Introduction
Urothelial carcinoma (UC) is the fourth most common
malignant tumor. Upper urinary tract UC is rare and accounts for
only 5.0-10% of all diagnosed UCs in Europe (1). The lifetime risk of developing UC in
developed countries is 3.9 and 1.2% in men and women, respectively
(2). Additionally, the majority of
patients diagnosed with UC are >70 years of age (1).
Approximately 4% of patients with UC display
metastasis at diagnosis and ~50% of patients with UC undergoing
surgery develop tumor recurrence during subsequent follow-up
examinations, with 70-90% of relapses represented by distant
failure (3). In patients with
distant failure, there are few therapeutic options available and
systemic therapies are currently the first-line treatment option
(3). However, some retrospective
studies have suggested the possibility of an improved outcome
following metastasectomy in selected patients with oligometastatic
disease (4-7).
Surgical resection of the metastatic site is not always feasible,
either due to technical issues or the presence of any concomitant
comorbidities (3).
A non-surgical approach based on stereotactic body
radiotherapy (SBRT) may be proposed in patients when surgical
resection is not feasible, given the efficacy and tolerability of
this irradiation technique. In particular, lymph node metastases
(LNMs) represent an ideal target for SBRT. The small volume,
minimum organ motion, regular shape and well-defined contours make
LNMs particularly suitable for treatment using SBRT (8). However, data availability on the
effects of SBRT in patients with oligometastatic UC is limited.
The current study reports a case of a patient with
recurrent UC who was successfully treated with repeated courses of
SBRT on multiple and subsequent metachronous LNMs. The current case
report follows the CARE (CAse REports) guidelines (9).
Case report
The current study reports a case of a 58-year-old
male patient with UC who was referred to our institution. A
physical examination and blood chemistry results demonstrated that
the patient exhibited no significant abnormalities. Additionally,
the patient did not report any neoplastic family history or
relevant comorbidities. The patient underwent ureteroileostomy in
September 2016 and pathological examination indicated a pT4N2Mx (7
out of 11 resected regional lymph nodes were metastatic) high-grade
(G3) UC based on the TNM (Union for International Cancer Control;
8th edition) (10) and WHO/ISUP
2004-2016 grading systems (11),
respectively. Based on the histological examination performed on
the patient, adjuvant therapy was undertaken (atezolizumab; 1,200
mg every 21 days; 16 cycles).
No local or distant relapse was observed in the
patient at subsequent follow-up checks until August 2018. A
whole-body 18F-labeled fluoro-2-deoxyglucose (FDG)
positron emission tomography (PET)/CT scan indicated six LNMs in
the para-aortic (2 LNMs), right common iliac (2 LNMs) and presacral
(2 LNMs) regions. All detected LNMs were treated with SBRT and the
prescribed dose was 35-40 Gy in five daily fractions, depending on
the dose delivered to the organs at risk. A subsequent
18F-FDG-PET/CT scan, which was performed in
December 2018, indicated a complete radiological and metabolic
response of all treated sites.
In November 2019, a subsequent
18F-FDG-PET/CT scan revealed a LNM in the
pre-aortic region, which was treated with SBRT (40 Gy in five
fractions). In January 2020, the
18F-FDG-PET/CT scan indicated a complete
response of this lesion. In April 2020, a
18F-FDG-PET/CT indicated a new LNM in the
left obturatory lymph node region. Additionally, this LNM was
treated with SBRT (40 Gy in five fractions) and the subsequent
18F-FDG-PET/CT (performed in June 2020)
indicated a complete response of this LNM. Furthermore, the latter
18F-FDG-PET/CT also indicated further LNMs in
the interaortocaval lymph nodes and on two left internal iliac
lymph nodes, all of which were treated with SBRT (35 and 40 Gy in
five fractions, respectively). A
18F-FDG-PET/CT was performed in October 2020
and indicated a complete radiological and metabolic response in the
aforementioned LNMs. The last follow-up performed in December 2020
confirmed a complete response with no evidence of any relapse in
the patient.
