Introduction
Predicting the prognosis of soft tissue sarcomas
remains a major challenge for orthopaedic oncologists. Several
reports regarding the prognostic factors for soft tissue sarcoma,
including age and tumour size, depth, location, and histological
grade, have already been published (1). However, except for serum C-reactive
protein (CRP) levels (2-4),
there are few reports on the prognostic biomarkers for soft tissue
sarcoma, including lactate dehydrogenase (LDH).
LDH is a ubiquitous enzyme among vertebrates that
catalyses the interconversion of pyruvate and lactate with the
concurrent interconversion of nicotinamide adenine dinucleotide
(NAD+) and reduced nicotinamide adenine dinucleotide (NADH). In
most cells, glucose is metabolized to pyruvate via glycolysis.
During oxidative phosphorylation, most of the pyruvate gets
completely oxidized to CO2 within the mitochondria in
the presence of abundant oxygen. During oxygen shortage, pyruvate
is redirected away from mitochondrial oxidative phosphorylation via
anaerobic glycolysis to generate lactate. In normal cells, lactate
is produced via anaerobic glycolysis only during oxygen deficiency.
However, in cancer cells, most of the glucose is converted to
lactate, regardless of the presence of oxygen. Aerobic glycolysis
is known as the Warburg effect (5-7).
LDH exists as five major isoenzymes, labelled LDH-1-5. It is formed
by the association between two different subunits, M and H, and
encoded by two different genes, ldh-a and ldh-b.
LDH-1 and LDH-5 are commonly known as LDHB and LDHA, respectively.
The isozyme profile ratio of the LDH isozyme is tissue specific.
Tumour tissues express LDH-4 and LDH-5, which play a role in
aerobic glycolysis (8).
Clinically, serum LDH levels are used for the late
detection of myocardial infarction and the diagnosis of haemolytic
anaemia (9); it also has clinical
importance in cancer. Several studies have reported an association
between high LDH levels and poor prognosis in several cancers,
including renal cell carcinoma, nasopharyngeal carcinoma, melanoma,
prostate cancer, colorectal cancer, and lung cancer (10). Reports suggest that a high serum
LDH level is a predictive factor for poor overall survival in a few
histological types, such as osteosarcoma and Ewing sarcoma
(11-16).
In past report, we revealed that serum LDH level was one of the
diagnostic factors for soft tissue sarcoma, however, we could not
make reference to whether serum LDH level was a prognostic factor
for soft tissue sarcoma (17).
There are few reports on the association between
serum LDH levels and the prognosis of soft tissue sarcoma and
whether it is a prognostic factor remains unclear. Thus, this study
evaluated the association between serum LDH levels and clinical
characteristics of soft tissue sarcomas, as well as the prognostic
impact of serum LDH levels.
Patients and methods
Patients
Medical records of 138 patients with soft tissue
sarcoma treated in our hospital between April 2003 and March 2019
were retrospectively reviewed. Tumours belonging to the
intermediate group were excluded, for example, atypical lipomatous
tumour/well differentiated liposarcoma. Thirty-five patients
treated after an unplanned resection or referred for additional
treatment were excluded; the remaining 103 patients were included.
Blood tests, including white blood cell (WBC) count, haemoglobin
(Hb) level, serum CRP level, and serum LDH level, were performed
for all patients during their first visit to our hospital. Tumour
size was defined as the maximum diameter of the tumour mass on
magnetic resonance imaging. Histological diagnosis and histological
grade determination were made using a core needle, incisional
biopsy, or excisional biopsy. Histologic grade 1 was classified as
low grade and grades 2 and 3 were classified as high grade.
Computed tomography was performed to screen for distant metastasis.
