Introduction
Prostate cancer (PCa) is the most common type of
solid neoplasm in males (1). When
radical prostatectomy (RP) is selected as a treatment for localized
PCa, the prognosis is generally favorable (2). However, postoperative biochemical
recurrence (BCR) occurs in 16-31% of patients within 5 years and in
25-53% within 10 years (3-5).
Certain cases develop into castration-resistant PCa after clinical
recurrence, frequently leading to poor outcomes. Thus, BCR is often
used to justify the application of salvage therapies, such as
endocrine therapy and radiotherapy.
Certain studies have identified various predictive
factors for BCR. BCR after prostatectomy has been associated with
multiple factors, including the preoperative prostate-specific
antigen (PSA) score, positive surgical margins (PSM), the Gleason
score (GS) at prostatectomy and pathological staging. Of these, PSM
is the most important predictive factor for BCR (6-13).
Certain patients with PSM have favorable prognosis after undergoing
surgery alone, while others require salvage therapy immediately
after surgery and have poor prognosis (14-16).
Therefore, patients with PSM are considered to be a highly diverse
group and the significance of PSM after RP remains controversial.
This finding suggests the requirement for further subclassification
of positive margins to identify patients with an elevated risk of
BCR. However, only a small number of studies have reported on
predictive factors for PSM and BCR in patients with PSM (17). In addition, studies evaluating the
usefulness of the GS of the tumor at the margin in RP are currently
scarce.
The present study aimed to investigate the
preoperative factors that predict PSM and the significant
predictive factors for BCR in cases with PSM. In addition, it was
examined whether documenting the GS of the tumor at the margin in
pathological reports is useful as a predictive factor for BCR.
Patients and methods
Patients and tissue samples
Patients (n=241) who underwent prostatectomy at
Kurume University Hospital (Kurume, Japan) between January 2007 and
December 2011 were enrolled in the present study. Most of the
surgeries during this period were open procedures. Patients who had
received preoperative hormone therapy and/or radiation therapy and
those with pathological T stage 0 (pT0) were excluded. As a part of
this study, the pathological diagnoses of the patients were
re-examined. All patients were pathologically diagnosed with
prostatic adenocarcinoma. Histopathological evaluations were
performed by three pathologists (HK, KU and HY). Pathological
diagnosis was made according to the 2016 World Health Organization
Classification of Tumors of the Urinary System and Male Genital
Organs (18).
The prostatectomy specimens were pinned to a
paraffin block and fixed in 10% formalin for a minimum of 48 h and
inked on the surface. Paraffin-embedded tissue samples were cut
into sections of 4-µm in thickness and examined on coated glass
slides. A positive margin was defined as tumor cells abutting the
inked surgical margin of the prostate apex, periphery and bladder
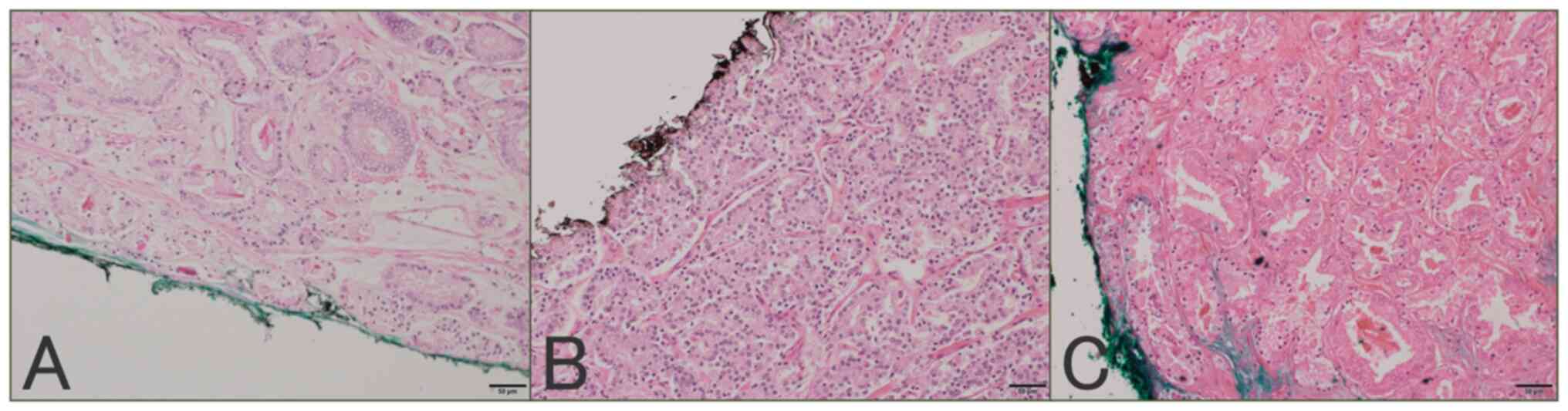
neck. The GS of the tumor at the PSM was evaluated at the site in
contact with the inked margin; when it was difficult to evaluate
the GS at the PSM due to heat denaturation, it was evaluated using
the continuous non-denaturing site GS (Fig. 1). PSM length was defined as the
total length of the tumor in contact with the inked margin. When
multiple PSMs were present, the added length of all margins was
calculated.