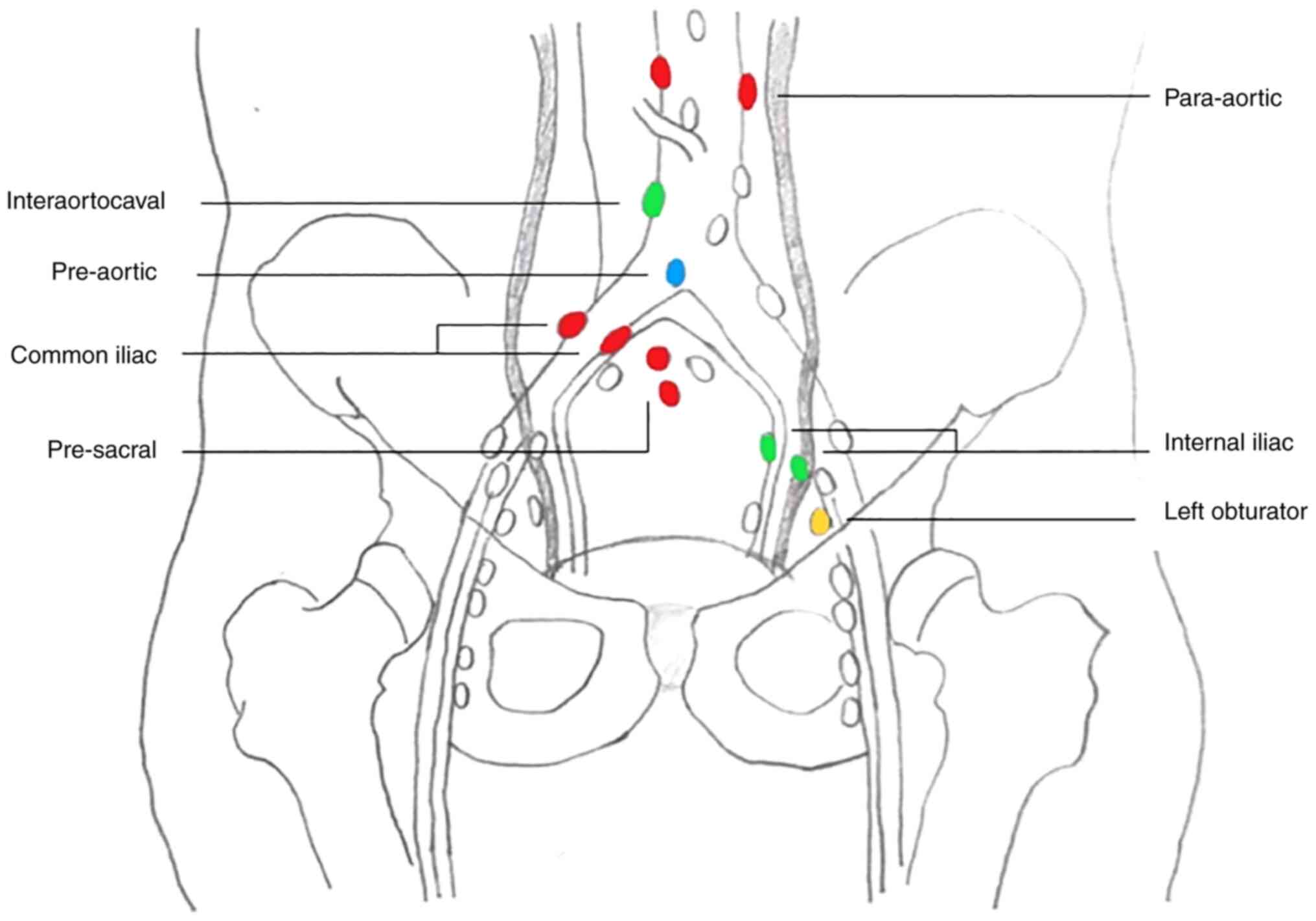
In the case discussed in the current study, a total
of 11 LNMs were irradiated over a period of 22 months, which led to
a complete radiological and metabolic response of all lesions
without systemic treatment. The anatomical site of the treated LNMs
is presented in Fig. 1. In April
2020, during the irradiation of an obturator LNM, the patient
reported mild anorexia and dyspnea, which resolved without the need
for supportive therapies. Additionally, no severe toxicity was
reported. Written informed consent was obtained from the patient
for the publication of the current case report.
