Introduction
Malignant melanoma (MM) is a cancer that develops in
the skin and mucosa. Five percent of female patients with cancer
have mucosal MM derived from the vulva, ovary, uterus, or cervix
(1). Cervical MM is rare, with
less than 90 reported cases since 1889(2). Although primary MM of the cervix is
localized to the cervix in the early stage, it infiltrates the
uterosacral ligaments, vaginal fornix, pelvic wall, and vulva, and
spreads to distant organs at advanced stages. Compared to vulval
and vaginal MM, primary cervical melanomas are sporadic and have a
poor prognosis (3). There are no
standard regimens for recurrent melanoma of the uterine cervix;
therefore, treatment regimens for cutaneous MM were followed.
Immune checkpoint inhibitors are used in patients as standard
therapy in MM; however, immune checkpoint inhibitors, such as PD1
and CTLA4, have been used in fewer patients with cervical malignant
melanoma. We report a case of recurrent uterine cervical MM treated
with anti-PD-1 antibodies and anti-CTLA4 antibodies.
Case report
A 73-year-old Japanese woman was admitted to a
gynecological clinic with genital bleeding. Her medical history
included aortic valve replacement. Gynecological examination
revealed a 5-mm diameter polypoid lesion at the uterine cervix. A
colposcopy-guided cervical biopsy was performed, and
immunohistochemical analysis revealed positive reactions for S-100
protein and Melan-A. Cervical MM was suspected, and she was
referred to the Department of Gynecology at the University of
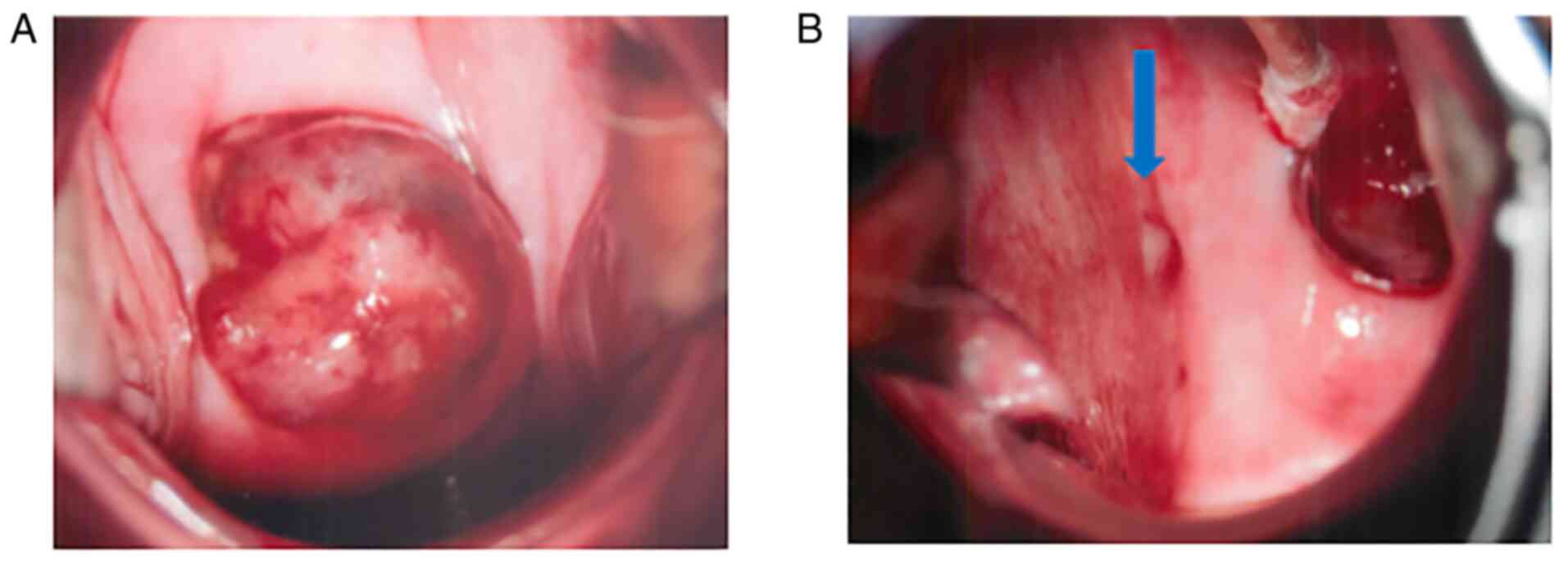
Tokyo. Colposcopy revealed a 2-cm mass in the uterine cervix and a
5-mm diameter skip lesion at the lateral vaginal fornix (Fig. 1). Findings from transvaginal
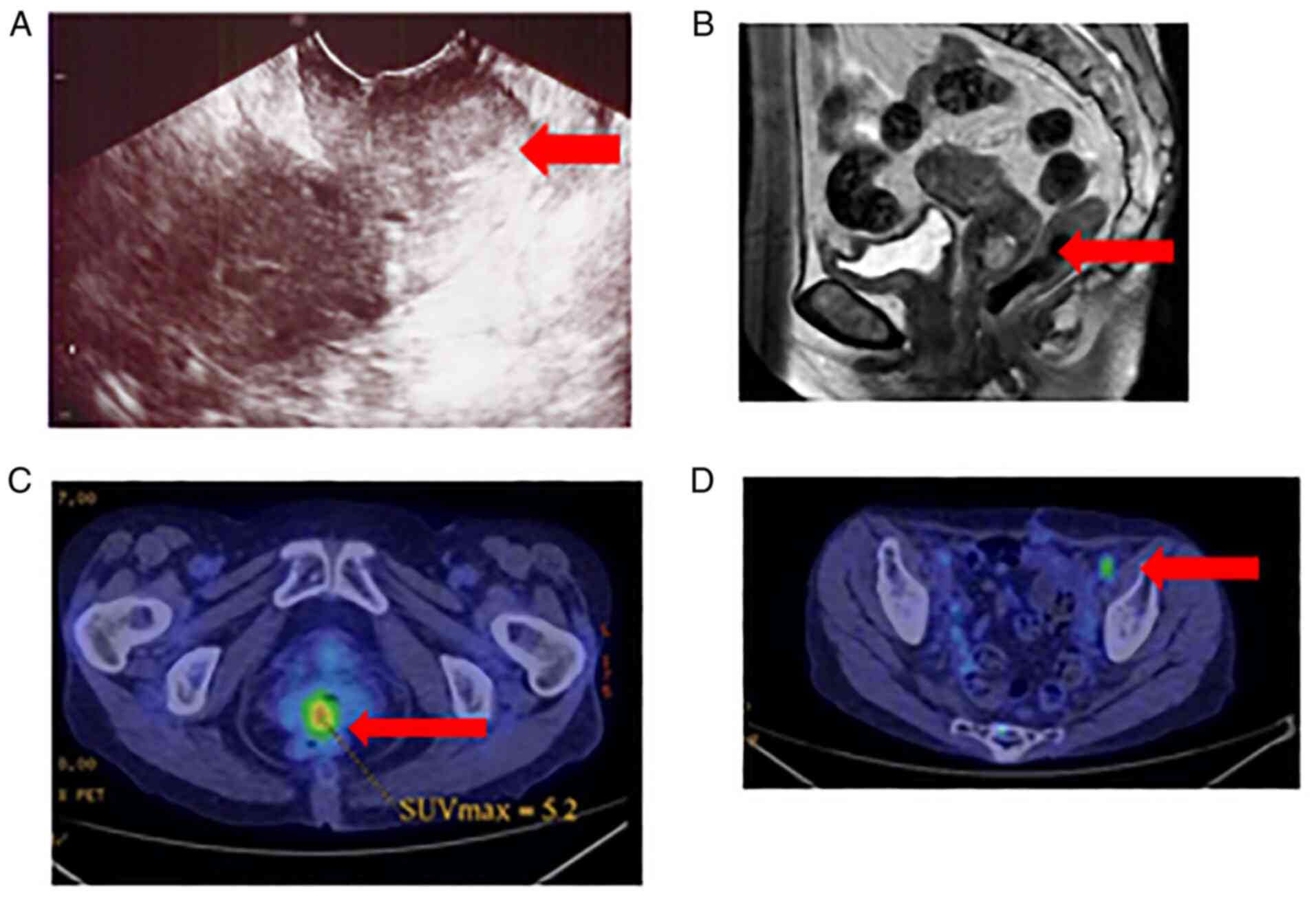
ultrasound and magnetic resonance imaging (MRI) revealed a mass
approximately 20-mm in size, confined to the uterine cervix area,
showing high signal intensity on T2-weighted images (Fig. 2A and B). Positron emission tomography and
computed tomography (PET-CT) revealed uptake of
fluoro-2-deoxy-D-glucose in the uterine cervix area (SUVmax: 6.5)
and minor uptake in the pelvic lymph node area (Fig. 2C and D). There were no metastatic lesions or
enlarged lymph nodes on CT scans of the brain, chest, abdomen, and
pelvis. To evaluate the primary sites, we performed a comprehensive
assessment of melanotic lesions in the skin, mucosal sites, and
uveal tract (ophthalmoscopy); the results were negative. The
patient was diagnosed with primary MM of the uterine cervix.