The present study was approved by the Research
Ethics Committee of Kurume University (Kurume, Japan) and conformed
to the guidelines of the Declaration of Helsinki.
Statistical analysis
The associations between the margin status and GS of
the tumor at the margin and clinicopathological characteristics
were examined using the χ2 test or Fisher's exact test.
Cancer survival analysis was performed using the Kaplan-Meier
method, log-rank test and Cox's proportional hazards model. The
threshold for statistical significance was set at P<0.05. BCR
was defined as an increase in PSA level (>0.2 ng/ml) after two
different measurements at least 3 months apart. For PSA, 10 ng/ml
was used as the cutoff value that was classified as indicative of
an intermediate risk in the D'Amico risk classification (7). For the positive core percentage and
PSA density, the median was used as the cutoff value.
JMP® Pro 14 software (SAS Institute, Inc.) was used to
perform all statistical analyses.
Results
Association between surgical margin
and clinicopathological characteristics
Of the 241 patients who had undergone RP, 122 had at
least one PSM. The median follow-up period was 72 months. The
characteristics of the entire RP cohort, the subset of patients
with PSM and the subset with negative surgical margins (NSM) are
provided in Table I. Higher PSA
level at diagnosis, GS at prostatectomy and pathological T stage,
as well as BCR, were more frequently identified in patients with
PSM than in those with NSM (all P<0.05).
 | Table IAssociation between surgical margin
and clinicopathological characteristics. |
Table I
Association between surgical margin
and clinicopathological characteristics.
| Parameter | Total (n=241) | Negative surgical
margin (n=119) | Positive surgical
margin (n=112) | P-value |
|---|
| Age at diagnosis,
years | 67 (50-77) | 67 (53-77) | 67 (50-76) | 0.697 |
| PSA level at
diagnosis, ng/ml | | | | |
|
Total | 7.90
(2.13-62.34) | 6.44
(2.13-62.34) | 9.42
(3.68-52.65) | 0.015 |
|
<10 | 163 (67.6) | 95 (79.8) | 68 (55.7) | 0.011 |
|
≥10 | 78 (32.4) | 24 (20.2) | 54 (44.3) | |
| Gleason score at
prostatectomy | | | | 0.013 |
|
6≥ | 27 (11.2) | 20 (16.8) | 7 (5.7) | |
|
3+4=7 | 103 (42.8) | 49 (41.2) | 54 (44.3) | |
|
4+3=7 | 75 (31.1) | 38 (31.9) | 37 (30.3) | |
|
8≤ | 36 (14.9) | 12 (10.1) | 24 (19.7) | |
| pT stage | | | | <0.0001 |
|
T2 | 176 (73.0) | 100 (84.0) | 76 (62.3) | |
|
T3a | 46 (19.1) | 15 (12.6) | 31 (25.4) | |
|
T3b | 19 (7.9) | 4 (3.4) | 15 (12.3) | |
| Lymphatic
invasion | 12 (5.0) | 4 (3.4) | 8 (6.6) | 0.067 |
| Peripheral nerve
invasion | 111 (46.1) | 41 (34.5) | 70 (57.4) | 0.081 |
| Biochemical
recurrence positive | 120 (49.8) | 45 (37.8) | 75 (61.5) | <0.0001 |
Preoperative predictive factors for
positive surgical margin in radical prostatectomy
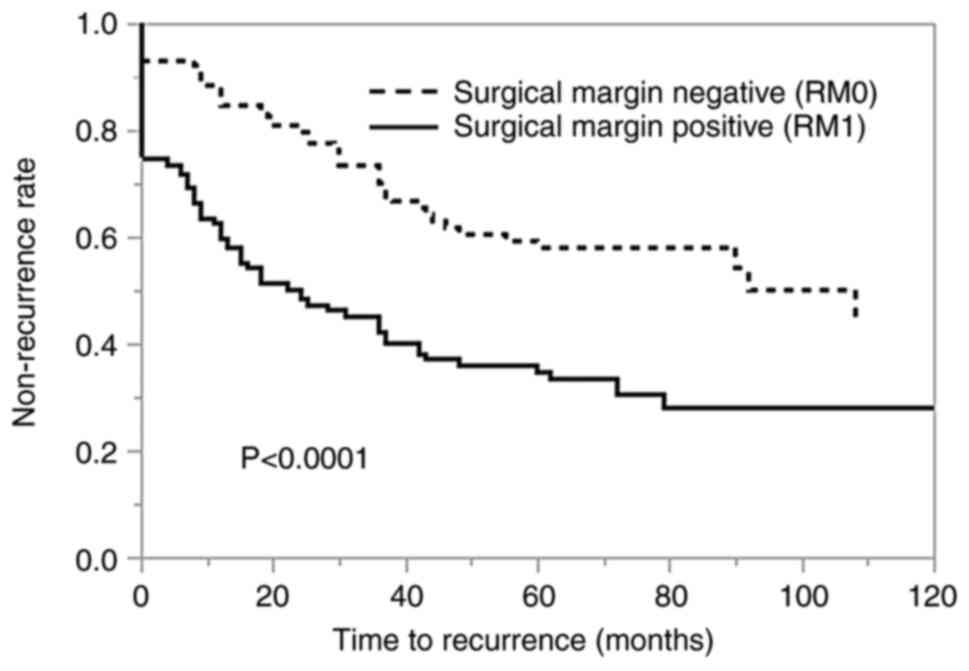
Kaplan-Meier curves demonstrated that the time to
BCR was significantly shorter in patients with PSM than in those
with NSM (Fig. 2). Univariate and
multivariate analyses for preoperative predictive factors for PSM
in RP are presented in Table II.