The timeline of events in this case were as follows:
i) Ureteroileostomy (Bricker) of high-grade (G3; WHO 2004-2016)
urothelial carcinoma, pT4N2M0, was performed on 2016-09-01; ii)
adjuvant therapy (atezolizumab; 16 cycles) was completed on
2017-09-27; iii) on 2018-07-29, a chest-abdomen-pelvis
contrast-enhanced CT was performed and indicated enlarged presacral
lymph nodes; iv) on 2018-08-14, a multidisciplinary evaluation was
performed and SBRT was planned on the oligometastatic sites; v) on
2018-08-24, 18F-FDG-PET/CT indicated pathological
enhancement in six lymph nodes [two in the paraortic region (SUV
8.4), two in the right common iliac region (SUV 6.8) and two in the
presacral region (SUV 8.4 and 2.8)]; vi) on 2018-09-11, SBRT was
performed on para-aortic nodes (40 Gy/5 fractions), right common
iliac nodes (35 Gy/5 fractions) and presacral nodes (40 Gy/5
fractions); vii) on 2018-12-03, a
18F-FDG-PET/CT indicated a complete
radiological and metabolic response of all treated sites; viii) on
2019-11-08, a chest-abdomen-pelvis contrast enhanced CT indicated
nodal oligorecurrence at preaortic/iliac bifurcation; ix) on
2019-11-21, a 18F-FDG-PET/CT indicated
recurrence in a preaortic/iliac bifurcation lymph node and three
lymph nodes in the left obturator region and left internal iliac
region; x) on 2019-11-28, SBRT was performed on the latter sites at
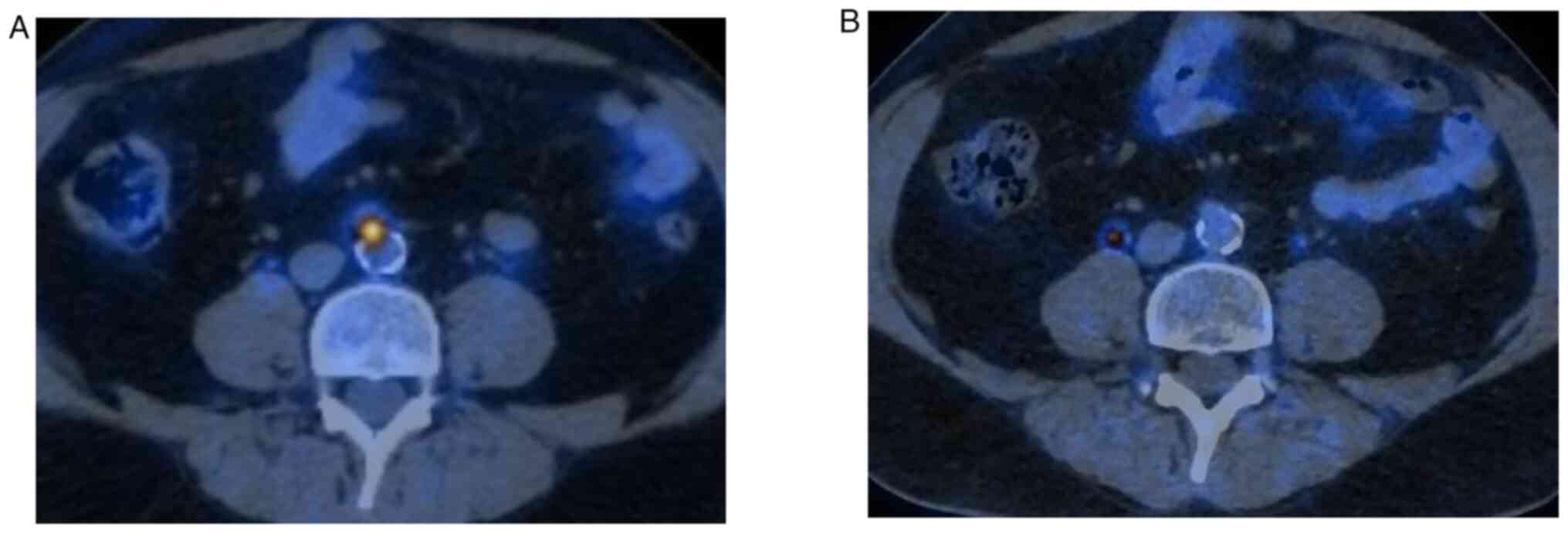
40 Gy/5 fractions; xi) on 2020-01-23, a
18F-FDG-PET/CT indicated a complete
radiological response of the treated sites (Fig. 2); xii) on 2020-04-17, a
18F-FDG-PET/CT indicated left obturator nodal
oligorecurrence; xiii) on 2020-05-06, SBRT was performed on the
latter site at 40 Gy/5 fractions; xiv) on 2020-06-30, a
18F-FDG-PET/CT indicated one interaortocaval
and two left internal iliac nodal oligometastases; xv) on
2020-07-13, SBRT was performed on the interaortocaval lymph node
(35 Gy/5 fractions) and left internal iliac lymph nodes (40 Gy/5
fractions); xvi) on 2020-10-05, a
18F-FDG-PET/CT indicated a complete
radiological and metabolic response of the treated lesions; and
xvii) on 2020-12-15 and at the last follow-up, all treated lesions
exhibited complete metabolic and radiological response with no
evidence of relapse.
Discussion
The current study reported a 58-year old male
patient exhibiting multiple and recurrent LNMs from UC and
undergoing repeated SBRT, with tumor control lasting up to 2 years
without the use of invasive or systemic therapies. The
aforementioned patient case is original for a number of different
reasons. Firstly, isolated LNMs are rare in UCs (12). In the patient discussed in the
present study, the tumor only recurred in the nodes for an extended
period of time. Furthermore, the patient underwent SBRT despite not
being fully classified as oligometastatic, as only patients with
