Introduction
NTRK gene fusions are consistently detected
in rare types of cancers (secretory breast carcinoma, mammary
analogue secretary carcinoma, congenital infantile fibrosarcoma,
and congenital mesoblastic nephroma) and they are novel therapeutic
targets across multiple tumor types (1-3).
On the other hand, these gene fusions are rare in common adult
cancers (1,2). In gynecologic oncology, NTRK
gene fusion is also rare, although there are several reports of
uterine sarcoma with this fusion gene (4-7).
A previous cohort study showed that TPM3-NTRK1 is most
frequent in NTRK1 fusions across multiple histologies
(2). Immunohistochemistry (IHC)
staining, fluorescence in situ hybridization, reverse transcriptase
polymerase chain reaction, DNA-based next-generation sequencing
(NGS) and RNA-based NGS are used to identify patients with
NTRK gene fusion cancer. Each method to detect NTRK
gene fusion has its own characteristics (3).
NTRK1, NTRK2 and NTRK3 encode
TRKA, TRKB, and TRKC, respectively (1). Entrectinib is a potent inhibitor of
TRKA, TRKB, TRKC, ROS1, and ALK, and is specifically designed to
have systemic activity. In gynecologic oncology, treatment using
entrectinib is rare because of the low frequency of NTRK
fusions (1). Here, we report a
case of recurrent ovarian cancer (OC) with TPM3-NTRK1 gene
fusion, which was treated with entrectinib.
Case report
In September 2013, a 56-year-old woman was referred
to Fukushima Medical University Hospital (Fukushima, Japan) with
bilateral ovarian tumors, multiple disseminations in the
peritoneum, bilateral pleural effusion, and multiple swellings of
the pelvic and paraaortic lymph nodes. Her serum level of cancer
antigen 125 (CA125) was elevated to 1,740 U/ml. She was diagnosed
as having stage IV OC according to the International Federation of
Gynecology and Obstetrics (FIGO) 1988 because pleural effusion
cytology was positive. Paclitaxel (175 mg/m2) and
carboplatin (area under the curve 6), TC therapy, were started as
neoadjuvant chemotherapy. After four courses of chemotherapy,
computed tomography (CT) revealed a reduction in tumor size.
Interval debulking surgery including abdominal hysterectomy,
bilateral salpingo-oophorectomy, omentectomy, and pelvic and
paraaortic lymphadenectomy, was performed. Histopathological
diagnosis was high-grade serous carcinoma. Following this surgery,
another three courses of the same regimen were administered, and
the patient achieved clinical complete response.
A total of 10 months after the last therapy, CT
showed multiple disseminations around the liver. TC therapy was
administered again. At the time of the third course,
carboplatin-related hypersensitivity reaction occurred. After the
third course, stable disease (SD) was shown. Thus, TC therapy was
converted to TP therapy (135 mg/m2 paclitaxel and 75
mg/m2 cisplatin). After three courses of TP therapy, SD
was maintained. As the disseminations were located only around the
liver, partial hepatectomy was performed. At that time,
postoperative chemotherapy was not administered as there was no
detectable disease in the abdominal cavity.
A total of 5 months after the surgery, CT showed
multiple lesions in the peritoneum. Therefore, TP therapy with 15
mg/m2 bevacizumab (BV) was started. At the ninth course
of this chemotherapy, cisplatin-related hypersensitivity reaction
occurred. After the ninth course, CT showed progressive disease
(PD). Subsequently, chemotherapy with pegylated liposomal
doxorubicin with BV, gemcitabine with BV, and nogitecan with BV was
administered. However, the tumors remained; mesentery dissemination
resection was performed.
A total of 8 months after mesentery dissemination
resection, CT showed multiple peritoneal lesions. Subsequently,
weekly paclitaxel, oral etoposide, weekly nedaplatin and
gemcitabine were administered in this order, however, none of the
regimens were effective. Microsatellite stability was detected in
specimens from the mesentery dissemination resection.