Serum CRP and LDH levels were tested using an automated clinical
chemistry analyser TBA-200SR (Toshiba Medical Systems) from April
2003 to February 2011 and TBA-c16000 (Canon medical systems
corporation, Tochigi, Japan) from March 2011 to March 2019. The
associations between serum LDH levels with age, sex, tumour depth,
tumour size, presence or absence of distant metastases,
histological grade, histological diagnosis, WBC count, Hb level,
and serum CRP level were analysed. Disease-specific survival (DSS)
and disease-free survival (DFS) were analysed using stratified
clinical characteristics. Age was stratified as <71 and ≥71
years according to the median value of analysed patients, and
tumour sizes were stratified as <5.0 and ≥5.0 cm according to a
past report (18). Regarding
laboratory test values, WBC count was stratified as ≤9,100/µl and
>9,100/µl, Hb level as <11.3 and ≥11.3 g/dl, serum CRP level
as ≤0.20 and >0.20 mg/dl, and serum LDH level as ≤253 and
>253 IU/l, which are the standard values used at our
institution. Survival rate analysis was performed for high-grade
soft tissue sarcomas. Patients with distant metastasis at the first
visit were excluded from the survival rate analysis and only
patients with a tumour-free status during treatment initiation were
included in the DFS analysis. DSS was defined as the interval
between the date of the first visit to our hospital and the date of
death. DFS was defined as the interval between the initiation of
primary treatment and the diagnosis of local recurrence or distant
metastasis.
Statistical analysis
Associations between serum LDH levels and clinical
characteristics were evaluated using the Mann-Whitney U test or
Kruskal-Wallis test for categorical data and Spearman's rank
correlation coefficient for continuous data. Survival curves were
constructed using the Kaplan-Meier method. The log-rank test was
used to compare the survival of patients with clinical
characteristics. Multivariate analyses for DSS were performed using
the Cox proportional hazards model. Significant variables
identified in the univariate analysis were evaluated in a
multivariable analysis. Statistical analyses were performed using
JMP® 14 (SAS Institute Inc.). P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient demographics of all 103
patients
This study included 61 men and 42 women.
Twenty-eight of the included tumours were superficial, and 75 were
deep. The median age was 66.0 (range, 2-96) years. The median
tumour size was 8.9 (1.0-31.6) cm. The median WBC count was 6,400
(1,900-18,100)/µl. The median Hb level was 13.2 (6.3-17.4) g/dl.
The median serum CRP level was 0.26 (0.01-21.2) mg/dl. The median
serum LDH level was 182 (21-2,014) IU/l. The most diagnosed tumour
was malignant fibrous histiocytoma/undifferentiated pleomorphic
sarcoma, followed by liposarcoma; the most common location was the
thigh, followed by the lower leg (Table I). Of all 103 patients, there were
86 patients with high histological grades, and 12 patients with low
histological grades. Data on the histological grade of five
patients were not available. Treatment methods are shown in
Table I.
 | Table IHistological diagnosis, location of
tumours, and treatment of all 103 patients. |
Table I
Histological diagnosis, location of
tumours, and treatment of all 103 patients.
| Characteristics | Number of
patients |
|---|
| Histological
diagnosis | |
|
MFH/UPS | 23 |
|
Liposarcoma | 20 |
|
Myxoid
liposarcoma | 9 |
|
Pleomorphic
liposarcoma | 6 |
|
Dedifferentiated
liposarcoma | 5 |
|
Leiomyosarcoma | 18 |
|
Myxofibrosarcoma | 11 |
|
MPNST | 8 |
|
Synovial
sarcoma | 6 |
|
Rhabdomyosarcoma | 5 |
|
Ewing
sarcoma | 3 |
|
Other
histologic types | 9 |
| Tumour location | |
|
Thigh | 52 |
|
Lower
leg | 8 |
|
Buttock | 7 |
|
Retroperitoneum | 6 |
|
Upper
arm | 5 |
|
Back | 4 |
|
Shoulder
girdle | 3 |
|
Chest
wall | 3 |
|
Abdominal
wall | 3 |
|
Forearm | 3 |
|
Hand | 2 |
|
Other
locations | 7 |
| Treatment | |
|
Surgery
alone | 64 |
|
Surgery +
RT | 7 |
|
Surgery +
CT | 17 |
|
Surgery + RT
+ CT | 2 |
|
RT
alone | 3 |
|
CT
alone | 3 |
|
RT + CT | 3 |
|
BST | 4 |
Serum LDH levels and clinical
characteristics
There was a significant association between the
presence/absence of distant metastasis at the first visit and serum
LDH levels (P<0.001) and tumour grade (P=0.040) (Table II). Age, sex, tumour depth, tumour
size, laboratory test results, and histological diagnosis did not
correlate with serum LDH levels (Table III).
