Introduction
In both developed and developing countries, cancer
is a major health issue and a leading cause of mortality that is
still on the increase, worldwide. According to the International
Agency for Research on Cancer, in 2018, 9.6 million individuals
died from cancer, an increase from 8.2 million in 2012 and 7.6
million in 2008 (1-3).
Tumorigenesis is a multi-step, multi-factorial disease described by
genetic and epigenetic changes, which is difficult to control and
prevent (4). In 2012, the WHO's
International Agency for Research on Cancer predicted that by 2030,
worldwide, there would be 21.7 million newly diagnosed cancer cases
and 13 million cancer deaths as a result of population growth and
the increase in life expectancy (5).
Cancer is a major health problem that affects
individuals globally. Several types of cancer can be avoided if
diagnosed early enough. However, tumors such as lung, colon, and
breast cancers frequently have late-stage diagnosis. Despite
efforts to ensure survival is prolonged, only a moderate
improvement has been achieved in cancer patients. Failure to
diagnose cancer early generally leads to ineffective treatment and
an even worse prognosis. The availability of robust diagnostic
biomarkers is critical for diagnosing cancer patients at an early
stage and thereby greatly reducing overall mortality rates.
(6).
Circulating molecular biomarkers have increasingly
been used as a liquid biopsy in the peripheral blood and have the
benefit of being easily accessible, with early detection, and
reproducibility (7). Circulating
tumor cells, circulating DNA, and microRNAs have been studied as a
detection tool and prognosis of various cancer types (8-13).
DNA is a molecule that may be found inside and
outside of cells. Extracellular DNA can be found in blood and other
body fluids. Cell-free DNA refers to the degraded DNA fragments
floating in the circulation (cfDNA). DNA in the bloodstream
releases apoptotic or necrotic cells. The length of DNA fragments
and distribution of DNA size could signify cfDNA source (14). Apoptosis of the cell naturally
occurs, and DNA is divided into similar fragments of 185-200 bp.
However, tumor necrosis produces similar fragments of DNA in
variable lengths generally >200 bp (15). Circulating tumor DNAs (ctDNAs)
based DNA integrity index served as a possible indicator of
prognosis in hepatocellular carcinoma, lymphoma, colorectal, lung,
and breast cancer (15-19).
DNA analysis can be conducted on the basis of ctDNA (from a liquid
biopsy) as well as directly isolated DNA from tumor tissue acquired
by biopsy or excision (20).
According to Iqbal et al (21) presence of cfDNA in blood, although
reported in 1948 by Mandel and Metais (22), was rediscovered after 30 years in
autoimmune disorders by Tan et al in 1966(23) and in cancer by Leon et al in
1977(24).
Apoptosis is the source of cfDNA in a healthy
person, raising shorter and evenly sized DNA fragments.
Furthermore, in cancer, necrosis results in unequal longer DNA
fragments in addition to the shorter apoptotic fragment (25-27).
As a result, higher levels of longer DNA fragments in the blood
have been identified as a useful indicator of the existence of
malignant tumor DNA (26-28).
The cfDNA concentration in serum is higher in patients with cancer
when compared to healthy individuals (5,29-31).
The variability of cfDNA levels in patients is most
probably associated with tumor stage, burden, cellular turnover,
vascularity, and response to therapy with the highest levels
reported in patients with metastatic and advanced disease (32).
cfDNA levels have been found to be elevated in a
variety of cancers (7). One
measure of cfDNA fragmentation is cfDNA integrity (cfDI), which is
calculated as the ratio of longer to shorter DNA fragment
concentrations at the same genetic location (29).
A liquid biopsy is a viable alternative consisting
of the circulating analysis (cfDI). This main advantage of this
method is that it is less invasive, using just a sample of
peripheral blood. During the past two decades, cfDI analysis has
emerged as a promising tool for cancer diagnosis and prognosis
(33,34).
The Arthrobacter luteus (ALU) repeats are the
most predominant repetitive sequences in the human genome, 300 bp
in length, with 1.4x106 copy number per genome. Most
studies used DNA integrity, defined as the ratio of ALU 247 long
fragments released from necrotic cells and ALU 115 short fragments
released from normal cells (35).
ALU-quantitative PCR (qPCR) has become the most
widely used technology for detecting the DNA integrity index
(32). ALU covers over 10% of the
human genome (36). Research has
been conducted to assess the potential use of cfDI from the ALU
variable as a diagnostic biomarker for a variety of cancers, such
as breast and prostate cancer (37).
A higher portion of longer DNA fragments has been
recommended as a cancer detection biomarker (26). Several formulae have been presented
to objectively calculate the ‘DNA integrity index’ as a ratio of
longer and smaller fragments. Umetani et al (27,38)
determined the pure ratio of ALU 247 and ALU 115 concentrations in
patients' blood, while Wang et al (39) assessed DNA integrity in patient
plasma using a calculation based on delta-Cp values. Patients with
ovarian, breast and colorectal cancer had higher DNA integrities in
serum and plasma than controls, according to both authors (27,38).
Other studies, on the other hand, could not find a difference in
DNA integrity values in the same tumor types (40-42).
However, in different studies, the performance of
ALU repeat as a biomarker for cancer diagnosis varied widely.
Therefore, this systematic study, to the best of our knowledge, is
the first to clarify the diagnostic and prognostic role of ALU
elements as a molecular marker of cancer.
Materials and methods
Strategy of search and study
selection
A search for potentially suitable articles was
performed on the PubMed online databases up to September 2021, for
research articles. The following keyword combinations were included
in the detailed search strategy: (‘ALU’ OR ‘cfDNA’) AND (‘cancer’
OR ‘tumor’). Studies were considered for selection if they included
information on ALU sequences and their potential role in the
diagnosis or prognosis of different types of cancer. Studies not in
English or where only the abstract was available were excluded.