Univariate analysis revealed that >10 ng/ml PSA at diagnosis,
PSA density of >0.29 ng/ml/ml, GS at biopsy, clinical T stage,
PSA density >0.29 and >25% positive core at biopsy were
significant predictors for PSM. Furthermore, multivariate analysis
demonstrated that >10 ng/ml PSA at diagnosis and >25%
positive core at biopsy were independent prognostic preoperative
factors for PSM.
 | Table IIUnivariate and multivariate analysis
for preoperative predictive factor for positive surgical margin in
radical prostatectomy. |
Table II
Univariate and multivariate analysis
for preoperative predictive factor for positive surgical margin in
radical prostatectomy.
| | Univariate | Multivariate |
|---|
| Parameter | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age at diagnosis,
>67 years | 1.05
(0.63-1.74) | 0.857 | 1.04
(0.71-1.52) | 0.840 |
| PSA level at
diagnosis, >10 ng/ml | 3.14
(1.77-5.57) | <0.0001 | 2.28
(1.11-3.89) | 0.024 |
| CRP, mg/l | 0.76
(0.37-1.58) | 0.455 | 1.09
(0.65-1.81) | 0.745 |
| NLR, >1.58 | 1.67
(0.96-2.91) | 0.069 | 1.22
(0.79-1.89) | 0.368 |
| Gleason score at
biopsy | | | | |
|
6≥ | 1 | 0.0482 | 1 | 0.739 |
|
3+4=7 | 1.82
(0.96-3.44) | | 1.16
(0.73-1.84) | |
|
4+3=7 | 1.22
(0.53-2.83) | | 1.21
(0.46-1.46) | |
|
8≤ | 2.40
(1.18-4.88) | | 1.31
(0.61-1.83) | |
| Clinical T
stage | | | | |
|
T2 | 1 | 0.017 | 1 | 0.425 |
|
T3a | 2.01
(1.20-3.37) | | 1.77
(0.23-13.5) | |
|
T3b | 4.20
(0.42-41.5) | | 1.90
(0.28-18.6) | |
| PSA density
>0.29 ng/ml/ml | 2.81
(1.67-4.74) | <0.0001 | 1.48
(0.93-2.35) | 0.103 |
| Positive core at
biopsy, >25% | 2.16
(1.28-3.65) | 0.0035 | 1.42
(0.96-2.10) | 0.041 |
Correlation of GS between the tumor at
the margin and the main tumor
The GS of the tumor at the margin was 6 in 14
patients (11.5%), 7 in 69 patients (56.6%; 35 with GS 3+4 and 34
with GS 4+3), 8 in 30 patients (24.6%; all with GS 4+4), 9 in 8
patients (6.5%; 6 with GS 4+5 and 2 with GS 5+4) and 10 in 1
patient (0.8%). The GS of the tumor at the margin was equal, lower
and higher than that of the main tumor in 74 (60.7%), 16 (13.1%)
and 32 (26.2%) RPs, respectively (Table III).
 | Table IIICorrelation of GS between the tumor
at the margin and the main tumor. |
Table III
Correlation of GS between the tumor
at the margin and the main tumor.