 | Table IIAssociations between serum LDH levels
and clinical characteristics with categorical data of all 103
patients. |
Table II
Associations between serum LDH levels
and clinical characteristics with categorical data of all 103
patients.
| | Number of
patients | Mean serum LDH
levels (IU/l) | P-value |
|---|
| Sex | | | 0.098 |
|
Male | 61 | 201.4 | |
|
Female | 42 | 288.1 | |
| Tumour depth | | | 0.69 |
|
Superficial | 28 | 219.3 | |
|
Deep | 75 | 243.3 | |
| Metastasis | | | <0.001 |
|
M0 | 82 | 199.9 | |
|
M1 | 21 | 380.6 | |
| Histological
grade | | | 0.040 |
|
Low
grade | 12 | 194.0 | |
|
High
grade | 86 | 232.4 | |
| Histological
diagnosis | | | 0.41 |
|
MFH/UPS | | 195.8 | |
|
Liposarcoma | | 189.8 | |
|
Leiomyosarcoma | | 214.9 | |
|
Myxofibrosarcoma | | 228.0 | |
|
MPNST | | 191.0 | |
|
Synovial
sarcoma | | 243.3 | |
|
Rhabdomyosarcoma | | 402.8 | |
|
Ewing
sarcoma | | 842.3 | |
|
Other
histologic types | | 242.0 | |
 | Table IIICorrelations between serum LDH levels
and clinical characteristics with continuous data. |
Table III
Correlations between serum LDH levels
and clinical characteristics with continuous data.
| Characteristic | Spearman's ρ | P-value |
|---|
| Age | -0.0060 | 0.55 |
| Tumour size | 0.0625 | 0.53 |
| WBC count | 0.0724 | 0.47 |
| Hb level | -0.0344 | 0.73 |
| Serum CRP
level | -0.0140 | 0.89 |
DSS and clinical characteristics
There were 21 patients with distant metastasis and
12 patients with low-grade tumours. As a result, DSS analysis was
performed for 70 patients with high-grade soft tissue sarcomas
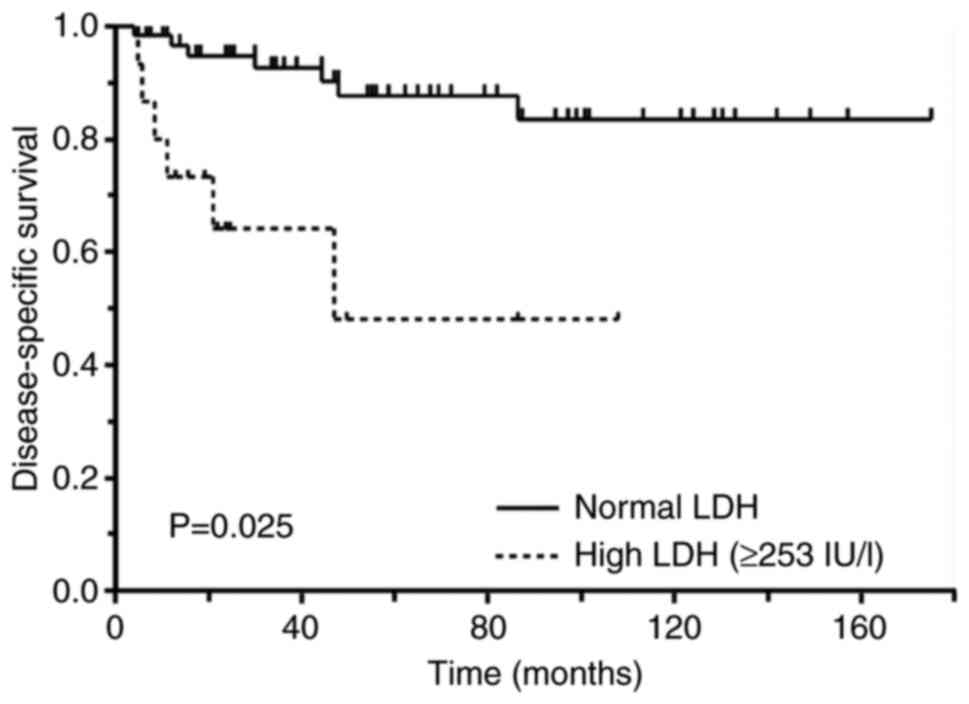
without distant metastasis at the first visit. In the univariate
analysis, patients with high serum LDH levels had significantly
worse DSS than patients with normal serum LDH levels (P=0.025)
(Fig. 1). Older patients had worse
DSS than younger patients (P=0.016) and patients with high-grade
soft tissue sarcomas (P=0.035). Sex, tumour depth, tumour size, WBC
count, Hb levels, and serum CRP levels were not associated with
DSS. In the multivariate analysis, older age (hazard ratio [HR],
5.86; 95% confidence interval [CI], 1.35-25.3; P=0.018) and high
serum LDH levels (HR, 4.60; 95% CI, 1.16-18.2; P=0.030) were poor