In August 2019, because there was no more standard
therapy, FoundationOne® CDx (Foundation Medicine,
Cambridge, MA), which is DNA based NGS and covers 324 genes, was
performed based on the patient's archival tumor tissue from the
mesentery dissemination resection. This revealed a missense variant
of TP53 (c.731G>A) and TPM3-NTRK1
rearrangement between somewhere around exon 2-3 of TPM3
(pos1=‘chr1:156844554-156844771’, pos2=’chr1:154155588-154155822’)
and exon 11 of NTRK1 (NM_002529). Oral entrectinib (600
mg/day) was started after discussing with experts. A total of 6
weeks after initiation of entrectinib, the patient's serum CA125
level elevated to 4,360 U/ml, which was 1,712 U/ml before
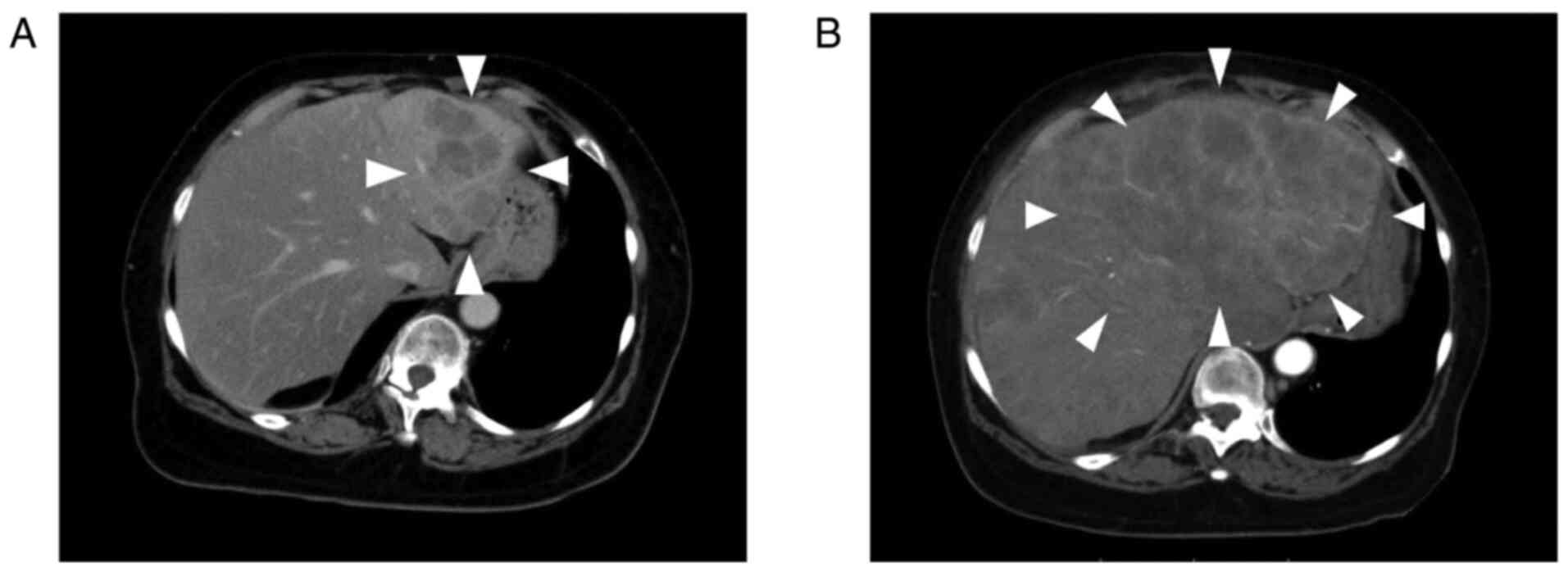
initiation of entrectinib, and CT revealed progression of liver
metastasis (Fig. 1). Adverse
events during entrectinib administration comprised grade 2
dysgeusia. A total of 1 month after discontinuation of entrectinib,
the patient died from disease progression (Fig. 2).