Initially, data extraction was carried out by two of the authors
(AS and SS). The full-text articles were then obtained for more
evaluation. The reference lists of all of the studies were manually
checked by the authors to identify additional publications that may
be of interest.
The studies that were determined to be eligible were
as follows: a plan for an observational, assessing the relationship
between ALU and the role in diagnosis or prognosis of cancer, there
was enough information to assess the difference in ALU levels
between the patients and the controls and between cancer stage.
Collection of data and quality
assessment
Authors extracted data independently from each
eligible study and abstracted the following information including
cancer type, sample type, DNA size ratio. The data were evaluated
for each group, and the main results identified.
Included studies
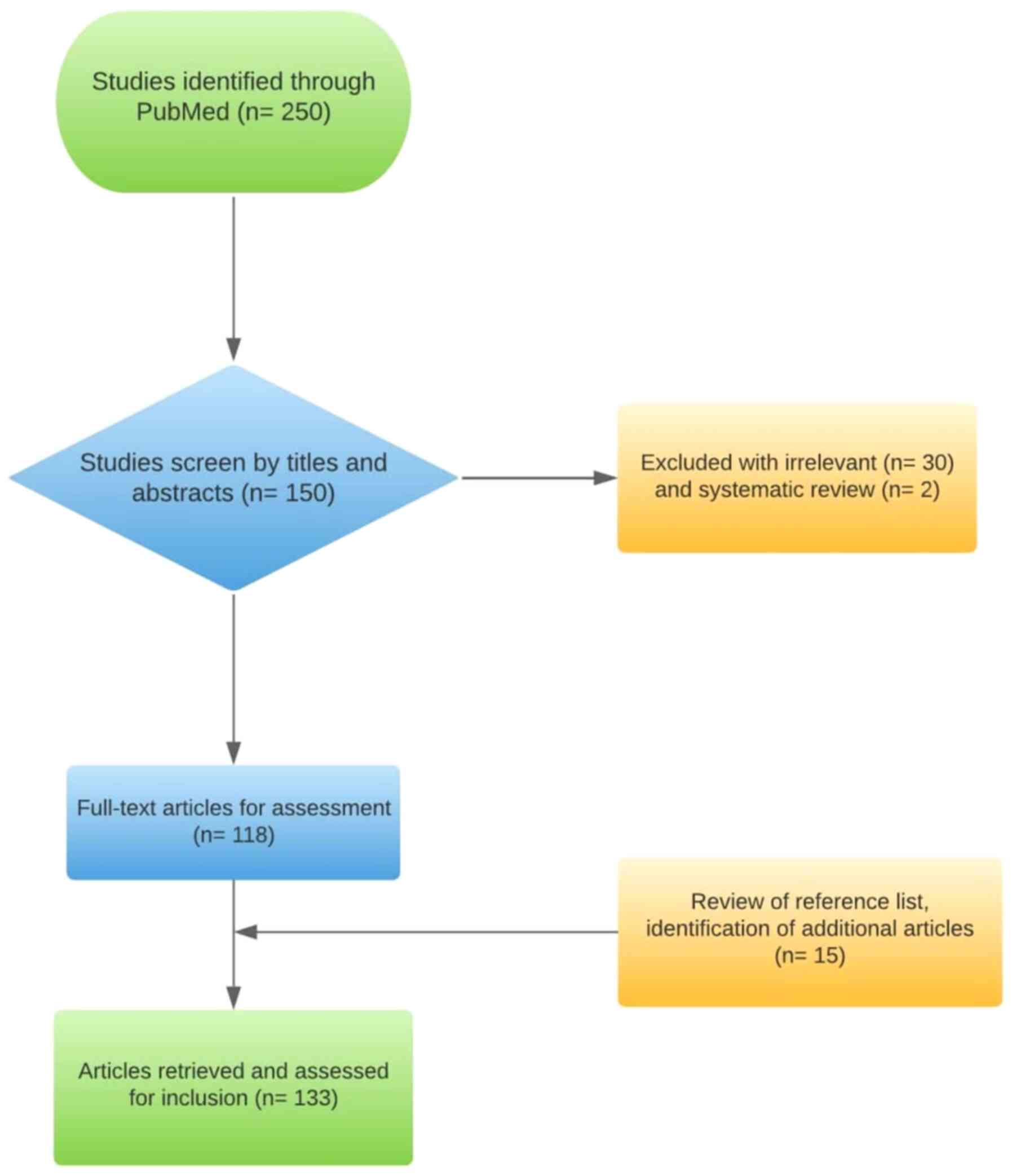
The PubMed search identified 250 articles. After the
initial inclusion of 150 articles (based on title and abstract were
selected for assessment), 32 articles were excluded due to not
meeting the criteria for inclusion (including ALU methylation,
comparison between ALU and LINE, and studies in other languages). A
manual search of the references of studies on the subject yielded
15 additional articles. A total of 133 articles were included for
the full text assessment, in English. The selection of the study
flowchart is presented in Fig.
1.
Results
Included studies
The PubMed search initially identified 250 articles.
Based on title and abstract 150 articles were selected for
assessment, of which 32 articles were excluded due to not meeting
the criteria for inclusion. Further manual search of the references
yielded 15 additional articles. A total of 133 articles were
included for the full text assessment (Fig. 1).