prognostic factors (Table
IV).
 | Table IVDisease-specific survival and
clinical characteristics. |
Table IV
Disease-specific survival and
clinical characteristics.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Characteristic | Number of
patients | 5-year DSS (%) | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age | | | 0.016 | | | |
|
Younger
(<71 years) | 32 | 92.1 | | | | |
|
Older (≥71
years) | 38 | 61.0 | | 5.86 | 1.35-25.3 | 0.018 |
| Sex | | | 0.81 | | | |
|
Male | 38 | 76.7 | | | | |
|
Female | 32 | 76.3 | | | | |
| Tumour depth | | | 0.39 | | | |
|
Superficial | 21 | 74.2 | | | | |
|
Deep | 49 | 78.5 | | | | |
| Tumour size | | | 0.44 | | | |
|
Small
(<5.0 cm) | 19 | 88.9 | | | | |
|
Large (≥5.0
cm) | 61 | 75.2 | | | | |
| Grade | | | 0.035 | | | |
|
2 | 30 | 92.7 | | | | |
|
3 | 40 | 65.3 | | 3.09 | 0.67-14.3 | 0.15 |
| WBC count | | | 0.84 | | | |
|
Normal
(≤9,100/µl) | 25 | 76.8 | | | | |
|
High
(>9,100/µl) | 57 | 80.0 | | | | |
| Hb level | | | 0.59 | | | |
|
Low
(<11.3 g/dl) | 15 | 68.8 | | | | |
|
Normal
(≥11.3 g/dl) | 55 | 79.6 | | | | |
| Serum CRP
level | | | 0.28 | | | |
|
Normal
(≤0.20 mg/dl) | 28 | 84.5 | | | | |
|
High
(>0.20 mg/dl) | 42 | 72.7 | | | | |
| Serum LDH
level | | | 0.025 | | | |
|
Normal (≤253
IU/l) | 58 | 82.1 | | | | |
|
High
(>253 IU/l) | 12 | 60.6 | | 4.60 | 1.16-18.2 | 0.030 |
Clinical characteristics of patients
with high serum LDH level
There were 12 patients with high serum LDH level
(>253 IU/l) in the group of patients analysed for DSS rate. The
most common histological diagnosis was myxofibrosarcoma (four
patients), followed by malignant fibrous
histiocytoma/undifferentiated pleomorphic sarcoma (two patients).
Pleomorphic liposarcoma, leiomyosarcoma, rhabdomyosarcoma, Ewing
sarcoma, synovial sarcoma, and CIC rearranged sarcoma were one case
each. There were seven older patients, eight women, seven
deep-seated tumours, 10 large tumours, three patients with high WBC
counts, two with low Hb levels, and eight with high serum CRP
levels in this group. During the observation period, local
recurrence or distant metastasis was observed in six patients, and
four patients died of disease.