| | GS of the tumor at
the margin | |
|---|
| GS of the main
tumor | 6 | 7 | 8 | 9 | 10 | Total |
|---|
|
6 | 7 | 0 | 0 | 0 | 0 | 7 |
|
7 | 6 | 66 | 19 | 0 | 0 | 91 |
|
8 | 1 | 2 | 11 | 0 | 0 | 14 |
|
9 | 0 | 1 | 0 | 8 | 0 | 9 |
| 10 | 0 | 0 | 0 | 0 | 1 | 1 |
| Total | 14 | 72 | 30 | 8 | 1 | 122 |
Association of the GS of the positive
surgical margin with other variables
The association of the GS of the tumor at the margin
and other variables in 122 patients with PSM is presented in
Table IV. The GS of the tumor at
the margin was highly associated with PSA at diagnosis (P=0.048),
pathological T stage (P=0.0445), the GS of the main tumor
(P<0.0001) and BCR (P=0.0017). Within a median follow-up of 72
months, BCR was observed in 75 (61.5%) of the 122 patients. The BCR
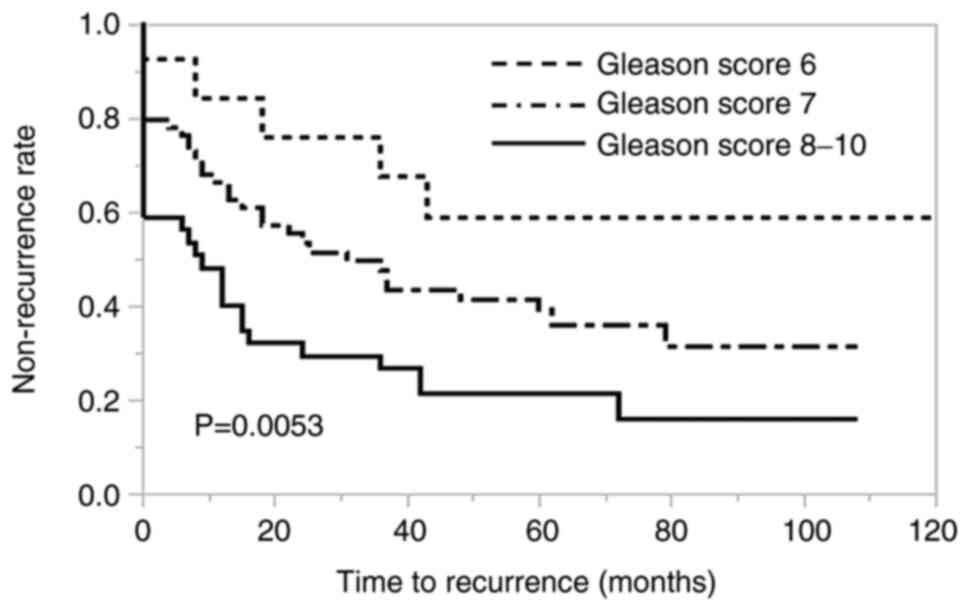
rates were 35.7, 55.1 and 82.1% in patients whose GS of the tumor
at the margin was 6, 7 and 8-10, respectively. The difference in
recurrence-free survival among these three groups of patients was
significant (P=0.0053; Fig. 3). In
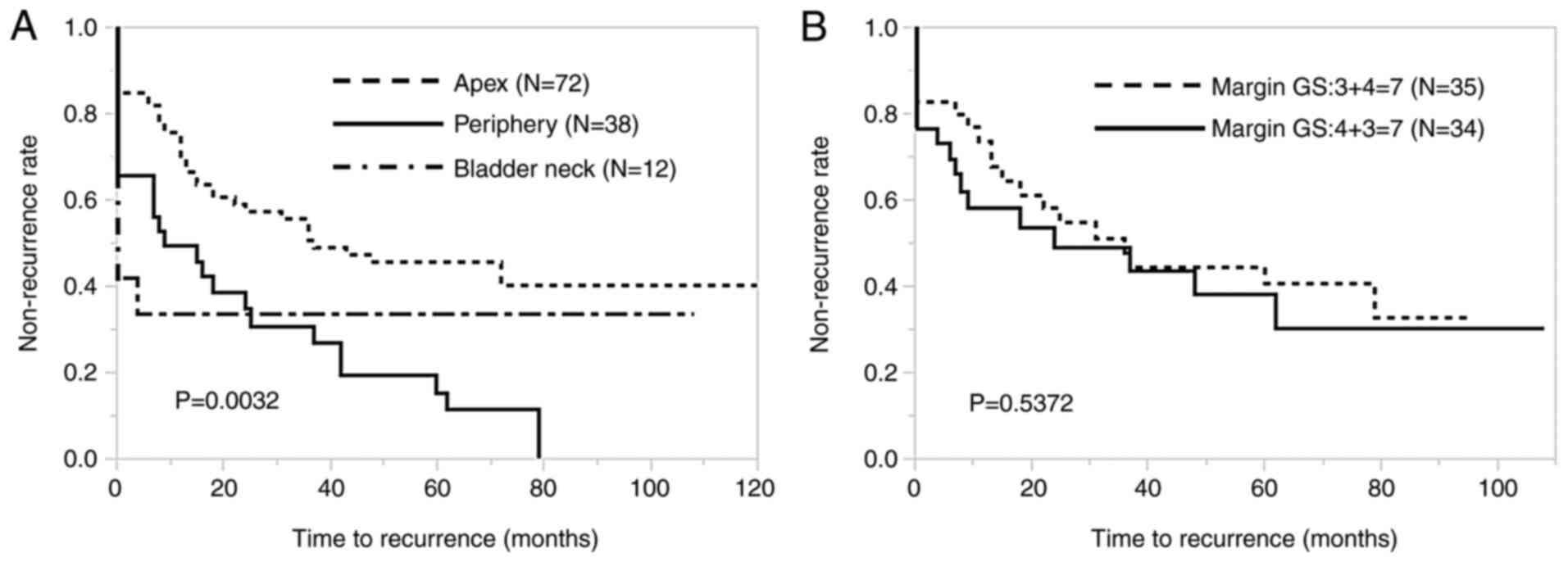
addition, the BCR rates were 52.7, 76.3 and 66.7% in patients whose
anatomic locations at the PSM were apex, periphery and bladder
neck, respectively. The difference in recurrence-free survival
among the three groups (apex, periphery and bladder neck) of
patients was also significant (P=0.0032; Fig. 4A). Among the 69 patients with GS 7
of the tumor at the margin, no significant difference was observed
in recurrence-free survival between those 35 with GS 3+4 and those
34 with GS 4+3 (P=0.537; Fig.