According to the International Federation of Gynecology and
Obstetrics (FIGO) classification 2018, the preoperative disease
stage was IIA1. Considering the patient's age, history of aortic
valve replacement, and poor prognosis, we chose less invasive
surgery. The patient underwent a modified radical hysterectomy,
bilateral salpingo-oophorectomy, pelvic lymph node dissection, and
partial vaginectomy because the tumor was grossly close to the
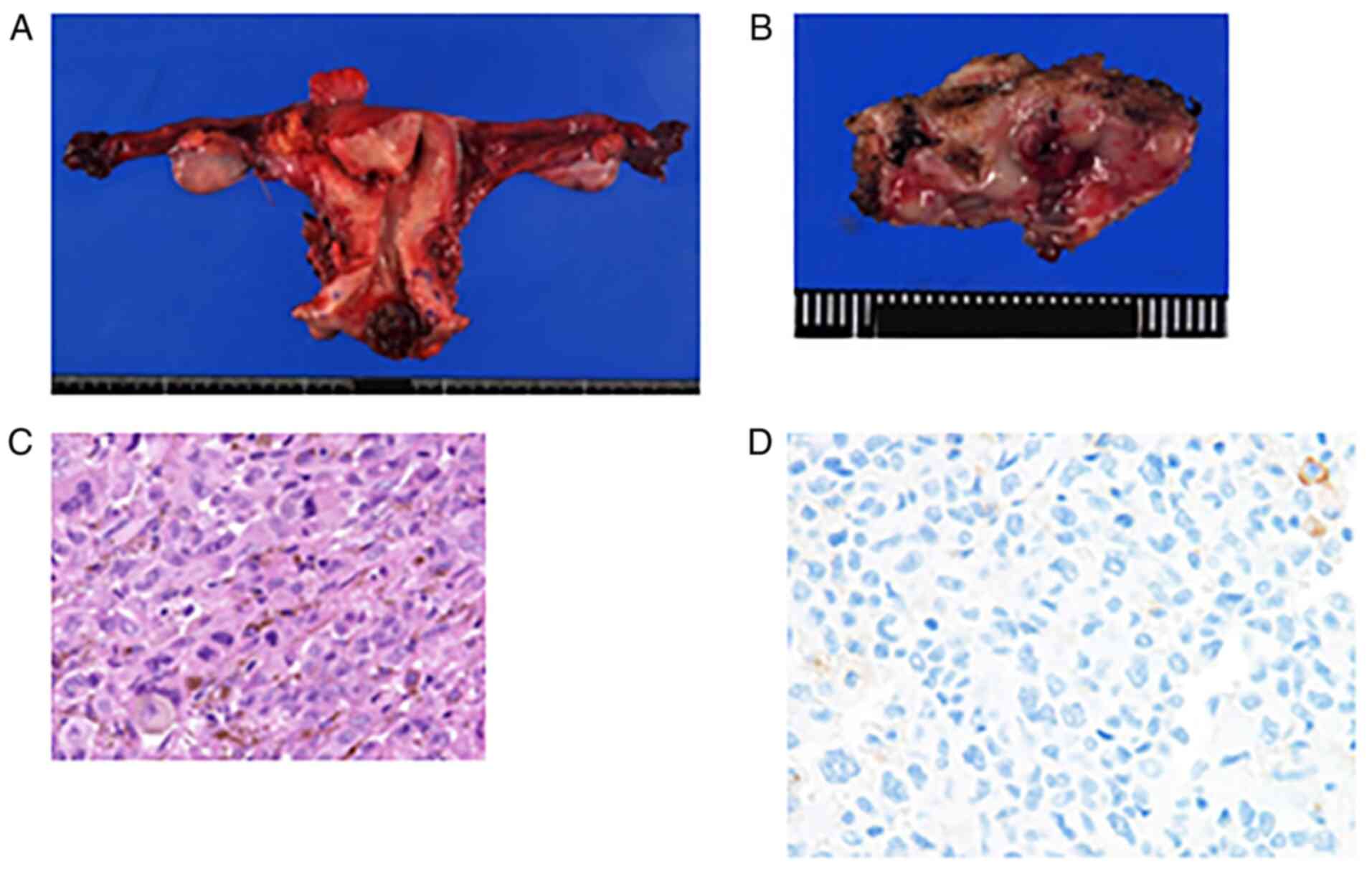
stump. Histopathological examination revealed a mass, which was
2.4x2.0 cm in size, invading the uterine cervix, with proliferation
of atypical melanocytes with bizarre nuclei and focal melanin
production (Fig. 3). These tumor
cells were immunohistochemically positive for Melan-A, confirming
the diagnosis of malignant melanoma. Furthermore, 20% of the tumor
cells were positive for C-kit, whereas all tumor cells were
negative for PD-L1 (Figs. S1 and
3D). Surgical margins of the
vagina and resected pelvic lymph nodes (16/36) were positive. The
postoperative disease stage was ⅢC1. After surgery, the patient
underwent adjuvant radiotherapy with a remote afterloading system
(RALS) due to margin positivity (30 Gy/5 Fr).