DFS and clinical characteristics
Only best supportive care was given to two of 70
patients who underwent DSS analysis. Therefore, DFS was analysed
for 68 patients. Patients with large tumour sizes had significantly
worse DFS than those with small tumour size (P=0.026). Age, sex,
tumour depth, tumour grade, WBC count, Hb levels, serum CRP level,
and serum LDH level were not associated with DFS. The multivariate
analysis was not performed because the only variable that showed a
significant value in univariate analysis was tumour size (Table V).
 | Table VDisease-free survival and clinical
characteristics. |
Table V
Disease-free survival and clinical
characteristics.
| | Univariate
analysis |
|---|
| Characteristic | Number of
patients | 5-year DFS (%) | P-value |
|---|
| Age | | | 0.15 |
|
Younger
(<71 years) | 32 | 54.9 | |
|
Older (≥71
years) | 36 | 39.2 | |
| Sex | | | 0.41 |
|
Male | 37 | 42.1 | |
|
Female | 31 | 51.6 | |
| Tumour depth | | | 0.96 |
|
Superficial | 21 | 42.7 | |
|
Deep | 47 | 46.2 | |
| Tumour size | | | 0.026 |
|
Small
(<5.0 cm) | 9 | 88.9 | |
|
Large (≥5.0
cm) | 59 | 39.3 | |
| Grade | | | 0.37 |
|
2 | 29 | 51.0 | |
|
3 | 39 | 42.9 | |
| WBC count | | | 0.061 |
|
Normal
(≤9,100/µl) | 59 | 50.5 | |
|
High
(>9,100/µl) | 9 | 19.1 | |
| Hb level | | | 0.27 |
|
Low
(<11.3 g/dl) | 14 | 35.9 | |
|
Normal
(≥11.3 g/dl) | 54 | 50.0 | |
| Serum CRP
level | | | 0.19 |
|
Normal
(≤0.20 mg/dl) | 28 | 56.0 | |
|
High
(>0.20 mg/dl) | 40 | 39.2 | |
| Serum LDH
level | | | 0.33 |
|
Normal (≤253
IU/l) | 56 | 48.8 | |
|
High
(>253 IU/l) | 12 | 44.4 | |
Discussion
This study demonstrated that soft tissue sarcoma
patients with metastases at the first visit and high histological
grade showed high serum LDH levels. Patients with high serum LDH
levels showed poor DSS in both the univariate and multivariate
analyses.
Several previous studies and reviews have
investigated the prognostic factors associated with soft tissue
sarcomas. Tumour size and grade are well-known prognostic factors
(1,19). Older adults had worse survival
compared with adolescents and young adults of all histologic
subtypes (20,21). Regarding biomarkers, the
pre-treatment serum CRP levels were correlated with the prognosis
(2,22), and the neutrophil-to-lymphocyte
ratio was also associated with the prognosis of soft tissue sarcoma
(23,24). Thus, pre-treatment high systemic
inflammation is implicated in the poor prognosis of sarcoma
(25). Furthermore,
fibrinogen/albumin rate (26) and
Hb levels (27) were reported as
prognostic biomarkers for soft tissue sarcomas. Regarding serum LDH
level, there were some reports of an association between LDH levels
and prognosis in certain sarcomas. High LDH levels were a
significant predictive factor for DFS or overall survival in
patients with osteosarcoma (11-13)
and Ewing sarcoma (15,28). In addition, LDH levels are
reportedly diagnostic, prognostic, and predictive markers of the
therapeutic response in many cancers (8,10,29),
including renal cell carcinoma (30), nasopharyngeal carcinoma (31), melanoma (32), prostate cancer (33), colorectal cancer (34), and lung cancer (35).