Overall characteristics
The characteristics of the studies that were
included are shown in Table I,
which shows the number of tumor cases (16-268) and healthy controls
(12-110). The subjects' age range was 18-71 years. There were 16
studies from European countries (44.4%), 12 from Asia (33.3%), 6
from Africa (16.7%), and 2 from the USA (5.6%). These studies
focused on carcinoma, including breast, lung, prostate, ovarian,
endometrial, pancreatic, and thyroid cancer. To determine the value
of cfDI, all of the included studies used the quantitative PCR
(qPCR) method, 14 of them were evaluated in serum and 19 in plasma,
1 (in serum and plasma), 1 in tissue, and 1 in urine. cfDI was
calculated as the ratio of longer DNA fragment concentrations to
shorter ones in the same locus. The reference list included 35
articles, published between 2006 and 2021.
 | Table IList of studies evaluating ALU in
cancer. |
Table I
List of studies evaluating ALU in
cancer.
| Cancer type | No. of
subjects | Source of DNA | Ratio of DNA
size | Conclusions | (Refs.) |
|---|
| Breast cancer | (51) Controls | Serum | ALU 247/115 | Serum circulating
cfDI is a potential molecular biomarker for detecting the
progression of BC and lymph node metastases | (27) |
| (83)
Preoperative |
| (Stage 0 to IV
primary BC) |
| Breast cancer | (49) Controls | Plasma | ALU 247/115 | According to the
results, necrosis could be a possible source of cfDNA ALU 247; and
a tumor biology phenotypic feature | (43) |
| (39) BC
patients |
| Breast cancer | (65) BC patients
undergoing neoadjuvant chemotherapy | Plasma | ALU 247/115 | The study indicates
circulating DNA biomarkers ALU (115 and 247) as two potential
future markers for the assessment of neoadjuvant chemotherapy
response in BC patients | (44) |
| No controls
reported |
| Breast cancer | (28) Controls | Plasma | ALU 247/115 | Plasma DNA is
helpful in the diagnosis of locally BC, however, in MBC,
established tumor markers are the most informative | (45) |
| (12) Benign BC
patients, |
| (65) Locally
confined BC patients |
| (47) Metastatic
breast cancer patients |
| Breast cancer | (100) Controls | Plasma | ALU 260/111 | Study shows that
cfDI was reduced and cfDNA level increased can be used as
diagnostic biomarkers for both primary and metastatic breast
cancer, and cfDI as an MBC prognostic marker, as a result, they're
good candidates for blood-based multi-marker tests | (10) |
| (82>) Primary BC
patients |
| (201) MBC
patients |
| Breast cancer | (51) Controls | Serum | ALU 247/115 | In patients with
primary BC, cfDNA level and cfDI were found to represent potential
prognostic markers | (21) |
| (148) BC
patients |
| (148) Baseline |
| (47)
Postoperative |
| Breast cancer | (175) Non-recurrent
BC patients | Plasma | ALU 260/111 | In the clinic, cfDI
could be a helpful biomarker for prognosis of BC recurrence when
combined with other molecular markers | (46) |
| (7) recurrent-BC
patients |
| No controls
reported |
| Breast cancer | (268) MBC
patients | Plasma | ALU 260/111 | At baseline and
during systematic therapy, cfDNA variables can serve as attractive
prognostic markers for MBC patients, especially when combined with
other markers | (18) |
| No controls
reported |
| Breast cancer | (10) Controls | Plasma | ALU 247/115 | Both ALU 247 and
ALU 115 appear to be prognostic markers for BC preoperative | (47) |
| (40) BC patients
(2: stage I, 31: stage II, 2: stage III, and 5: stage IV) |
| Breast and prostate
cancers | (64) Females | Serum | ALU 247/115 | cfDI increased with
disease severity and higher staging in the prostate but not in
BC | (5) |
| (Consisting of 32
controls 32 and BC patients) and (61) Males |
| (Consisting of 30
controls and 31 prostate cancer patients) |
| Breast and lung
cancers | (64) Controls | Plasma | ALU 263/58 | This study
suggested ALU index could be used as a test to discriminate cancer
patients from healthy individuals | (34) |
| (64) BC
patients |
| (64) Lung cancer
patients |
| Prostate
cancer | (96) PC
patients | Plasma | ALU 247/115 | cfDNA and cfDI
could be used to differentiate PC from BPH in patients with serum
PSA C 4 ng/ml | (48) |
| (112) Benign
prostate hyperplasia |
| Prostate
cancer | (30) Controls | Serum | ALU 247/115 | ALU 115 could be a
useful biomarker for identifying patients that are at high risk,
pointing to early tumor cell spread as a possible seed for future
metastases | (49) |
| (50) PC
patients |
| (25) BPH |
| Prostate
cancer | (30) Controls | Plasma | ALU 247/115 | A significant
relationship between cfDNA concentration, its integrity, and PC
suggests that the liquid biopsy can be usedas a non- invasive early
diagnostic biomarker | (50) |
| (30) PC
patients |
| (40) BPH |
| Ovarian cancer | (12) Controls | Plasma | ALU 219/115 | Monitoring ALU
concentrations, alone or in combination with other tumor markers,
could be used for subsidiary diagnosis and prognosis of ovarian
cancer | (51) |
| (24) Ovariancancer
patients |
| (12) Benign ovarian
cysts patients |
| Ovarian cancer | (28) Controls | Plasma | ALU 260/111 | In combination with
other molecular markers, cfDNA variables could be used as
diagnostic biomarkers in ovarian cancer | (29) |
| (37) Ovarian cancer
patients |
| Endometrial
cancer | (15) Controls | Plasma | - | Although cfDNA
measurement is not effective for EC screening, the change in cfDNA
in a patient could be a prognostic biomarker for EC | (52) |
| (53) EC
patients |
| (9) Benign
gynecologic disease patients |
| Endometrial
cancer | (60) Controls and
EC patients | Serum | ALU 247/115 | The study noted the
potential use of serum cfDI as a noninvasive molecular biomarker in
EC. And a correlation analysis between cfDNA quantitative and
qualitative content and clinicopathologic characteristics, such as
body mass index, blood pressure level, and lymphovascular space
invasion status | (32) |
| Endometrial
cancer | (32) EC
patients | Plasma | ALU 247/115 | Decreased plasma
cfDI during vaccination and the cfDI was related to prognosis.