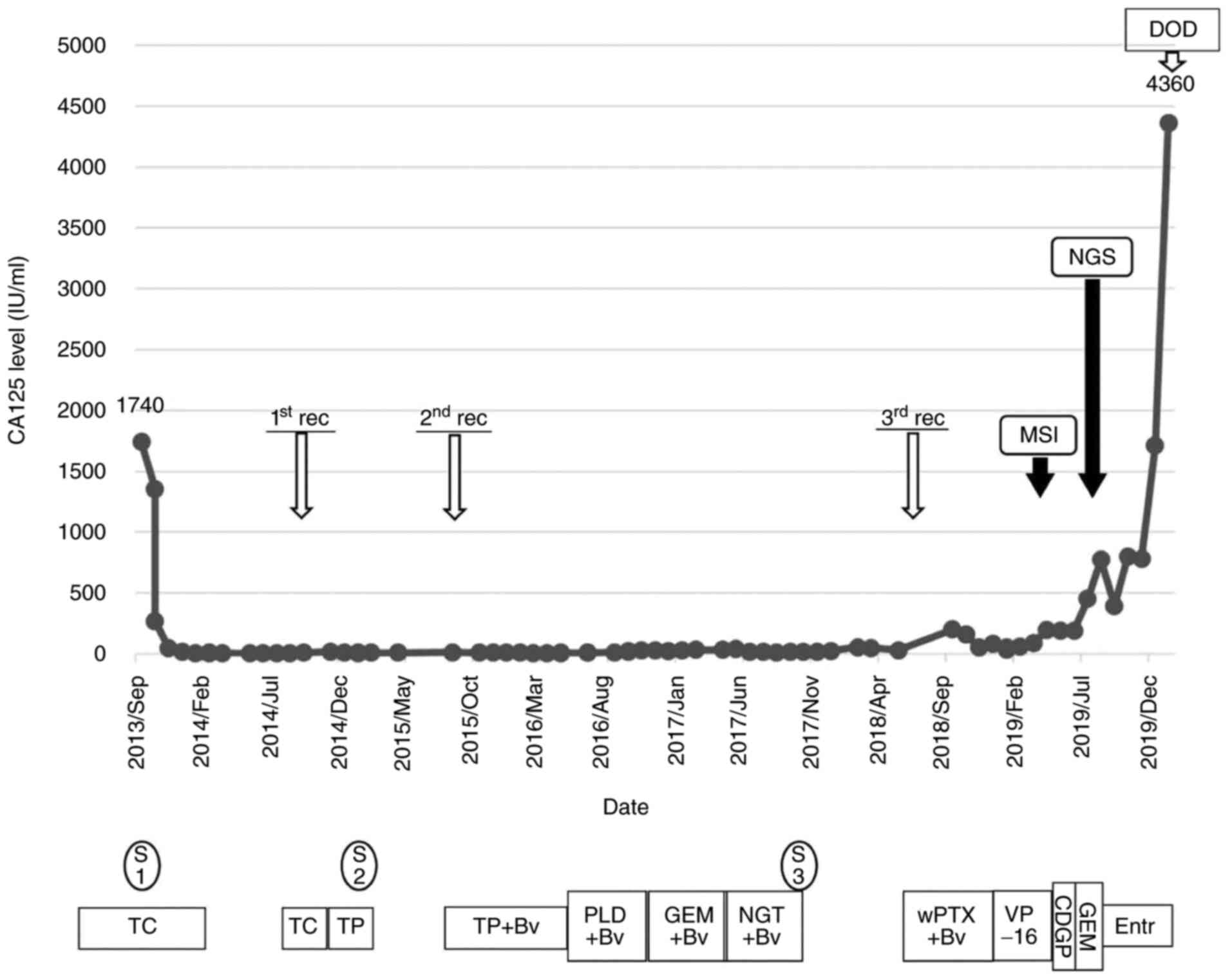 | Figure 2Clinical flowchart of the patient.