In this study, the univariate analysis demonstrated
that DSS was associated with age, tumour grade, and serum LDH
levels. In the multivariate analysis, older age and high serum LDH
levels were predictors of poor DSS. As for DFS, significant
variables were not found. Some variables have been reported as
prognostic factors of soft tissue sarcoma. There were a few
discrepancies between our results and past reports. Soft tissue
sarcoma is a group of tumours which are highly heterogeneous and
have various characteristics. Differences in the distribution of
histological type, study sizes, patient recruitment criteria, and
biomarker thresholds might have resulted in inconsistencies. There
was one report showing that a high serum LDH level was not a poor
prognostic factor for soft tissue sarcoma. Although it is probably
the median value, the paper showing different results did not
clearly stipulate the threshold of LDH level (36). Our results revealed that a high
serum LDH level above our laboratory standard of 253 IU/l is a poor
prognostic factor for DSS in patients with soft tissue sarcoma. And
we are in a position to support this result.
LDH plays a significant role in the Warburg effect
that occurs during cancer cell metabolism. In normal cells, LDH
activity and pyruvate production increases during certain stress
conditions, specifically tissue injury, necrosis, hypoxia,
haemolysis, and myocardial infarction. In contrast, the
upregulation of LDH activity in cancer cells is not associated with
stress conditions. Thus, the oxygen dependency of cancer cells is
reduced (5-7).
Additionally, the damage of surrounding soft tissue due to tumour
growth can elevate LDH levels (8,29).
Activity of soft tissue sarcoma cell and tumour growth effect serum
LDH levels, therefore, high serum LDH levels may be correlated with
presence of distant metastasis, high histological grade, and poor
prognosis in patients with soft tissue sarcoma.
This study had some limitations. First, our sample
size was smaller than that of a similar existing study on other
prognostic factors. Secondly, there were some uncertainties about
the clinical significance of high serum LDH levels in patients with
soft tissue sarcoma. For example, serum LDH level is affected by
general conditions other than soft tissue sarcoma such as tissue
injury, necrosis, hypoxia, haemolysis, and myocardial infarction.
However, LDH isozymes were not distinguished in this study; tumour
tissues expressed LDH-4 and LDH-5 specifically. Therefore, the
levels of other isoenzymes may have been elevated owing to other
mechanisms. As another uncertainty, this study defined our
laboratory standard of 253 IU/l as a threshold, which varies
according to reports. Therefore, it is impossible to define high
serum LDH levels strictly and it should be aware of this point when
describing the word ‘high serum LDH levels’. Finally, soft tissue
sarcoma is a group of tumours with many histological types and
heterogeneous characteristics, though, this study could not analyse
the significance of serum LDH level for each histological type.
These points should be addressed in future studies.
In conclusion, this study revealed that high serum
LDH levels predict the presence of distant metastasis, high
histological grade, and worse DSS in patients with high-grade soft
tissue sarcomas. In patients with high serum LDH levels at the
first visit, we should keep these risks in mind during pretreatment
examinations and post-treatment follow-up.
Acknowledgements
The authors are grateful to the late Dr Joji
Miyawaki (Department of Bone and Joint Surgery, Ehime University
Graduate School of Medicine) for valuable discussions.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF designed this study and drafted the manuscript.
HI and HM gave advice on study design. TF and TK treated patients
and collected patient data. TF and TK confirm the authenticity of
all raw data. TM performed statistical analysis. TF, TK, HI and HM
analysed and interpreted data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Institutional
Review Board of the Ehime University Hospital (approval no.
1510010) and all study procedures were performed following the
Declaration of Helsinki. The requirement for informed consent was
waived owing to the retrospective nature of the study and the lack
of identifiable patient information.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maretty-Nielsen K: Prognostic factors in
soft tissue sarcoma. Dan Med J. 61(B4957)2014.PubMed/NCBI
|
|
2
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Uchida A and Sudo A: Clinical significance of
pretreatment serum C-reactive protein level in soft tissue sarcoma.
Cancer. 118:1055–1061. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Uchida A and Sudo A: The combined use of the
neutrophil-lymphocyte ratio and C-reactive protein level as
prognostic predictors in adult patients with soft tissue sarcoma. J
Surg Oncol. 108:481–485. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Choi ES, Kim HS and Han I: Elevated
preoperative systemic inflammatory markers predict poor outcome in
localized soft tissue sarcoma. Ann Surg Oncol. 21:778–785.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Warburg O: On the orign of cancer cells.