Another cancer study has confirmed some of these findings, as a
result, the cfDI could be a potential biomarker for future cancer
vaccination therapies | (53) |
| No controls
reported |
| Pancreatic
malignancies | (23) Controls | Plasma | ALU 244/83 | The lack of
detectable cfDNA levels in pancreatic diseases has a significant
impact on the clinical usage of such a biomarker in pancreatic
ductal adenocarcinoma patients When evaluating the diagnostic value
of cfDNA in pancreas pathology, different methods of analysis
should be used | (54) |
| (50) Pancreatic
ductal adenocarcinoma patients |
| (23) Pancreatic
neuroendocrine tumor patients |
| (20) Chronic
pancreatitis patients |
| Pancreatic
cancer | (19) Controls | Serum | ALU 247/115 | cfDI is not a
useful biomarker to detect premalignant pancreatic tumors | (55) |
| (19) Pancreatic
cancer patients |
| Pancreatic
cancer | (32) Control
adjacent pancreatic tissue specimens | Tissue | ALU 247/115 | cfDI (ALU 247/115
ratio) was no significant difference between pancreatic cancer
patients and controls | (14) |
| (42) The tumors
samples |
| Colorectal cancer
and periampullary cancer | (51) Controls | Serum | ALU 247/115 | cfDI is a promising
serum biomarker for colorectal and periampullary cancer detection
and evaluation | (38) |
| (32) CRC
patients |
| (19) Periampullary
cancer patients |
| Colorectal
cancer | (35) Patients
without endoscopic abnormality | Serum and
plasma | ALU 247/115 | In patients with
positive fecal occult blood tests, the circulating marker, in
combination with other markers, offers the possibility of a simple
blood test as a secondary screen for CRC and polyps | (56) |
| (26) Benign
colorectal adenomas patients |
| (24) CRC
patients |
| Colorectal
cancer | (24) Controls | Serum | ALU 247/115 | cfDI is
significantly higher in CRC patients and could be useful in future
studies | (57) |
| (24) CRC
patients |
| (11) Benign
gastrointestinal diseases patients |
| Colorectal
cancer | (110) Controls | Serum | ALU 247/115 | Combined with ALU
115, the ratio of ALU 247/115 and carcinoembryonic antigen
detection could enhance CRC diagnostic efficiency. Serum cfDNA and
cfDI may be valuable in early diagnosis and monitoring of CRC
progression and prognosis | (30) |
| (104) Primary CRC
patients |
| (85) Operated
colorectal cancer patients |
| (16)
Recurrent/metastatic CRC patients |
| (63) Intestinal
polyps' patients |
| Colorectal
cancer | (20) Controls | Serum | ALU 247/115 | As a potential
serum biomarker, the cfDI outperforms the absolute DNA level for
CRC diagnosis. It could also be used as a marker for monitoring the
progression of CRC patients | (58) |
| (50)
CRCpatients |
| (10) Benign colonic
polyp's patients |
| Colorectal
cancer | (56) Controls | Serum | ALU 244/83 | Serum cfDNA
concentrations may be an effective source of non-invasive cancer
biomarkers | (59) |
| (114) CRC
patients |
| (22) Adenomatous
lesion patients |
| Colorectal
cancer | (30) Controls | Serum | ALU 247/115 | According to the
study, cfDI is better to carcinoembryonic antigen as an early
biomarker for detecting CRC and its potential to be employed as a
biomarker for malignancy | (35) |
| (90) CRC
patients |
| (30)
Benigncolorectal mass patients |
| Colorectal
cancer | (76) Primary CRC
patients who underwent surgery, including (60) with chemotherapy
and (43) with follow-up | Serum | ALU 247/115 | Serum cfDI may be a
promising candidate biomarker for prognostic prediction in CRC
patients who have had chemotherapy and are being followed-up for a
short time | (60) |
| No controls
reported |
| Thyroid cancer | (29) Benign nodules
patients | Plasma | ALU 247/115 | Measured the
integrity index in the vein draining the thyroid is similar to that
measured in the antecubital vein, using a peripheral liquid biopsy
to validate cfDI measurements. In opposition to its diagnostic
efficacy in aggressive cancers, cfDI has limited utility as a
biomarker of malignancy in cytologically indeterminate thyroid
nodules | (33) |
| (38) Malignant
lesions patients |
| No controls
reported |
| Non-small cell lung
cancer | (40) Controls | Serum | ALU 247/115 | Serum cfDNA level,
its integrity may be an effective tool of NSCLC early diagnosis and
prognosis of the disease | (61) |
| (60) Non-small cell
lung cancer patients |
| (40) Chronic
obstructive pulmonary disease patients |
| Non-small cell lung
cancer | (107) Controls | Plasma | ALU 247/115 | NSCLC may be
identified from tuberculosis with cfDNA and cfDI as indicators.