CA125, cancer antigen-125; MSI, microsatellite instability test;
NGS, next-generation sequencing; DOD, dead of disease; rec,
recurrence; S, surgery; S1, abdominal hysterectomy, bilateral
salpingo-oophorectomy, omentectomy, and pelvic and paraaortic
lymphadenectomy; S2, partial hepatectomy; S3, mesentery
dissemination resection; TC, paclitaxel and carboplatin; TP,
paclitaxel and cisplatin; BV, bevacizumab; PLD, pegylated liposomal
doxorubicin; GEM, gemcitabine; NGT, nogitecan; wPTX, weekly
paclitaxel; VP-16, etopocide; CDGP, nedaplatin; Entr,
entrectinib. |
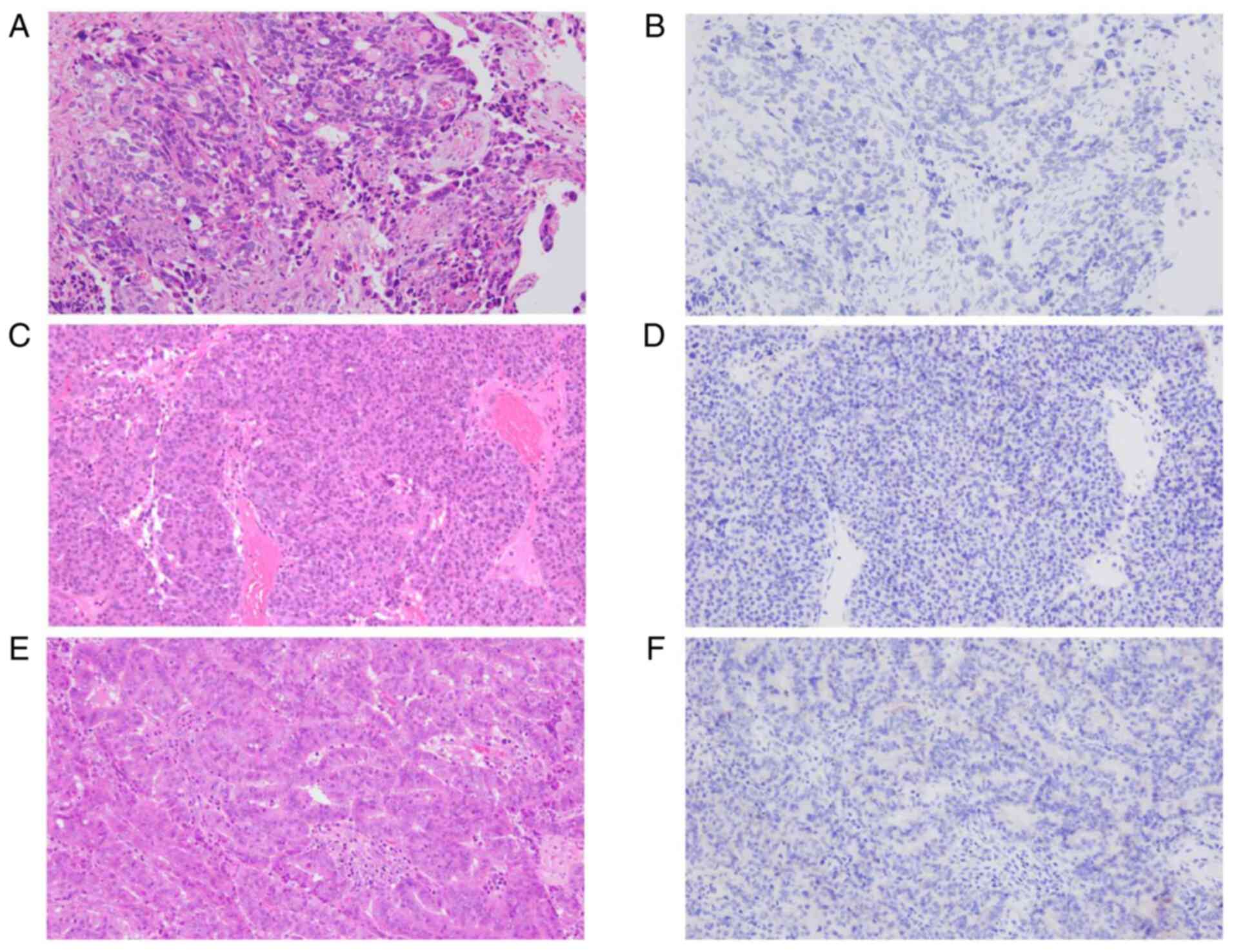
After the patient's death, IHC staining with a
pan-Trk monoclonal antibody (mAB) clone EPR17341 (Abcam, Cambridge,
MA) was performed to assess TRKA, TRKB, and TRKC expression as
previously described (8). This mAB
clone is most commonly used and has been investigated thoroughly.