4B).
 | Table IVAssociation of the GS of the positive
surgical margin with other variables in the cohort (n=122). |
Table IV
Association of the GS of the positive
surgical margin with other variables in the cohort (n=122).
| Parameter | GS of 6 at the
margin (n=14) | GS of 7 at the
margin (n=69) | GS of 8-10 at the
margin (n=39) | P-value |
|---|
| Age at diagnosis,
years | 68 (55-76) | 67 (50-75) | 63 (53-75) | 0.216 |
| PSA level at
diagnosis, ng/ml | 7.0
(4.16-16.5) | 9.41
(4.05-52.6) | 10.4
(3.68-50.1) | 0.048 |
| Pathological
stage | | | | 0.0445 |
|
T2 | 13 | 40 | 23 | |
|
T3a | 1 | 21 | 9 | |
|
T3b | 0 | 8 | 7 | |
| GS of the main
tumor | | | | <0.0001 |
|
6 | 7 | 0 | 0 | |
|
7 | 6 | 66 | 19 | |
|
8 | 1 | 3 | 20 | |
| Biochemical
recurrence | 5 (35.7) | 38 (55.1) | 32 (82.1) | 0.0017 |
| Median FU time,
months | 58 | 69 | 72 | 0.081 |
Identification of the GS of the tumor
at the margin as a prognostic factor of biochemical recurrence
The results of univariate and multivariate analyses
for time to BCR are provided in Table
V. Univariate analysis indicated that PSA level at diagnosis,
pathological T stage, GS of the main tumor, GS of the tumor at the
margin and anatomic location of PSM were strong predictive factors
of BCR. On multivariate analysis, the GS of the tumor at the margin
(P=0.038) and anatomic location of PSM (P=0.040) were identified as
independent prognostic preoperative factors for BCR, whereas the GS
of the main tumor was not (P=0.661).
 | Table VUnivariate and multivariate analysis
for time to biochemical recurrence. |
Table V
Univariate and multivariate analysis
for time to biochemical recurrence.
| | Univariate | Multivariate |
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age at diagnosis
>67 years | 1.04
(0.99-1.08) | 0.056 | 1.03
(0.99-1.09) | 0.099 |
| PSA level at
diagnosis >10 ng/ml | 1.02
(1.00-1.04) | 0.026 | 2.61
(0.93-8.23) | 0.063 |
| Pathological T
stage | | | | |
|
T2 | 1 | | 1 | |
|
T3a | 1.36
(0.78-2.29) | 0.265 | 1.32
(0.68-2.56) | 0.210 |
|
T3b | 2.44
(1.21-4.55) | 0.014 | 1.51
(0.63-3.63) | 0.130 |
| GS of the main
tumor | | | | |
|
6 | 1 | | 1 | |
|
7 | 3.09
(0.96-18.9) | 0.137 | 2.22
(0.35-2.86) | 0.678 |
|
8-10 | 5.15
(1.47-32.5) | 0.029 | 1.94
(0.49-3.62) | 0.661 |
| GS of the tumor at
the PSM | | | | |
|
6 | 1 | | 1 | |
|
7 | 1.92
(0.82-5.58) | 0.137 | 1.15
(0.36-3.98) | 0.124 |
|
8-10 | 3.29
(1.39-9.66) | 0.005 | 1.78
(0.43-5.22) | 0.038 |
| Linear length of
tumor at the margin, >3 mm | 1.31
(0.89-1.17) | 0.656 | 1.08
(0.91-1.25) | 0.359 |
| Anatomic location
of the positive margin | | | | |
|
Apex | 1 | | 1 | |
|
Periphery | 2.12
(1.28-3.45) | 0.003 | 2.24
(1.16-4.30) | 0.040 |
|
Bladder
neck | 1.84
(0.79-3.77) | 0.118 | 1.19
(0.47-3.06) | 0.115 |
Discussion
To date, several studies have demonstrated the
usefulness of the GS of the tumor at the PSM as a predictor of BCR.
Cao et al (17) indicated
that both the GS of the main tumor and that of the tumor at the
margin were predictors of BCR. However it was previously suggested
that there was a difference between the two factors (17). In the present study, it was
demonstrated that the GS of the main tumor is not a prognostic
factor for BCR, whereas that of the tumor at the PSM is an
independent prognostic predictor for BCR on multivariate analysis.
This result suggested that GS of the tumor at the PSM is a more
important factor than that at the main tumor.