1-5 metastases are traditionally defined as oligometastatic
(13). However, considering the
excellent general condition of this patient, their willingness to
postpone systemic therapy and the fact that the tumor was spreading
only to the lymph nodes, which is a condition known for its
favorable prognosis, the multidisciplinary team selected SBRT
treatment for this case (12). It
may be observed that all subsequent disease relapses in this
patient may be defined as oligorecurrences, as the number of new
LNMs was <5. The data collected in the current study suggest
that there can be some flexibility in the selection of patients
referred for SBRT. Furthermore, previous data have indicated that
SBRT is less effective in UCs compared with other cancer types and
in terms of local control (14,15).
However, all 11 lesions treated in the current case had a complete
response following SBRT, confirming the effectiveness of this
technique in the treatment of LNMs (8).
The current case report highlights a relevant issue
in the treatment of lymph node oligometastases. A well-known
problem following therapies that focus only on macroscopic
metastatic sites is the risk of further regional LNMs (8). The risk of this is estimated to be
21.4% (range, 10.5-58.0%) following SBRT, which is a figure that is
based on a small amount of data (16). A number of different strategies
have been proposed to address this problem. Firstly, SBRT may be
used as a boost on macroscopic LNMs following regional prophylactic
irradiation. For example, Seo et al (17) reported significantly higher disease
control on regional nodes in patients treated with external beam
radiotherapy on the paraaortic region followed by SBRT boost
compared with patients treated with SBRT alone (5-year regional
control: 92% vs. 34%; P=0.006). The first tumor recurrence in the
patient discussed in the present case study involved pelvic and
abdominal lymph nodes. Therefore, it may be suggested that a
prophylactic treatment of the pelvic lymph nodes that was extended
to the para-aortic region and associated with a SBRT-boost on LNMs
may have led to avoidance of subsequent relapses, which all
occurred in the same areas. Another strategy to reduce the risk of
further regional LNMs is less extensive prophylactic irradiation,
with SBRT at ‘adjuvant’ doses delivered to the macroscopic disease
and to the contiguous part of the lymphatic pathway, combined with
a high dose-simultaneous integrated boost to the macroscopic LNM
only (18). However, the results
of this irradiation strategy are not fully elucidated in terms of
improved regional control. Furthermore, it should be noted that the
aforementioned technique would not have been useful in the patient
outlined in the current study as all new relapses occurred at some
distance from treated sites (Fig.