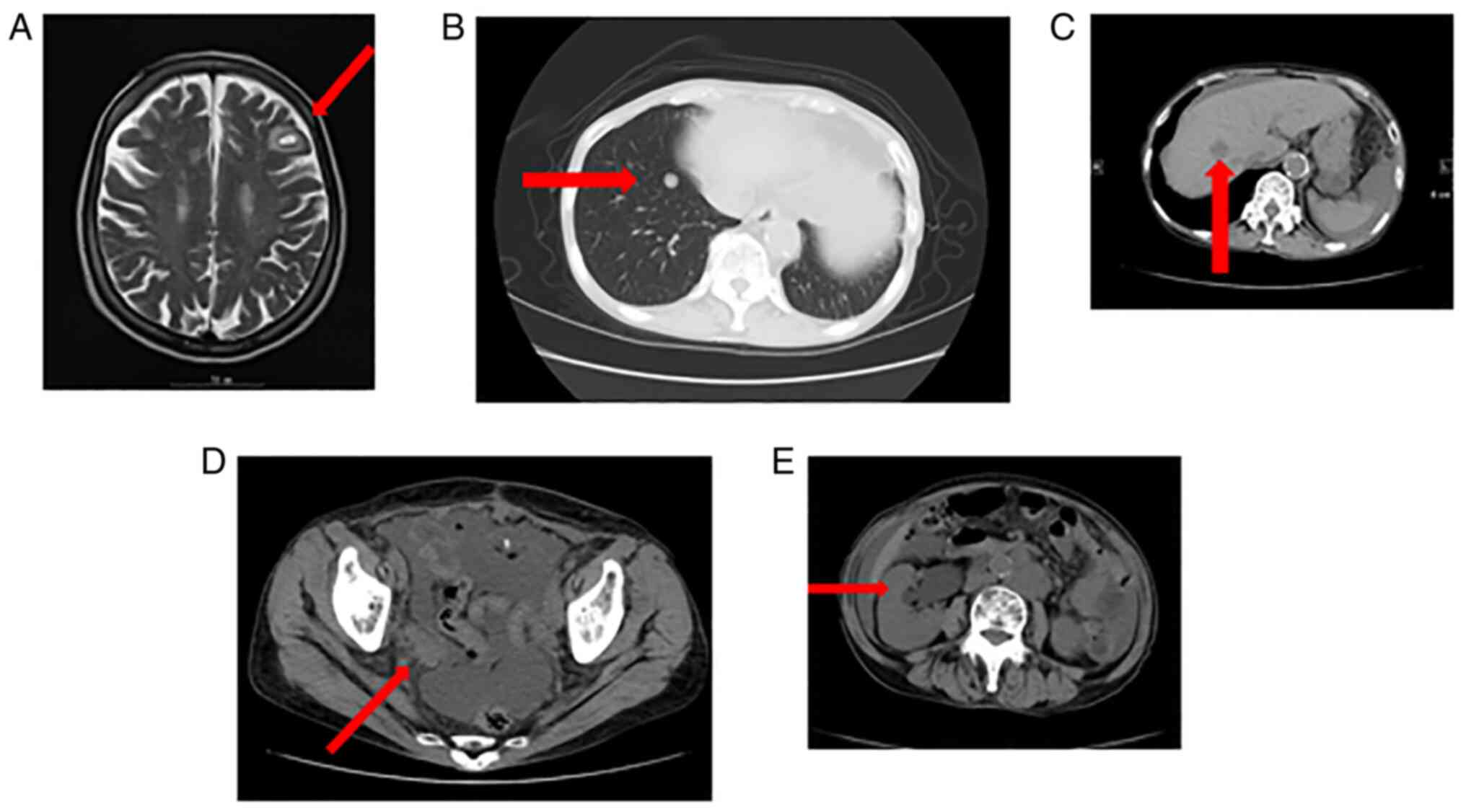
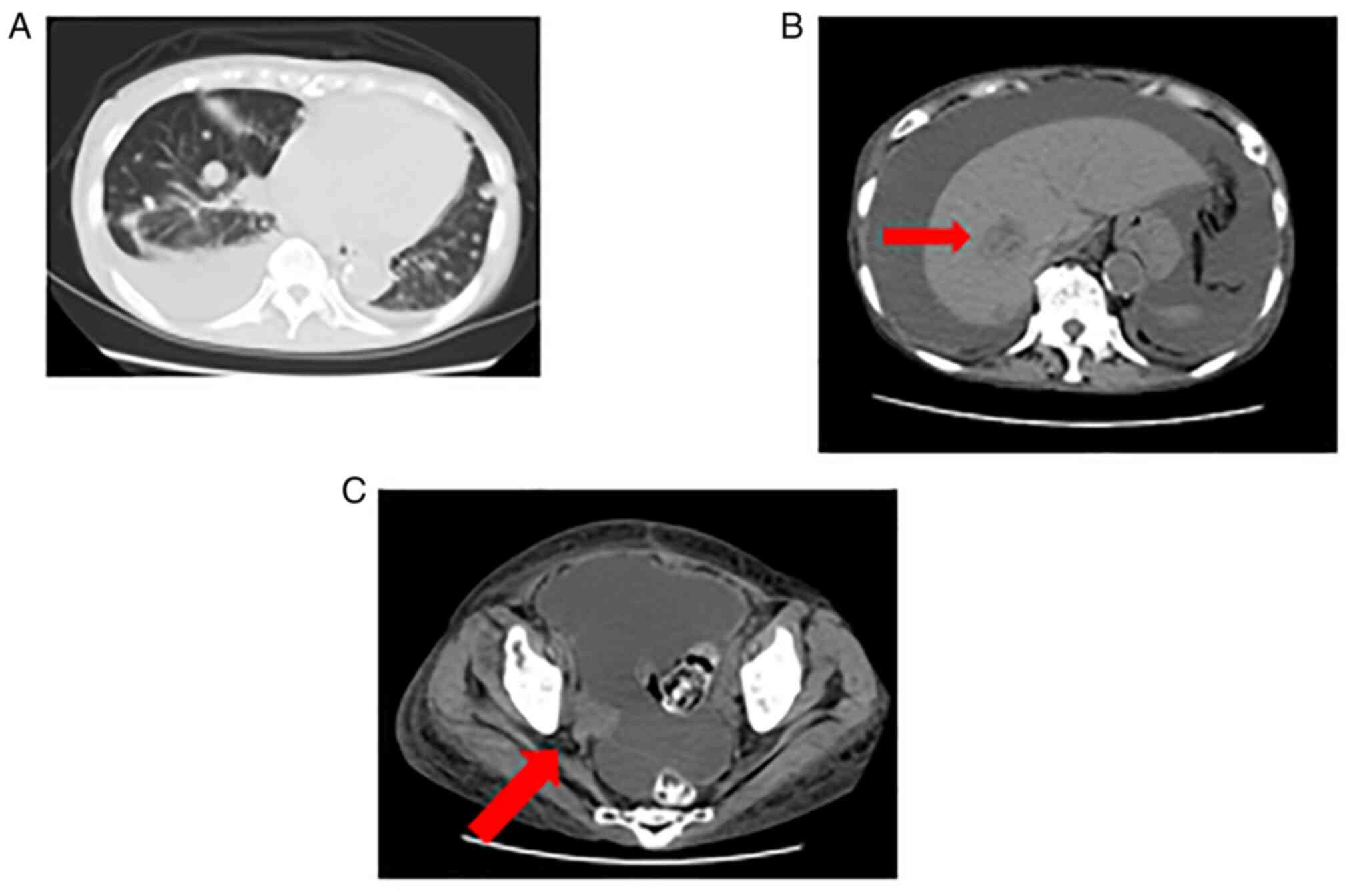
CT revealed brain and multiple lymph node metastases
four months after surgery, and the patient underwent γ-knife
radiotherapy (44 Gy/1 Fr) (Fig.
4A). No mutations were found in BRAF; therefore, the patient
received the immune checkpoint inhibitor anti-PD-1 antibodies
(nivolumab) at 3.0 mg/kg biweekly, according to treatment
guidelines for recurrent cutaneous MM. Five months after surgery,
multiple metastases were detected by MRI in the lungs, liver,
bones, and hydronephrosis due to pelvic recurrence (Fig. 4B-E). The patient underwent
palliative hole pelvis irradiation (20 Gy/4Fr) for hydronephrosis.
Six months after surgery, the patient received the immune
checkpoint inhibitor anti-CTLA-4 antibodies (ipilimumab, 3.0 mg/kg)
after three cycles of the anti-PD-1 antibodies. The patient was
admitted to the hospital one week after anti-CTLA-4 administration
because of deterioration of her general condition caused by
aggravation of lesions and ascites (Fig. 5A-C). Renal function also
deteriorated significantly. Consequently, we decided to provide the
best supportive care. Seven months after surgery, the patient died
of multiple organ failure.
Discussion
We encountered a case of MM of the uterine cervix,
which was treated with radiotherapy after surgery, but multiple
recurrences were observed. Two types of immune checkpoint
inhibitors were administered to the recurrent lesion, but they were
ineffective, and the patient died. There are four notable points to
be drawn from this case.
First, diagnosis of cervical MM was performed by
pelvic examination, other gynecologic examinations, and
pathological diagnosis. To determine the diagnosis of primary
cervical melanoma, metastasis of melanoma needs to be ruled out
elsewhere (4). In this case, the
lesion had extended to the vagina beyond the preoperative pelvic
examination findings. MM is characterized by exudative growth, and
attention must be paid to the possibility that vaginal invasion
cannot be accurately judged by palpation and inspection alone.