PSM is considered the most significant risk factor
for BCR after RP. Stephenson et al (19) reported that the PSM was a predictor
for BCR based on the result of a multivariate analysis in a
large-scale multicenter study involving >7,000 individuals. In
addition, Wright et al (20) reported that in cases with PSM, the
risk of BCR is 3.7 times higher and the risk of PCa-associated
death is 1.7 times higher.
Among the predictors of PSM, identifying the
preoperative risk factors may prevent BCR through the
implementation of more careful surgical manipulation during
surgery. Some preoperative factors have been reported previously,
as in previous studies, PSA >10 ng/ml and a biopsy-positive rate
of >25% were the factors identified in the present study.
Preston et al (21) reported that among patients with
localized PCa with a PSM, the disease-free survival in
extraprostatic extention (EPE)+ patients with a negative resection
margin was short. The importance of determining the appropriate
dissection layer and resection site was confirmed. In the present
study, the biochemical non-recurrent survival curves of 122
patients with PSM and 119 with NSM were compared and the incidence
of BCR was indicated to be significantly higher in those with
PSM.
The frequency of progression to clinical recurrence
from BCR without treatment was 34% (22). In addition, hormone and radiation
therapy were performed as salvage therapy for BCR. For radiotherapy
in particular, improvement in cancer-specific survival has been
reported (23,24). The results of the EORTC22911 trial
reported improved clinical progression in the postoperative
adjuvant radiotherapy group over a follow-up period of >5 years
after RP (25). Furthermore, to
identify the factors related to the effects of postoperative
adjuvant radiotherapy, GS, seminal vesicular infiltration, pT
stage, EPE and PSM were re-examined using isolated RP specimens of
522 individuals (26). They
reported that only PSM was a prognostic factor. With this
background, the present study examined risk factors for the purpose
of extracting the PSM cases, particularly those that were highly
likely to have BCR.
GS is generally used as an indicator of the grade of
histopathological malignancy. GS in isolated specimens, in
particular, exhibits a more accurate malignancy grade (7). Resnick et al (27) reported that GS in the excised
specimen is a risk factor for BCR, even in PSM cases. In recent
years, certain studies have reported that the GS of the tumor at
the PSM is also a predictor for BCR (28,29).
Udo et al (30) reported
that the incidence of BCR increases when the PSM site contains
Gleason grade 4 or higher tissues. In particular, cases with
Gleason grade 4 tumors at the PSM have more BCRs than those with
only grade 3 tumors (31).
In ~40% of the present cases, the GS of the tumor at
the margin was different from that of the main tumor (lower in
13.1% and higher in 26.2% of cases). The multivariate analysis
indicated that the GS of the main tumor was not a prognostic factor
for BCR, whereas that of the tumor at the PSM was an independent
prognostic predictor for BCR. In addition, the GS at the site of a
positive margin was significantly associated with the PSA value and
pT stage. The higher the GS at the positive margin, the earlier the
BCR was observed. Evaluation of the GS of the tumor at the margin
may be difficult; however, as no significant difference was
observed in BCR-free survival between patients whose tumor at the
margin had GS 3+4=7 and those whose tumor at the margin had GS
4+3=7, the GS of the tumor at the positive margin was simply
classified into stages 6, 7 and 8 or more.
In addition, a positive margin site is more common
in the apex of the tumor (32); in
the present study, of all patients with a PSM, ~60% had a positive
margin at the apex, which is almost equivalent to the proportion
reported in a previous study (32). In the present study of positive
sites, BCR occurred significantly earlier in patients with positive
margins located laterally than in those with positive margins in
the apex and bladder neck sites. PSM located laterally was an
independent prognostic predictor of BCR in PSM cases alongside GS
at the PSM. This finding demonstrated that documenting the GS of
the tumor at the positive margin and at the PSM in the pathology
report may accurately identify the presence of BCR.
According to the margin length, Marks et al
(33) reported no significant
difference between the extent of PSM and BCR. By contrast, Cao
et al (34) indicated that
the linear length of a PSM was an independent prognostic factor for
BCR in stage pT2 cancers. Certain studies have reported a standard
linear PSM length of 1 mm, while others have reported a standard
length of 3 mm; the impact of the PSM length is still under debate.
From the viewpoint of pathologists, measuring the length of
multiple positive sites is time-consuming and labor-intensive. Even
in the present study, the length of the positive margin site was
not a predictor for BCR.
Several limitations of the present study should be
acknowledged. First, in this study, PSM was present in 122/241
patients and this proportion is high. The reasons for this may be
that about one-third of these cases were at high-risk according to
the D'Amico classification and that most of the surgical procedures
were open surgeries. Further studies on other surgical procedures
(endoscopic/robot-assisted surgery) are required. Furthermore, the
length of the tumor was evaluated; however, the width of the tumor
was not considered when evaluating the GS of the tumor at the
margin in the present study. At our hospital, the width is unified
at 3.5 mm according to Japanese guidelines (35). As the width of all specimens was
almost the same, the width was not considered in the present
study.
In conclusion, the present study suggested that the
GS of the tumor at the PSM in RP is a more significant prognostic
factor for BCR than the GS of the main tumor. For a PSM in RP, the
GS of the tumor at the margin must be documented, in addition to
the anatomic location.