1).
In the case of the patient discussed in the current
study, SBRT was used in order to postpone the start of systemic
therapy. However, it should be noted that this technique was used
for a variety of therapeutic purposes, including the local
consolidation of metastatic lesions treated with systemic therapy
and the local control of oligoprogressive lesions during the course
of systemic therapy, in order to postpone the initiation of
subsequent treatment lines (17,19).
The choice between these treatments may depend on a number of
varied factors, such as the presence of primary cancer,
availability of effective systemic therapies and patient
preferences. In the case outlined in the current study, the
patient's initial willingness to avoid chemotherapy was considered
a priority.
In conclusion, the case reported in the present
study suggests the possibility of achieving a complete response in
subsequent metachronous LNMs from UC treated with SBRT. Further
studies on this subject should aim to investigate the following: i)
Multicenter databases to evaluate the SBRT impact on both local
control and progression-free survival in a large dataset; ii) the
comparison of strategies based on SBRT combined with systemic
treatments with strategies based on SBRT alone followed by systemic
treatments only in case of widespread metastases; iii) the most
effective combination of systemic therapies and SBRT in terms of
timing, types of drugs and SBRT dose/fractionation regimens. Given
the theoretical advantages of a SBRT-immunotherapy combination
treatment, it may be noted that a recent study has indicated the
tolerability of the SBRT-pembrolizumab combination in patients with
UC (20).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors conceptualized the study. FM, GS and SB
wrote the manuscript and prepared the original draft. MB, SC, PC
and AGM reviewed and edited the manuscript. LS, SF, FM and AGM
supervised the study. FM and AGM confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient has been correctly informed and provided
written informed consent for the publication of any associated data
and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 update. Eur
Urol. 68:868–879. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Noone AM, Howlader N, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR (eds), et
al: SEER Cancer Statistics Review 1975-2015. National Cancer
Institute, Bethesda, MD, 2018.
|
|
3
|
Spiess PE, Agarwal N, Bangs R, Boorjian
SA, Buyyounouski MK, Clark PE, Downs TM, Efstathiou JA, Flaig TW,
Friedlander T, et al: Bladder Cancer, Version 5.2017, NCCN Clinical
Practice Guidelines in Oncology. J Natl Compr Canc Netw.
15:1240–1267. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siefker-Radtke AO, Walsh GL, Pisters LL,
Shen Y, Swanson DA, Logothetis CJ and Millikan RE: Is there a role
for surgery in the management of metastatic urothelial cancer? The
M. D. Anderson experience. J Urol. 171:145–148. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lehmann J, Suttmann H, Albers P, Volkmer
B, Gschwend JE, Fechner G, Spahn M, Heidenreich A, Odenthal A, Seif
C, et al: Surgery for metastatic urothelial carcinoma with curative
intent: The German experience (AUO AB 30/05). Eur Urol.