Second, surgical treatment is recommended for
cervical MM, similar to other MMs. Hysterectomy and bilateral
salpingo-oophorectomy have been recommended in previous reports.
Other reports recommend a radical hysterectomy to ensure adequate
margins (5). On the other hand,
reports suggest a less invasive operative procedure such as total
hysterectomy because the prognosis is extremely poor (6). Moreover, in patients with stage IIIA
MM of the cervix, radical hysterectomy and total vaginal wall
resection have been associated with rapid recurrence and death.
Therefore, aggressive vaginal wall resection should be considered
with caution because of its invasiveness and curability (7). We selected a modified radical
hysterectomy considering the patient's age and prognosis. However,
because of the rare occurrence of this condition, to the best of
our knowledge, no standard guidelines are available for its
treatment and management. Radical hysterectomy and simple
hysterectomy were therefore considered to be acceptable
procedures.
Lymph node dissection without lymphadenopathy on MRI
or CT images remain controversial. Jones et al (8) reported that 30% of patients with
clinically normal lymph nodes had microscopic lymph node metastases
and recommended lymph node dissection. In contrast, Cantuaria et
al (5) suggested that pelvic
and para-aortic lymph node dissection is recommended when lymph
nodes are grossly enlarged or have invaded beyond the uterus
(5). We performed pelvic lymph
node dissection because pelvic lymph node metastasis was suspected
on preoperative PET/CT, but multiple organ metastases were found
several months later. Thus, the significance of lymph node
dissection remains to be determined.
Third, although MM is resistant to radiation
therapy, radiation therapy is considered an option for patients
with positive lymph node metastasis, patients who do not have a
sufficient surgical margin, or patients with palliative intent
(4). We also performed RALS given
that the surgical margin was positive for the vaginal stump. In
this case, external beam radiation to the pelvic wall was
considered at first because of lymph node metastasis. However,
pelvic irradiation was not performed considering the complications
of pelvic irradiation, the patient's age, and the effects of
radiation. At the time of recurrence, gamma knife irradiation for
brain metastasis and palliative irradiation for hydronephrosis were
performed. As there are no chemotherapeutic regimens or molecularly
targeted drugs that improve prognosis in cases of advanced or
recurrent cervical MM, chemotherapy and molecularly targeted
treatments are administered as per cutaneous melanoma protocols
(9). Dacarbazine is the most
commonly used drug for MM, showing recurrence rates of 15-20%
(10). Although other chemotherapy
regimens such as cisplatin and vinblastine combined with
dacarbazine may produce a 20-35% response rate, they may not
prolong life compared with dacarbazine alone (11).
Fourth, targeted drugs indicated for recurrent or
unresectable MM include BRAF inhibitors, MEK inhibitors, and immune
checkpoint inhibitors. BRAF inhibitors plus MEK inhibitors can be
used only in patients with BRAF V600 mutations. They act more
rapidly than immune checkpoint inhibitors and have an early tumor
response but have not been reported in cases of cervical MM
(12). MM is a cancer that is
easily recognized by the immune system, and research and
development of cancer immunotherapy have advanced, particularly in
melanoma. In recent years, improved knowledge of tumor control in
the immune system has led to the development of novel immunotherapy
targeting immune checkpoint factors. Immune checkpoint molecules
that are treatment targets include CTLA-4 and PD-1. Ipilimumab
(Yervoy®) is an anti-CTLA-4 antibodies, while nivolumab
(Opdivo®) and pembrolizumab (Keytruda®) are
anti-PD-1 antibodies; these have been adopted for the treatment of
MM. Several reports have described the use of immune checkpoint
inhibitors for the treatment of cervical MM. Noguchi et al
(7) administered nivolumab to a
patient with FIGO stage IIIA cervical MM, but the patient died
without any response to therapy. Kim et al used
pembrolizumab as postoperative therapy for a patient with stage IIA
cervical melanoma, but the disease recurred rapidly, and the
patient died (13). Ipilimumab was
also administered to four patients with cervical MM, but all four
patients had progressive disease (14). While many reports have suggested
that immune checkpoint inhibitors are not effective against
cervical MM, some reports have also supported their effectiveness.
Anko et al (15) treated
patients with recurrent cervical MM with nivolumab, and most
recurrent pelvic tumors disappeared.