Acknowledgements
The authors would like to thank Ms. Sachiko
Ogasawara (Department of Pathology, Kurume University School of
Medicine, Kurume, Japan) for providing technical assistance.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysis during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK, KU, MM, SS and TI designed the study. HK, NO,
KC, MN and KN performed the research. HK, JA and HY contributed to
the pathological analysis. HK analyzed the data and wrote the
manuscript. MM, SS, and TI agree to be accountable for all aspects
of the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated.
HK and KU checked and approved the authenticity of the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
committee at Kurume University (Kurume, Japan). The Ethics
Committee waived the requirement for obtaining written informed
consent for the cases as the data of these patients were
retrospectively analyzed.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative
intent-update 2013. Eur Urol. 65:124–137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nichol AM, Warde P and Bristow RG: Optimal
treatment of intermediate-risk prostate carcinoma with
radiotherapy: Clinical and translational issues. Cancer.
104:891–905. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lowrance WT, Eastham JA, Savage C,
Maschino AC, Laudone VP, Dechet CB, Stephenson RA, Scardino PT and
Sandhu JS: Contemporary open and robotic radical prostatectomy
practice patterns among urologists in the United States. J Urol.
187:2087–2092. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hull GW, Rabbani F, Abbas F, Wheeler TM,
Kattan MW and Scardino PT: Cancer control with radical
prostatectomy alone in 1,000 consecutive patients. J Urol.
167:528–534. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stephenson AJ, Kattan MW, Eastham JA,
Dotan ZA, Bianco FJ Jr, Lilja H and Scardino PT: Defining
biochemical recurrence of prostate cancer after radical
prostatectomy: A proposal for a standardized definition. J Clin
Oncol. 24:3973–3978. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
D'Amico AV, Chen MH, Roehl KA and Catalona
WJ: Preoperative PSA velocity and the risk of death from prostate
cancer after radical prostatectomy. N Engl J Med. 351:125–135.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
D'Amico AV, Whittington R, Malkowicz SB,
Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA,
Kaplan I, Beard CJ and Wein A: Biochemical outcome after radical
prostatectomy, external beam radiation therapy, or interstitial
radiation therapy for clinically localized prostate cancer. JAMA.
280:969–974. 1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Horiguchi A, Nakashima J, Horiguchi Y,
Nakagawa K, Oya M, Ohigashi T, Marumo K and Murai M: Prediction of
extraprostatic cancer by prostate specific antigen density,
endorectal MRI, and biopsy Gleason score in clinically localized
prostate cancer. Prostate. 56:23–29. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ohori M, Kattan MW, Koh H, Maru N, Slawin
KM, Shariat S, Muramoto M, Reuter VE, Wheeler TM and Scardino PT:
Predicting the presence and side of extracapsular extension: A
nomogram for staging prostate cancer. J Urol. 171:1844–1849.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stephenson AJ, Shariat SF, Zelefsky MJ,
Kattan MW, Butler EB, Teh BS, Klein EA, Kupelian PA, Roehrborn CG,
Pistenmaa DA, et al: Salvage radiotherapy for recurrent prostate
cancer after radical prostatectomy. JAMA. 291:1325–1332.
2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yossepowitch O, Briganti A, Eastham JA,
Epstein J, Graefen M, Montironi R and Touijer K: Positive surgical
margins after radical prostatectomy: A systematic review and
contemporary update. Eur Urol. 65:303–313. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Negishi T, Kuroiwa K, Hori Y, Tomoda T,
Uchino H, Tokuda N, Furubayashi N, Nagase K, Iwai H and Nakamura M:
Predictive factors of late biochemical recurrence after radical
prostatectomy. Jpn J Clin Oncol. 47:233–238. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Blute ML, Bostwick DG, Bergstralh EJ,
Slezak JM, Martin SK, Amling CL and Zincke H: Anatomic
site-specific positive margins in organ-confined prostate cancer
and its impact on outcome after radical prostatectomy. Urology.
50:733–739. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Simon RM, Howard LE, Freedland SJ, Aronson
WJ, Terris MK, Kane CJ, Amling CL, Cooperberg MR and Vidal AC:
Adverse pathology and undetectable ultrasensitive prostate-specific
antigen after radical prostatectomy: Is adjuvant radiation
warranted? BJU Int. 117:897–903. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pettenati C, Neuzillet Y, Radulescu C,
Hervé JM, Molinié V and Lebret T: Positive surgical margins after
radical prostatectomy: What should we care about? World J Urol.
33:1973–1978. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Simon MA, Kim S and Soloway MS: Prostate
specific antigen recurrence rates are low after radical retropubic
prostatectomy and positive margins. J Urol. 175:140–145.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cao D, Kibel AS, Gao F, Tao Y and Humphrey
PA: The Gleason score of tumor at the margin in radical
prostatectomy is predictive of biochemical recurrence. Am J Surg
Pathol. 34:994–1001. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Humphrey PA: WHO clasification of tumors
of the prostate, pp138-162, 2016.
|
|
19
|
Stephenson AJ, Wood DP, Kattan MW, Klein
EA, Scardino PT, Eastham JA and Carver BS: Location, extent and
number of positive surgical margins do not improve accuracy of
predicting prostate cancer recurrence after radical prostatectomy.