Furthermore, the integrity index had a significant effect on
traditional tumor markers in distinguishing NSCLC from
tuberculosis | (31) |
| (106)
NSCLCpatients |
| (105) Tuberculosis
patients |
| Non-small cell lung
cancer | (130) NSCLC
patients | Plasma | ALU 247/115 | The findings show
that cfDI could be used as a prognostic biomarker in patients who
received a personalized peptidevaccine | (62) |
| Lung cancer | (19) Controls | Plasma | ALU 247/115 | The study suggests
that ALU repeat ratios could be used for prognostic purposes in the
advanced setting for patients of lung cancer patients | (15) |
| (29) Lung cancer
patients |
| Lung cancer | (35) Controls | Urine | ALU-60, 115 and
247 | cfDNA concentration
index could serve as promising diagnostic biomarkers for lung
cancer | (63) |
| (55) Lung cancer
patients |
ALU as diagnostic or prognostic
biomarker in cancer
When comparing cancer cases with control in 36
articles, in 12 studies, the levels of ALU 247 and ALU 115 were
higher in patients than in controls. Thus, in total, 11 studies
were retained, with cfDI higher in cancer patients than the healthy
controls (an association between a higher cfDI and tumor stage, as
well as high sensitivity was identified in 3 of 12 articles) and 4
had no cfDI difference.
Table I shows 11
studies had only diagnostic and 8 had only prognostic information,
and 5 articles from the cancer group had significantly higher
concentrations of ALU sequences and cfDI than the benign disease
group. Six studies monitoring the ALU level could be applied for
subsidiary cancer diagnosis, either alone or in combination with
additional tumor markers.
For other ALU, including ALU 260/111, the cfDNA
variables can serve as attractive prognostic markers for metastatic
cancer during therapy. In addition, although ALU 244/83 is a
potential biomarker, there are currently no extensive studies to
verify this hypothesis (Table
I).
Discussion
Cancer is a major global health problem due to the
increasing incidence and fatality rates. An early cancer diagnosis
is crucial as it can improve the chances of survival for cancer
patients and decrease the mortality rate. At present, liquid
biopsies are promising due to their potential advantages, including
reliability, easy access, and reproducibility (11).
In the present review, several studies supported the
use of liquid biopsy in cancer as being innovative. To determine
whether this liquid biopsy could assist the diagnostic or
assessment of treatment response, 36 articles were included to
identify the ALU sequences as a biomarker in cancer. The
limitations of data retrieved are mostly related to the 36 articles
as there is great heterogeneity in studies, it is difficult to
analyse the subgroup study, the articles constitute a small sample
size, little research highlighting the differences of ALU and cfDI
at different stages is available, and the cut-off values vary
widely between studies and were missing in other studies.
The present review identified the role of ALU
element in cancer progression. Collectively, our data indicated
that ALU elements can be used as a biomarker (29,35,44,47,49,52,54,63).
The use of cfDNA, for early diagnosis, prognosis biomarkers and
monitoring of therapy have been a significant advancement in
clinical medicine (18,47,51,52,60-62).
As mentioned previously, not all studies have
confirmed that ALU levels vary with tumor development and
progression, which may elucidate that cancer is a heterogeneous
disease.
cfDI was subsequently evaluated for its usefulness
in cancer diagnosis and prognosis (5,15,31,32,34,48,58).
Higher cfDI values in cancer patients vs. healthy controls were
identified in many studies (31,34,57,58,61).
By contrast, lower cfDI was observed in different studies; however,
some articles with a focus on metastatic breast cancer (10,45),
recurrent breast cancer (46), or
first cycle of vaccination (53,62)
were few and inconsistent.
There are few studies highlight of the ALU 260/111
in cancer (10,18,29,46)
so is not possible to determinate the role of it as biomarker.
Several studies have identified an altered cfDI in
patients compared to controls. However, these studies are
heterogeneous, some studies showed a reduced cfDI in patients,
while others reported an increased cfDI. Various hypotheses have
been posited to understand the underlying reason. cfDNA from
healthy individuals had 3- to 5-fold multiples of
nucleosome-associated DNA length, and longer fragments than cfDNA
from pancreatic cancer patients using direct visualization by gel
electrophoresis (64). Jiang et
al (64) found increased
levels of shorter mitochondrial DNA molecules in the plasma of
cancer patients compared to healthy people using massively parallel
sequencing.
The present study included 36 studies with many
discrepancies. This was due to the fact that they were highly
heterogeneous studies and not all studies included different
stages. In addition, not enough data were available for a specific
cancer type and occasionally subjects were limited, there were
differences in molecular methods used and source of samples could
influence the results. This heterogeneity may affect clarification
and whether ALU can act as a biomarker in cancer disease. Thus,
further studies are needed to better clarify the role of the ALU
element in specific subgroups.
In general, the results obtained from the present
systematic review show that the expression of ALU-247 and ALU-115,
and cfDI concentration is a rising trend associated with cancer and
cancer stage. As a result, circulating cfDNA may be significantly
related to tumor cell turnover and tumor progression, indicating
biologic tumor aggressiveness. Thus, the circulating cfDI could be
suitable for monitoring cancer progression. In addition, large and
multicenter sample groups must be studied to corroborate the
findings.
Liquid biopsy is becoming the focus of future tumor
diagnosis and treatment research. It is less invasive and painful,
can be collected in a short period of time, can be collected
regardless of where the target organ is located, can be repeated
continually, and can represent cancer volume in real-time. However,
there are disadvantages, such as if the amount of cfDNA is
insufficient, correct information may not be collected. Thus,
analytical technology must be established, and the patient's
condition or underlying disease may influence the results. Liquid
biopsies, as with any other test method, involve large cohort
studies to determine their efficacy and sensitivity to various
disorders.
The limitation of the present study was mainly to
mention the results of ALU and cfDI in blood samples without
mentioning the in vitro information regarding ALU and cfDI.