In addition, this mAB clone reacts with a conserved proprietary
peptide from the C-terminus of TRKA, TRKB and TRKC, and is
therefore reactive to any oncogenic NTRK fusion (3). IHC was negative for all specimens
from the primary site, as well as the first and second recurrent
sites (Fig. 3).
Discussion
Here we presented a case of recurrent OC with
TPM3-NTRK1 rearrangement. Additionally, the present case
demonstrates the discrepancy between gene rearrangement detected by
NGS and protein expression. This discrepancy may be an indicator
for predicting the ineffectiveness of entrectinib for cancers with
NTRK rearrangement detected by NGS.
In the current case, NGS revealed TPM3-NTRK1
rearrangement and a missense variant of TP53. There are few
approved therapies for TP53 variants, although almost all
cases of ovarian high-grade serous carcinoma (95%) have somatic
TP53 variants (9). On the
other hand, NTRK fusions are oncogenic drivers and novel
targets. Doebele et al (1)
reported the safety and activity of entrectinib in adult patients
with advanced or metastatic NTRK fusion-positive cancers
across three clinical trials (ALKA-372-001, STARTRK-1 and
STARTRK-2). In these trials, only one ovarian cancer patient was
included. They showed that the objective response rate, which
included complete response and partial response, was 57% (95% CI
43.2-70.8). The median duration of response was 10 months (95% CI
7.1 to not estimable) and the percentage of PD was only 7%.
However, the characteristics of cases with PD remained unclear in
their report (1).
In the present case, entrectinib was administered
because NGS revealed TPM3-NTRK1 rearrangement and
entrectinib was recommended after a discussion among experts.
However, this novel target drug was ineffective. TRK protein was
not expressed as a result of IHC testing with a pan-Trk mAB clone
(EPR17341). A previous study reported that gene fusions involving
NTRK1, 2, and 3 and their partner genes result in a
constitutive activation or overexpression of TRK receptors,
potentially leading to oncogenesis (10). Additionally, other reports have
shown that pan-Trk IHC yielded a sensitivity of 75-95.2%, and a
specificity of 92-100% and that the sensitivity of pan-Trk IHC for
TRKA was 96.2% (3,8,11,12).
Pan-Trk IHC is a reliable screening method for the detection of
NTRK gene fusions based on these data. Moreover, pan-Trk IHC
is used to assess rapidly assess malignancies which may harbor
possible NTRK gene fusions in order to determine eligibility
of patients for targeted therapy with TRK inhibitors (8). However, it should be considered that
there are NTRK rearrangements which are found to be negative
by IHC, and can only be detected by NGS, such as in the present
case.
Drilon et al (13) reported the efficacy of
larotrectinib, which is a selective inhibitor of TRKA, TRKB and
TRKC. In their study, six of an initial 55 patients showed primary
resistance to larotrectinib. Three of the six patients had tumor
material available for pan-Trk IHC, by which TRK protein expression
was not detected in all three. This indicated that the
rearrangements detected by NGS were false positives or that the
identified fusion genes were not expressed at the protein level
(13). It is considered that
entrectinib has the same characteristics as larotrectinib with
regard to discrepancy between gene fusion and protein expression,
as observed in the current case, and that this finding may be a key
to predict the ineffectiveness of entrectinib for cancers with
NTRK rearrangement detected by NGS.