J Urol. 182:1357–1363. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wright JL, Dalkin BL, True LD, Ellis WJ,
Stanford JL, Lange PH and Lin DW: Positive surgical margins at
radical prostatectomy predict prostate cancer specific mortality. J
Urol. 183:2213–2218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Preston MA, Carrière M, Raju G, Morash C,
Doucette S, Gerridzen RG, Bella AJ, Eastham JA, Scardino PT and
Cagiannos I: The prognostic significance of capsular incision into
tumor during radical prostatectomy. Eur Urol. 59:613–618.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pound CR, Partin AW, Eisenberger MA, Chan
DW, Pearson JD and Walsh PC: Natural history of progression after
PSA elevation following radical prostatectomy. JAMA. 281:1591–1597.
1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Trock BJ, Han M, Freedland SJ, Humphreys
EB, DeWeese TL, Partin AW and Walsh PC: Prostate cancer-specific
survival following salvage radiotherapy vs observation in men with
biochemical recurrence after radical prostatectomy. JAMA.
299:2760–2769. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wiegel T, Bottke D, Steiner U, Siegmann A,
Golz R, Störkel S, Willich N, Semjonow A, Souchon R, Stöckle M, et
al: Phase III postoperative adjuvant radiotherapy after radical
prostatectomy compared with radical prostatectomy alone in pT3
prostate cancer with postoperative undetectable prostate-specific
antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 27:2924–2930.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bolla M, van Poppel H, Collette L, van
Cangh P, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset
JF, van Velthoven R, et al: Postoperative radiotherapy after
radical prostatectomy: A randomised controlled trial (EORTC trial
22911). Lancet. 366:572–578. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Van der Kwast TH, Bolla M, Van Poppel H,
Van Cangh P, Vekemans K, Da Pozzo L, Bosset JF, Kurth KH, Schröder
FH and Collette L: EORTC 22911. Identification of patients with
prostate cancer who benefit from immediate postoperative
radiotherapy: EORTC 22911. J Clin Oncol. 25:4178–4186.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Resnick MJ, Canter DJ, Guzzo TJ,
Magerfleisch L, Tomaszewski JE, Brucker BM, Bergey MR, Sonnad SS,
Wein AJ and Malkowicz SB: Defining pathological variables to
predict biochemical failure in patients with positive surgical
margins at radical prostatectomy: Implications for adjuvant
radiotherapy. BJU Int. 105:1377–1380. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chapin BF, Nguyen JN, Achim MF, Navai N,
Williams SB, Prokhorova IN, Wang X, Tapia EM, Davis JW and Troncoso
P: Positive margin length and highest Gleason grade of tumor at the
margin predict for biochemical recurrence after radical
prostatectomy in patients with organ-confined prostate cancer.
Prostate Cancer Prostatic Dis. 21:221–227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Preisser F, Coxilha G, Heinze A, Oh S,
Chun FK, Sauter G, Pompe RS, Huland H, Graefen M and Tilki D:
Impact of positive surgical margin length and Gleason grade at the
margin on biochemical recurrence in patients with organ-confined
prostate cancer. Prostate. 79:1832–1836. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Udo K, Cronin AM, Carlino LJ, Savage CJ,
Maschino AC, Al-Ahmadie HA, Gopalan A, Tickoo SK, Scardino PT,
Eastham JA, et al: Prognostic impact of subclassification of
radical prostatectomy positive margins by linear extent and Gleason
grade. J Urol. 189:1302–1307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Viers BR, Sukov WR, Gettman MT, Rangel LJ,
Bergstralh EJ, Frank I, Tollefson MK, Thompson RH, Boorjian SA and
Karnes RJ: Primary Gleason grade 4 at the positive margin is
associated with metastasis and death among patients with Gleason 7
prostate cancer undergoing radical prostatectomy. Eur Urol.
66:1116–1124. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Walz J, Joniau S, Chun FK, Isbarn H,
Jeldres C, Yossepowitch O, Chao-Yu H, Klein EA, Scardino PT,
Reuther A, et al: Pathological results and rates of treatment
failure in high-risk prostate cancer patients after radical
prostatectomy. BJU Int. 107:765–770. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Marks RA, Koch MO, Lopez-Beltran A,
Montironi R, Juliar BE and Cheng L: The relationship between the
extent of surgical margin positivity and prostate specific antigen
recurrence in radical prostatectomy specimens. Hum Pathol.
38:1207–1211. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cao D, Humphrey PA, Gao F, Tao Y and Kibel
AS: Ability of linear length of positive margin in radical
prostatectomy specimens to predict biochemical recurrence. Urology.
77:1409–1414. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kawai H: Grneral rule for clinical and
pathological studies on prostate cancer: 70-71, 2010.
|