This aspect should be investigated in future work.
In summary, findings of the review suggest that cfDI
can be a significant predictor of developing cancer in patients and
could be a useful marker in a molecular, blood-based multi-marker
assay.
The current systematic review assessed the total ALU
sequence levels and its index are promising biomarkers for the
purpose of investigation or prognosis of cancer. However, because
of the heterogeneity between studies, the difference of ALU value
in different cancers (type or stage) therefore makes it difficult
to compare between different types of cancer. Further studies and
meta-analyses are needed for the final conclusion to explore the
diagnostic role of ALU in malignant diseases, especially when
combined with other cancer biomarkers.
Acknowledgements
The authors would like to express deep thanks to the
University of Anbar, College of Science, Department of
Biotechnology for their assistance.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
SAS, AMA-R and AAS conceived and planned the review.
Data extraction was carried out by SAS and AAS. SAS, AMA-R and AAS
evaluated the articles, manually checked references and wrote the
review. All authors have read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable for systematic reviews.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parkin DM, Pisani P and Ferlay J: Global
Cancer Statistics. CA Cancer J Clin. 49:33–640. 1. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Y, Gu M, Jing F, Cai S, Bao C, Wang J,
Jin M and Chen K: Association between physical activity and all
cancer mortality: Dose-response meta-analysis of cohort studies.
Int J Cancer. 138:818–832. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ye D, Jiang D, Zhang X and Mao Y: Alu
methylation and risk of cancer: A meta-analysis. Am J Med Sci.
359:271–280. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Arko-Boham B, Aryee NA, Blay RM, Owusu
EDA, Tagoe EA, Doris Shackie ES, Debrah AB and Adu-Aryee NA:
Circulating cell-free DNA integrity as a diagnostic and prognostic
marker for breast and prostate cancers. Cancer Genet.
235-236:65–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pan Y, Liu G, Zhou F, Su B and Li Y: DNA
methylation profiles in cancer diagnosis and therapeutics. Clin Exp
Med. 18:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Shen J, Stass SA and Jiang F: MicroRNAs as
potential biomarkers in human solid tumors. Cancer Lett.
329:125–136. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Paterlini-Brechot P and Benali NL:
Circulating tumor cells (CTC) detection: Clinical impact and future
directions. Cancer Lett. 253:180–204. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Madhavan D, Wallwiener M, Bents K,
Zucknick M, Nees J, Schott S, Cuk K, Riethdorf S, Trumpp A, Pantel
K, et al: Plasma DNA integrity as a biomarker for primary and
metastatic breast cancer and potential marker for early diagnosis.
Breast Cancer Res Treat. 146:163–174. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cuk K, Zucknick M, Heil J, Madhavan D,
Schott S, Turchinovich A, Arlt D, Rath M, Sohn C, Benner A, et al:
Circulating microRNAs in plasma as early detection markers for
breast cancer. Int J Cancer. 132:1602–1612. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cuk K, Zucknick M, Madhavan D, Schott S,
Golatta M, Heil J, Marmé F, Turchinovich A, Sinn P, Sohn C, et al:
Plasma MicroRNA panel for minimally invasive detection of breast
cancer. PLoS One. 8(e76729)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Madhavan D, Zucknick M, Wallwiener M, Cuk
K, Modugno C, Scharpff M, Schott S, Heil J, Turchinovich A, Yang R,
et al: Circulating miRNAs as surrogate markers for circulating
tumor cells and prognostic markers in metastatic breast cancer.
Clin Cancer Res. 18:5972–5982. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tuchalska-Czuroń J, Lenart J, Augustyniak
J and Durlik M: Clinical value of tissue DNA integrity index in
pancreatic cancer. Surgeon. 18:269–279. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chudasama DY, Aladag Z, Felicien MI, Hall
M, Beeson J, Asadi N, Gidron Y, Karteris E and Anikin VB:
Prognostic value of the DNA integrity index in patients with
malignant lung tumors. Oncotarget. 9:21281–21288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li M, Jia Y, Xu J, Cheng X and Xu C:
Assessment of the circulating cell-free DNA marker association with
diagnosis and prognostic prediction in patients with lymphoma: A
single-center experience. Ann Hematol. 96:1343–1351.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
El Messaoudi S, Mouliere F, Du Manoir S,
Bascoul-Mollevi C, Gillet B, Nouaille M, Fiess C, Crapez E, Bibeau
F, Theillet C, et al: Circulating DNA as a strong multimarker
prognostic tool for metastatic colorectal cancer patient management
care. Clin Cancer Res. 22:3067–3077. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng J, Holland-Letz T, Wallwiener M,
Surowy H, Cuk K, Schott S, Trumpp A, Pantel K, Sohn C, Schneeweiss
A and Burwinkel B: Circulating free DNA integrity and concentration
as independent prognostic markers in metastatic breast cancer.
Breast Cancer Res Treat. 169:69–82. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Habeeb WH, Suleiman AA and Al-Hitawee HT:
Exploration of the beta-actin DNA integrity index as early genetic
marker of presence of breast cancer. Electron J Gen Med.
17(em188)2020.
|
|
20
|
Akca H, Demiray A, Yaren A, Bir F, Koseler
A, Iwakawa R, Bagci G and Yokota J: Utility of serum DNA and
pyrosequencing for the detection of EGFR mutations in non-small
cell lung cancer. Cancer Genet. 206:73–80. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Iqbal S, Vishnubhatla S, Raina V, Sharma
S, Gogia A, Deo SS, Mathur S and Shukla NK: Circulating cell-free
DNA and its integrity as a prognostic marker for breast cancer.
