cryoablation (PCA) in patients
with primary and secondary lung tumors: A preliminary experience.
Eur J Radiol. 82:e246–e253. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li M, Liu J, Zhang SZ, Zhou Y, Guo YW,
Chen Q, Ke YQ, Jiang XD and Cai YQ: Cellular immunologic response
to primary cryoablation of C6 gliomas in rats. Technol Cancer Res
Treat. 10:95–100. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Das SK, Huang YY, Li B, Yu XX, Xiao RH and
Yang HF: Comparing cryoablation and microwave ablation for the
treatment of patients with stage IIIB/IV non-small cell lung
cancer. Oncol Lett. 19:1031–1041. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Martin RC, Scoggins CR and McMasters KM:
Microwave hepatic ablation: Initial experience of safety and
efficacy. J Surg Oncol. 96:481–486. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wright AS, Lee FT Jr and Mahvi DM: Hepatic
microwave ablation with multiple antennae results in
synergistically larger zones of coagulation necrosis. Ann Surg
Oncol. 3:275–283. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Berber E: Laparoscopic microwave
thermosphere ablation of malignant liver tumors: An initial
clinical evaluation. Surg Endosc. 30:692–698. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ma Y, Wallace AN, Waqar SN, Morgensztern
D, Madaelil TP, Tomasian A and Jennings JW: Percutaneous
image-guided ablation in the treatment of osseousmetastases from
non-small cell lung cancer. Cardiovasc Intervent Radiol.
41:726–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wei YT and Xiao YY: Expert consensus for
image-guided cryoblation of lung cancer (In Chinese). Chin J Interv
Imaging Ther. 15:259–263. 2018.
|
|
31
|
Yashiro H, Nakatsuka S, Inoue M, Kawamura
M, Tsukada N, Asakura K, Yamauchi Y, Hashimoto K and Kuribayashi S:
Factors affecting local progression after percutaneous cryoablation
of lung tumors. J Vasc Interv Radiol. 24:813–821. 2013.PubMed/NCBI View Article : Google Scholar
|