Science. 123:309–314. 1956.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Markert CL: Lactate dehydrogenase.
Biochemistry and function of lactate dehydrogenase. Cell Biochem
Funct. 2:131–134. 1984.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gallo M, Sapio L, Spina A, Naviglio D,
Calogero A and Naviglio S: Lactic dehydrogenase and cancer: An
overview. Front Biosci (Landmark Ed). 20:1234–1249. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Huijgen HJ, Sanders GTB, Koster RW,
Vreeken J and Bossuyt PMM: The clinical value of lactate
dehydrogenase in serum: A quantitative review. Clin Chem Lab Med.
35:569–580. 1997.PubMed/NCBI
|
|
10
|
Zhang J, Yao YH, Li BG, Yang Q, Zhang PY
and Wang HT: Prognostic value of pretreatment serum lactate
dehydrogenase level in patients with solid tumors: A systematic
review and meta-analysis. Sci Rep. 5(9800)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ferrari S, Bacci G, Picci P, Mercuri M,
Briccoli A, Pinto D, Gasbarrini A, Tienghi A and Brach del Prever
A: Long-term follow-up and post-relapse survival in patients with
nonmetastatic osteosarcoma of the extremity treated with
neoadjuvant chemotherapy. Ann Oncol. 8:765–771. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Durnali A, Alkis N, Cangur S, Yukruk FA,
Inal A, Tokluoglu S, Seker MM, Bal O, Akman T, Inanc M, et al:
Prognostic factors for teenage and adult patients with high-grade
osteosarcoma: An analysis of 240 patients. Med Oncol.
30(624)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen J, Sun MX, Hua YQ and Cai ZD:
Prognostic significance of serum lactate dehydrogenase level in
osteosarcoma: A meta-analysis. J Cancer Res Clin Oncol.
140:1205–1210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Riley RD, Burchill SA, Abrams KR, Heney D,
Sutton AJ, Jones DR, Lambert PC, Young B, Wailoo AJ and Lewis IJ: A
systematic review of molecular and biological markers in tumours of
the Ewing's sarcoma family. Eur J Cancer. 39:19–30. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bacci G, Forni C, Longhi A, Ferrari S,
Donati D, De Paolis M, Barbieri E, Pignotti E, Rosito P and Versari
M: Long-term outcome for patients with non-metastatic Ewing's
sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402
patients treated at Rizzoli between 1972 and 1992. Eur J Cancer.
40:73–83. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wan W, Lou Y, Hu Z, Wang T, Li J, Tang Y,
Wu Z, Xu L, Yang X, Song D and Xiao J: Factors affecting survival
outcomes of patients with non-metastatic Ewing's sarcoma family
tumors in the spine: A retrospective analysis of 63 patients in a
single center. J Neurooncol. 131:313–320. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fujibuchi T, Miyawaki J, Kidani T, Imai H
and Miura H: Prediction of soft tissue sarcoma from clinical
characteristics and laboratory data. Cancers (Basel).
12(679)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zagars GK, Ballo MT, Pisters PWT, Pollock
RE, Patel SR, Benjamin RS and Evans HL: Prognostic factors for
patients with localized soft-tissue sarcoma treated with
conservation surgery and radiation therapy: An analysis of 1225
patients. Cancer. 97:2530–2543. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maretty-Nielsen K, Aggerholm-Pedersen N,
Safwat A, Jørgensen PH, Hansen BH, Baerentzen S, Pedersen AB and
Keller J: Prognostic factors for local recurrence and mortality in
adult soft tissue sarcoma of the extremities and trunk wall. Acta
Orthop. 85:323–332. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Biau DJ, Ferguson PC, Turcotte RE, Chung
P, Isler MH, Riad S, Griffin AM, Catton CN, O'Sullivan B and Wunder
JS: Adverse effect of older age on the recurrence of soft tissue
sarcoma of the extremities and trunk. J Clin Oncol. 29:4029–4035.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Papworth KE, Arroyo VM, Styring E, Zaikova
O, Melin BS and Lupo PJ: Soft-tissue sarcoma in adolescents and
young adults compared with older adults: A report among 5000
patients from the Scandinavian Sarcoma Group Central Register.