Springerplus. 4(265)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mandel P and Metais P: Nuclear Acids In
Human Blood Plasma. C R Seances Soc Biol Fil. 14:241–243.
1948.PubMed/NCBI(In French).
|
|
23
|
Tan EM, Schur PH, Carr RI and Kunkel HG:
Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of
patients with systemic lupus erythematosus. J Clin Invest.
45:1732–1740. 1966.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Leon SA, Shapiro B, Sklaroff DM and Yaros
MJ: Free DNA in the serum of cancer patients and the effect of
therapy. Cancer Res. 37:646–650. 1977.PubMed/NCBI
|
|
25
|
Fleischhacker M and Schmidt B: Circulating
nucleic acids (CNAs) and cancer-A survey. Biochim Biophys Acta.
1775:181–232. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jahr S, Hentze H, Englisch S, Hardt D,
Fackelmayer FO, Hesch RD and Knippers R: DNA fragments in the blood
plasma of cancer patients: Quantitations and evidence for their
origin from apoptotic and necrotic cells. Cancer Res. 61:1659–1665.
2001.PubMed/NCBI
|
|
27
|
Umetani N, Giuliano AE, Hiramatsu SH,
Amersi F, Nakagawa T, Martino S and Hoon DSB: Prediction of breast
tumor progression by integrity of free circulating DNA in serum. J
Clin Oncol. 24:4270–4276. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Diehl F, Li M, Dressman D, He Y, Shen D,
Szabo S, Diaz LA Jr, Goodman SN, David KA, Juhl H, et al: Detection
and quantification of mutations in the plasma of patients with
colorectal tumors. Proc Natl Acad Sci USA. 102:16368–16373.
2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stamenkovic S, Cheng J, Surowy H,
Burwinkel B and Gündert M: Circulating cell-free DNA variables as
marker of ovarian cancer patients: A pilot study. Cancer Biomark.
28:159–167. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hao TB, Shi W, Shen XJ, Shen XJ, Qi J, Wu
XH, Wu Y, Tang YY and Ju SQ: Circulating cell-free DNA in serum as
a biomarker for diagnosis and prognostic prediction of colorectal
cancer. Br J Cancer. 111:1482–1489. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Leng S, Zheng J, Jin Y, Zhang H, Zhu Y, Wu
J, Xu Y and Zhang P: Plasma cell-free DNA level and its integrity
as biomarkers to distinguish non-small cell lung cancer from
tuberculosis. Clin Chim Acta. 477:160–165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vizza E, Corrado G, De Angeli M, Carosi M,
Mancini E, Baiocco E, Chiofalo B, Patrizi L, Zampa A, Piaggio G and
Cicchillitti L: Serum DNA integrity index as a potential molecular
biomarker in endometrial cancer. J Exp Clin Cancer Res.
37(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Thakur S, Tobey A, Daley B, Auh S, Walter
M, Patel D, Nilubol N, Kebebew E, Patel A, Jensen K, et al: Limited
utility of circulating cell-free dna integrity as a diagnostic tool
for differentiating between malignant and benign thyroid nodules
with indeterminate cytology (Bethesda Category III). Front Oncol.
9(905)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park MK, Lee JC, Lee JW and Hwang SJ: Alu
cell-free DNA concentration, Alu index, and LINE-1 hypomethylation
as a cancer predictor. Clin Biochem. 94:67–73. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Salem R, Ahmed R, Shaheen K, Abdalmegeed M
and Hassan H: DNA integrity index as a potential molecular
biomarker in colorectal cancer. Egypt J Med Hum Genet.
21(38)2020.
|
|
36
|
Lander ES, Linton LM, Birren B, Nusbaum C,
Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al:
Initial sequencing and analysis of the human genome. Nature.
409:860–921. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Cheng J, Tang Q, Cao X and Burwinkel B:
Cell-free circulating DNA integrity based on peripheral blood as a
biomarker for diagnosis of cancer: A systematic review. Cancer
Epidemiol Biomarkers Prev. 26:1595–1602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Umetani N, Kim J, Hiramatsu S, Reber HA,
Hines OJ, Bilchik AJ and Hoon DSB: Increased integrity of free
circulating DNA in sera of patients with colorectal or
periampullary cancer: Direct quantitative PCR for ALU repeats. Clin
Chem. 52:1062–1069. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang BG, Huang HY, Chen YC, Bristow RE,
Kassauei K, Cheng CC, Roden R, Sokoll LJ, Chan DW and Shih IeM:
Increased plasma DNA integrity in cancer patients. Cancer Res.
63:3966–3968. 2003.PubMed/NCBI
|
|
40
|
Holdenrieder S, Burges A, Reich O,
Spelsberg FW and Stieber P: DNA integrity in plasma and serum of
patients with malignant and benign diseases. Ann N Y Acad Sci.
1137:162–170. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Boddy JL, Gal S, Malone PR, Shaida N,
Wainscoat JS and Harris AL: The role of cell-free DNA size
distribution in the management of prostate cancer. Oncol Res.
16:35–41. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Schmidt B, Weickmann S, Witt C and
Fleischhacker M: Integrity of cell-free plasma DNA in patients with
lung cancer and nonmalignant lung disease. Ann N Y Acad Sci.