In this study, we administered two types of immune
checkpoint inhibitors, PD-1 antibodies and CTLA-4 antibodies, to
patients with uterine cervical MM. To the best of our knowledge, no
single patient with cervical melanoma has yet been treated with
PD-1 and CTLA-4 antibodies. At the time when the patient was
treated, nivolumab was not indicated as an adjuvant therapy for
malignant melanoma in the Japanese guidelines. RLARS has also been
used, but there has been no evidence supporting the use of
radiation combined with immune checkpoint inhibitors. Considering
the above facts, an immune checkpoint inhibitor was not used
postoperatively. Unfortunately, neither of the two types of immune
checkpoint inhibitors was effective against cervical MM. In this
case, we administered nivolumab followed by ipilimumab. Clinical
trials using nivolumab in combination with ipilimumab for malignant
melanoma have been reported. D'Angelo et al (16) pooled the data of 889 patients
treated with nivolumab alone and 665 patients treated with
nivolumab + ipilimumab in several clinical trials. Of these, 121
patients had mucosal melanoma, including 86 with nivolumab alone.
Nearly 35 patients were treated with Nivolumab + ipilimumab.
Combination therapy facilitated better outcomes for both melanomas
than a single agent in terms of prognosis and response rate.
Mucosal melanomas had a worse prognosis than cutaneous melanomas in
both the monotherapy and combination groups Since the combination
therapy of anti-PD -1 antibodies and anti-CTLA -4 antibodies
increases the likelihood of adverse events, the combination therapy
for elderly patients, such as this patient, was not performed
considering the high risk (16).
We also performed immunostaining for PD-L1 and c-kit, which
revealed that 20% of the tumor cells were positive for C-kit,
whereas all tumor cells were negative for PD-L1. Reportedly, the
higher the incidence of PD-L1 in pre-treatment cancer tissues, the
more likely it is that anti-PD-1 antibodies will be effective. The
National Comprehensive Cancer Network guidelines recommend the
Bcr-Abl inhibitor imatinib for malignant melanoma with a
c-kit-activating mutation; however, this drug has not been approved
for the treatment of malignant melanomas in Japan (17). In previous reports, the KIT
mutation was not recognized when the positivity rate for c-kit
expression was 10% or less on immunohistochemistry in cases of
mucosal malignant melanoma. In contrast, if the c-kit expression by
immunohistochemistry was positive in 50% or more, the KIT mutation
was recognized in 82% of cases (18). Based on these reports, we concluded
that our patient was unlikely to have a c-kit mutation. It is true
that the onset of the effect of immunotherapy is often slower than
that of other anticancer drugs. However, a higher effectiveness may
lead to rapid resolution of the patient's condition. In addition,
the size of this lesion increased rapidly after 1 course of
ipilimumab therapy in this case. Ipilimumab was not considered
highly effective in this case. If the performance status is bad,
there is a possibility that the immune system is in bad shape, and
immunotherapy will not be effective. However, there was no
conclusive evidence for this possibility. Mucosal malignant
melanoma often arises in the head and neck region (e.g., nasal
cavity and oral cavity), followed by the female genital tract. More
than 90% of malignant melanomas in the female reproductive tract
occur in the vulva and vagina, and only a few occur in the cervix
(1). Therefore, although cervical
malignant melanoma is not rare, its incidence is extremely low
among mucosal malignant melanomas. Of the 750 malignant melanomas
examined in CheckMate 218, 47 were mucosal malignant melanomas.
While the exact details are not available, we believe that there
will be very few, if any, patients with cervical malignant
melanoma. There are no detailed reports of patients being treated
with nivolumab followed by ipilimumab for cervical malignant
melanomas (19). The course of
this case suggests that the efficacy of these two agents may be
lower than that of other mucosal malignant melanomas. Negative
PD-L1 in this case may reflect why nivolumab was ineffective.
However, as mentioned above, it has been reported that PD-L1
expression is not related to the effect of nivolumab in cases of
mucosal malignant melanoma, and further investigation is required.
Considering our findings alongside previous reports, we believe
that immune checkpoint inhibitors may be less effective than other
treatments for MM, although the number of cases in the literature
is relatively small to draw this conclusion. Further development of
biomarkers to stratify efficacy is required. Therefore, it is
necessary to accumulate more experience and data on a greater
number of patients.
Supplementary Material
Approximately 20% of the tumor cells
were positive for c-kit in this case.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Conception and design of the study: KS, AKu, AKa,
AT. Acquisition of data: YMa, DY, YMi, MT. Analysis and/or
interpretation of data: TI, MMU, TT, YO, ASU. Drafting the
manuscript: KS, AKu. KS and AKu confirm the authenticity of all the
raw data. Revising the manuscript critically for important
intellectual content: TI, TT, YO, ASU. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The case report was approved by the ethics committee
at The University of Tokyo [approval no. 3084-(3)]. In patient application forms, it was
clearly stated that patients were allowed to opt out of the study
at any time. Information on how they could opt out was provided on
our website, or arrangements were made for patients to opt out.