Cancer. 125:3595–3602. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang X, Liu S and Zhao X, Fang E and Zhao
X: The value of C-reactive protein as an independent prognostic
indicator for disease-specific survival in patients with soft
tissue sarcoma: A meta-analysis. PLoS One.
14(e0219215)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Idowu OK, Ding Q, Taktak AFG, Chandrasekar
CR and Yin Q: Clinical implication of pretreatment neutrophil to
lymphocyte ratio in soft tissue sarcoma. Biomarkers. 17:539–544.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu G, Ke LC and Sun SR: Prognostic value
of pretreatment neutrophil-to-lymphocyte ratio in patients with
soft tissue sarcoma: A meta-analysis. Medicine (Baltimore).
97(e12176)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Y, Liu X, Zhang J and Yao W: Prognostic
role of elevated preoperative systemic inflammatory markers in
localized soft tissue sarcoma. Cancer Biomarkers. 16:333–342.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liang Y, Wang W, Que Y, Guan Y, Xiao W,
Fang C, Zhang X and Zhou Z: Prognostic value of the
fibrinogen/albumin ratio (FAR) in patients with operable soft
tissue sarcoma. BMC Cancer. 18(942)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Szkandera J, Gerger A, Liegl-Atzwanger B,
Stotz M, Samonigg H, Ploner F, Stojakovic T, Leithner A and Pichler
M: Pre-Treatment anemia is a poor prognostic factor in soft tissue
sarcoma patients. PLoS One. 9(e107297)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li S, Yang Q, Wang H, Wang Z, Zuo D, Cai Z
and Hua Y: Prognostic significance of serum lactate dehydrogenase
levels in Ewing's sarcoma: A meta-analysis. Mol Clin Oncol.
5:832–838. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase a in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Shinohara N, Nonomura K, Abe T, Maruyama
S, Kamai T, Takahashi M, Tatsugami K, Yokoi S, Deguchi T, Kanayama
H, et al: A new prognostic classification for overall survival in
asian patients with previously untreated metastatic renal cell
carcinoma. Cancer Sci. 103:1695–1700. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wan XB, Wei L, Li H, Dong M, Lin Q, Ma XK,
Huang PY, Wen JY, Li X, Chen J, et al: High pretreatment serum
lactate dehydrogenase level correlates with disease relapse and
predicts an inferior outcome in locally advanced nasopharyngeal
carcinoma. Eur J Cancer. 49:2356–2364. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Weide B, Richter S, Büttner P, Leiter U,
Forschner A, Bauer J, Held L, Eigentler TK, Meier F and Garbe C:
Serum S100B, lactate dehydrogenase and brain metastasis are
prognostic factors in patients with distant melanoma metastasis and
systemic therapy. PLoS One. 8(e81624)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Halabi S, Lin CY, Kelly WK, Fizazi KS,
Moul JW, Kaplan EB, Morris MJ and Small EJ: Updated prognostic
model for predicting overall survival in first-line chemotherapy
for patients with metastatic castration-resistant prostate cancer.
J Clin Oncol. 32:671–677. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mekenkamp LJ, Heesterbeek KJ, Koopman M,
Tol J, Teerenstra S, Venderbosch S, Punt CJ and Nagtegaal ID:
Mucinous adenocarcinomas: Poor prognosis in metastatic colorectal
cancer. Eur J Cancer. 48:501–509. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang X, Jiang R and Li K: Prognostic
significance of pretreatment laboratory parameters in combined
small-cell lung cancer. Cell Biochem Biophys. 69:633–640.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakamura T, Asanuma K, Hagi T and Sudo A:
Is serum lactate dehydrogenase useful for predicting oncological
outcome in patients with soft tissue sarcoma? Anticancer Res.
39:6871–6875. 2019.PubMed/NCBI View Article : Google Scholar
|