1137:207–213. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Agostini M, Enzo MV, Bedin C, Belardinelli
V, Goldin E, Del Bianco P, Maschietto E, D'Angelo E, Izzi L,
Saccani A, et al: Circulating cell-free DNA: A promising marker of
regional lymphonode metastasis in breast cancer patients. Cancer
Biomark. 11:89–98. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lehner J, Stötzer OJ, Fersching D, Nagel D
and Holdenrieder S: Circulating plasma DNA and DNA integrity in
breast cancer patients undergoing neoadjuvant chemotherapy. Clin
Chim Acta. 425:206–211. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Stötzer OJ, Lehner J, Fersching-Gierlich
D, Nagel D and Holdenrieder S: Diagnostic relevance of plasma DNA
and DNA integrity for breast cancer. Tumor Biol. 35:1183–1191.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cheng J, Cuk K, Heil J, Golatta M, Schott
S, Sohn C, Schneeweiss A, Burwinkel B and Surowy H: Cell-free
circulating DNA integrity is an independent predictor of impending
breast cancer recurrence. Oncotarget. 8:54537–54547.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hussein NA, Mohamed SN and Ahmed MA:
Plasma ALU-247, ALU-115, and cfDNA integrity as diagnostic and
prognostic biomarkers for breast cancer. Appl Biochem Biotechnol.
187:1028–1045. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Feng J, Gang F, Li X, Jin T, Houbao H, Yu
C and Guorong L: Plasma cell-free DNA and its DNA integrity as
biomarker to distinguish prostate cancer from benign prostatic
hyperplasia in patients with increased serum prostate-specific
antigen. Int Urol Nephrol. 45:1023–1028. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fawzy A, Sweify KM, El-Fayoumy HM and
Nofal N: Quantitative analysis of plasma cell-free DNA and its DNA
integrity in patients with metastatic prostate cancer using ALU
sequence. J Egypt Natl Canc Inst. 28:235–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Khani M, Hosseini J, Mirfakhraie R, Habibi
M, Azargashb E and Pouresmaeili F: The value of the plasma
circulating cell-free DNA concentration and integrity index as a
clinical tool for prostate cancer diagnosis: A prospective
case-control cohort study in an Iranian population. Cancer Manag
Res. 11:4549–4556. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang R, Pu W, Zhang S, Chen L, Zhu W,
Xiao L, Xing C and Li K: Clinical value of ALU concentration and
integrity index for the early diagnosis of ovarian cancer: A
retrospective cohort trial. PLoS One. 13(e0191756)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tanaka H, Tsuda H, Nishimura S, Nomura H,
Kataoka F, Chiyoda T, Tanaka K, Iguchi Y, Susumu N and Aoki D: Role
of circulating free Alu DNA in endometrial cancer. Int J Gynecol
Cancer. 22:82–86. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Waki K, Yokomizo K, Kawano K, Tsuda N,
Komatsu N and Yamada A: Integrity of plasma cell-free DNA as a
prognostic factor for vaccine therapy in patients with endometrial
cancer. Mol Clin Oncol. 14(29)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sikora K, Bedin C, Vicentini C, Malpeli G,
D'Angelo E, Sperandio N, Lawlor RT, Bassi C, Tortora G, Nitti D, et
al: Evaluation of cell-free DNA as a biomarker for pancreatic
malignancies. Int J Biol Markers. 30:e136–e141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Utomo WK, Janmaat VT, Verhaar AP, Cros J,
Lévy P, Ruszniewski P, den Berg MS, Jenster G, Bruno MJ, Braat H,
et al: DNA integrity as biomarker in pancreatic cyst fluid. Am J
Cancer Res. 6:1837–1841. 2016.PubMed/NCBI
|
|
56
|
Mead R, Duku M, Bhandari P and Cree IA:
Circulating tumour markers can define patients with normal colons,
benign polyps, and cancers. Br J Cancer. 105:239–245.
2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Leszinski G, Lehner J, Gezer U and
Holdenrieder S: Increased DNA integrity in colorectal cancer. In
Vivo. 28:299–303. 2014.PubMed/NCBI
|
|
58
|
El-Gayar D, El-Abd N, Hassan N and Ali R:
Increased free circulating DNA integrity index as a serum biomarker
in patients with colorectal carcinoma. Asian Pacific J Cancer Prev.
17:939–944. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bedin C, Enzo MV, Del Bianco P,
Pucciarelli S, Nitti D and Agostini M: Diagnostic and prognostic
role of cell-free DNA testing for colorectal cancer patients. Int J
Cancer. 140:1888–1898. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhu F, Ma J, Ru D, Wu N, Zhang Y, Li H,
Liu X, Li J, Zhang H, Xu Y, et al: Plasma DNA Integrity as a
prognostic biomarker for colorectal cancer chemotherapy. J Oncol.
2021(5569783)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Soliman SES, Alhanafy AM, Habib MSE, Hagag
M and Ibrahem RAL: Serum circulating cell free DNA as potential
diagnostic and prognostic biomarker in non small cell lung cancer.
Biochem Biophys Rep. 15:45–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Waki K, Yokomizo K, Yoshiyama K, Takamori
S, Komatsu N and Yamada A: Integrity of circulating cell-free DNA
as a prognostic biomarker for vaccine therapy in patients with
nonsmall cell lung cancer. Immunopharmacol Immunotoxicol.
43:176–182. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ren S, Ren X, Guo H, Liang L, Wei K, Guo
L, Qu X, Dai X and Huang Q: Concentration and integrity indexes of
urine cell-free DNA as promising biomarkers for early lung cancer
diagnosis. Per Med. 18:129–139. 2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Jiang P, Chan CW, Chan KC, Cheng SH, Wong
J, Wong VW, Wong GL, Chan SL, Mok TS, Chan HL, et al: Lengthening
and shortening of plasma DNA in hepatocellular carcinoma patients.
Proc Natl Acad Sci USA. 112:E1317–E1325. 2015.PubMed/NCBI View Article : Google Scholar
|