Patient consent for publication
In addition to the application form (with the
provision for ‘opt out’), written informed consent was obtained
from the patient in this case for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kedzia W, Sajdak S, Kedzia H and
Spaczyński M: Primary melanoma of the uterine cervix in a 19 year
old woman case report. Ginekol Pol. 68:386–389. 1997.PubMed/NCBI(In Polish).
|
|
2
|
Myriokefalitaki E, Babbel B, Smith M and
Ahmed AS: Primary malignant melanoma of uterine cervix FIGO IIa1: A
case report with 40 months ongoing survival and literature review.
Gynecol Oncol Case Rep. 5:52–54. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pusceddu S, Bajetta E, Carcangiu ML,
Formisano B, Ducceschi M and Buzzoni R: A literature overview of
primary cervical malignant melanoma: An exceedingly rare cancer.
Crit Rev Oncol Hematol. 81:185–195. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yuan G, Wu L, Li B and An J: Primary
malignant melanoma of the cervix: Report of 14 cases and review of
literature:. Oncotarget. 8:73162–73167. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cantuaria G, Angioli R, Fernandez-Abril A
and Penalver M: Primary malignant melanoma of the uterine cervix:
Case report and review of the literature. Prim Care Update Ob Gyns.
5:159–160. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kristiansen SB, Anderson R and Cohen DM:
Primary malignant melanoma of the cervix and review of the
literature Gynecol. Oncol. 47:398–403. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Noguchi T, Ota N, Mabuchi Y, Yagi S,
Minami S, Okuhira H, Yamamoto Y, Nakamura Y and Ino K: A case of
malignant melanoma of the uterine cervix with disseminated
metastases throughout the vaginal wall. Case Rep Obstet Gynecol.
2017(5656340)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jones HW III, Droegemueller W and Makowski
EL: Primary melanocarcinoma of the cervix. Am J Obstet Gynecol.
111:959–963. 1971.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Piura B: Management of primary melanoma of
the female urogenital tract. Lancet Oncol. 9:973–981.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Garbe C, Eigentler TK, Keilholz U,
Hauschild A and Kirkwood JM: Systematic review of medical treatment
for melanoma: Current status and future prospects. Oncologist.
16:5–24. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bajetta E, Del Vecchio M, Nova P, Fusi A,
Daponte A, Sertoli MP, Queirolo P, Taveggia P, Bernengo MG, Legha
SS, et al: Multicenter phase III randomized trial of
polychemotherapy (CVD regimen) versus the same chemotherapy (CT)
plus subcutaneous interleukin-2 and interferon-alpha2b in
metastatic melanoma. Ann Oncol. 17:571–577. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Robert C, Karaszewska B, Schachter J,
Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R,
Grange F, Mortier L, et al: Improved overall survival in melanoma
with combined dabrafenib and trametinib. N Engl J Med. 372:30–39.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim MS, Choi CH, Kim TJ, Lee JW, Lee J,
Bae D and Kim BG: Primary malignant melanoma of the uterine cervix
treated with pembrolizumab after radical surgery: A case report and
literature review. Obstet Gynecol Sci. 61:524–528. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Indini A, Di Guardo L, Cimminiello C,
Lorusso D, Raspagliesi F and Del Vecchio M: Investigating the role
of immunotherapy in advanced/recurrent female genital tract
melanoma: A preliminary experience. J Gynecol Oncol.
30(e94)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Anko M, Nakamura M, Kobayashi Y, Tsuji K,
Nakada S, Nakamura Y, Funakoshi T, Banno K and Aoki D: Primary
malignant melanoma of the uterine cervix or vagina was successfully
treated with nivolumab. J Obstet Gynaecol Res. 46:190–195.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
D'Angelo SP, Larkin J, Sosman JA, Lebbé C,
Brady B, Neyns B, Schmidt H, Hassel JC, Hodi FS, Lorigan P, et al:
Efficacy and safety of nivolumab alone or in combination with
Ipilimumab in patients with mucosal melanoma: A pooled analysis. J
Clin Oncol. 35:226–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines®) Cutaneous Melanoma Version
2.2019-March 12, 2019. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
in 2019.
|
|
18
|
Torres-Cabala CA, Wang WL, Trent J, Yang
D, Chen S, Galbincea J, Kim KB, Woodman S, Davies M, Plaza JA, et
al: Correlation between KIT expression and KIT mutation in
melanoma: A study of 173 cases with emphasis on the
acral-lentiginous/mucosal type. Mod Pathol. 22:1446–1456.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hodi FS, Chapman PB, Sznol M, Lao CD,
Gonzalez R, Smylie M, Daniels GA, Thompson JA, Kudchadkar R,
Sharfman W, et al: Safety and efficacy of combination nivolumab
plus ipilimumab in patients with advanced melanoma: Results from a
North American expanded access program (CheckMate 218). Melanoma
Res. 31:67–75. 2021.PubMed/NCBI View Article : Google Scholar
|