55:1293–1299. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dodd PM, McCaffrey JA, Herr H, Mazumdar M,
Bacik J, Higgins G, Boyle MG, Scher HI and Bajorin DF: Outcome of
postchemotherapy surgery after treatment with methotrexate,
vinblastine, doxorubicin, and cisplatin in patients with
unresectable or metastatic transitional cell carcinoma. J Clin
Oncol. 17:2546–2552. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abe T, Shinohara N, Harabayashi T, Sazawa
A, Maruyama S, Suzuki S and Nonomura K: Impact of multimodal
treatment on survival in patients with metastatic urothelial
cancer. Eur Urol. 52:1106–1113. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Deodato F, Ferro M, Cilla S, Ianiro A,
Buwenge M, Re A, Sallustio G, Valentini V, Morganti AG and Macchia
G: Stereobody radiotherapy for nodal recurrences in oligometastatic
patients: A pooled analysis from two phase I clinical trials. Clin
Exp Metastasis. 37:519–529. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Riley DS, Barber MS, Kienle GS, Aronson
JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG,
Sox H, et al: CARE guidelines for case reports: Explanation and
elaboration document. J Clin Epidemiol. 89:218–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM Classification of Malignant Tumours. 8th
edition. John Wiley & Sons, Inc. Hoboken, NJ, pp1-272,
2017.
|
|
11
|
Compérat EM, Burger M, Gontero P, Mostafid
AH, Palou J, Rouprêt M, van Rhijn BWG, Shariat SF, Sylvester RJ,
Zigeuner R and Babjuk M: Grading of urothelial carcinoma and the
new ‘World health organisation classification of tumours of the
urinary system and male genital organs 2016’. Eur Urol Focus.
5:457–466. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG:
Upper Tract Urothelial Carcinoma CollaborationThe Upper Tract
Urothelial Carcinoma Collaboration. Outcomes of radical
nephroureterectomy: A series from the Upper Tract Urothelial
Carcinoma Collaboration. Cancer. 115:1224–1233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hellman S and Weichselbaum RR:
Oligometastases. J Clin Oncol. 13:8–10. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Loi M, Frelinghuysen M, Klass ND, Oomen-De
Hoop E, Granton PV, Aerts J, Verhoef C and Nuyttens J: Locoregional
control and survival after lymph node SBRT in oligometastatic
disease. Clin Exp Metastasis. 35:625–633. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Francolini G, Desideri I, Detti B, Di
Cataldo V, Masi L, Caramia G, Visani L, Terziani F, Muntoni C, Lo
Russo M, et al: Stereotactic radiotherapy in oligoprogressive and
oligorecurrent urothelial cancer patients: A retrospective
experience. Cancer Treat Res Commun. 19(100124)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Deodato F, Macchia G, Buwenge M, Bonetti
M, Cilla S, Zamagni A, Re A, Pezzulla D, Cellini F, Strigari L, et
al: Systematic review of stereotactic body radiotherapy for nodal
metastases. Clin Exp Metastasis. 38:11–29. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Seo YS, Kim MS, Cho CK, Yoo HJ, Jang WI,
Kim KB, Lee DH, Moon SM and Lee HR: Stereotactic body radiotherapy
for oligometastases confined to the para-aortic region: Clinical
outcomes and the significance of radiotherapy field and dose.
Cancer Invest. 33:180–187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kneebone A, Hruby G, Ainsworth H, Byrne K,
Brown C, Guo L, Guminski A and Eade T: Stereotactic body
radiotherapy for oligometastatic prostate cancer detected via
prostate-specific membrane antigen positron emission tomography.
Eur Urol Oncol. 1:531–537. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Detti B, D'Angelillo RM, Ingrosso G,
Olmetto E, Francolini G, Triggiani L, Bruni A, Borghesi S, Fondelli
S, Carfagno T, et al: Combining abiraterone and radiotherapy in
prostate cancer patients who progressed during abiraterone therapy.
Anticancer Res. 37:3717–3722. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sundahl N, Vandekerkhove G, Decaestecker
K, Meireson A, De Visschere P, Fonteyne V, De Maeseneer D, Reynders
D, Goetghebeur E, Van Dorpe J, et al: Randomized phase 1 trial of
pembrolizumab with sequential versus concomitant stereotactic body
radiotherapy in metastatic urothelial carcinoma. Eur Urol.
75:707–711. 2019.PubMed/NCBI View Article : Google Scholar
|