To the best of our knowledge, this is the first case
report of OC with NTRK rearrangement. It is known that a
small percentage of common adult cancers carry fusions of
NTRK genes (2). Large
cohort studies revealed that the frequency of NTRK gene
fusions was 0.25% of general cancers (2,12).
Therefore, physicians have few chances to experience this molecular
characteristic; however, they should be aware of the pitfall that
TRK protein may not express even if NGS shows NTRK
rearrangement.
In conclusion, we here presented a rare case of
recurrent OC with TPM3-NTRK1 fusion. Physicians may need to
be aware of the discrepancy of DNA rearrangement and protein
expression, and IHC may be required for confirmation of TRK protein
expression before entrectinib administration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YE, TW and MS drafted the manuscript. YE, TW, RS,
HS, YN, ES, MU, NK, SF, SSo, SSa and KF contributed to the patient
management and manuscript editing. MS, KS and KK performed
immunohistochemical staining. YE and TW confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and the accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Doebele RC, Drilon A, Paz-Ares L, Siena S,
Shaw AT, Farago AF, Blakely CM, Seto T, Chow BC, Tosi D, et al:
Entrectinib in patients with advanced or metastatic NTRK
fusion-positive solid tumours: Integrated analysis of three phase
1-2 trials. Lancet Oncol. 21:271–282. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Getalica Z, Xiu J, Swensen J and Vranic S:
Molecular characterization of cancers with NTRK gene fusions. Mod
Pathol. 32:147–153. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Solomon JP, Benayed R, Hechtman JF and
Ladanyi M: Identifying patients with NTRK fusion cancer. Ann Oncol.
30 (Suppl 8):viii16–viii22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chiang S, Cotzia P, Hyman DM, Drilon A,
Tap WD, Zhang L, Hechtman JF, Frosina D, Jungbluth AA, Murali R, et
al: NTRK fusions define a novel uterine sarcoma subtype with
features of fibrosarcoma. Am J Surg Pathol. 42:791–798.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rabban JT, Devine WP, Sangoi AR, Poder L,
Alvarez E, Davis J, Rudzinski E, Garg K and Bean GR: NTRK fusion
cervical sarcoma: A report of three cases, emphasising
morphological and immunohistochemical distinction from other
uterine sarcomas, including adenosarcoma. Histopathology.
77:100–111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hodgson A, Pun C, Djordjevic B and
Turashvili G: NTRK-rearranged cervical sarcoma: Expanding the
clinicopathologic spectrum. Int J Gynecol Pathol. 40:73–77.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Croce S, Hostein I and McCluggage WG: NTRK
and other recently described kinase fusion positive uterine
sarcomas: A review of a group of rare neoplasms. Genes Chromosomes
Cancer. 60:147–159. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hechtman JF, Benayed R, Hyman DM, Drilon
A, Zehir A, Frosina D, Arcila ME, Dogan S, Klimstra DS, Ladanyi M
and Jungbluth AA: Pan-Trk immunohistochemistry is an efficient and
reliable screen for the detection of NTRK fusions. Am J Surg
Pathol. 41:1547–1551. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Boyarskikh UA, Gulyaeva LF, Avdalyan AM,
Kechin AA, Khrapov EA, Lazareva DG, Kushlinskii NE, Melkonyan A,
Arakelyan A and Filipenko ML: Spectrum of TP53 mutations in BRCA1/2
associated high-grade serous ovarian cancer. Front Oncol.
10(1103)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khotskaya YB, Holla VR, Farago AF, Mills
Shaw KR, Meric-Bernstam F and Hong DS: Targeting TRK family
proteins in cancer. Pharmacol Ther. 173:58–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rudzinski ER, Lockwood CM, Stohr BA,
Vargas SO, Sheridan R, Black JO, Rajaram V, Laetsch TW and Davis
JL: Pan-Trk immunohistochemistry identifies NTRK rearrangements in
pediatric mesenchymal tumors. Am J Surg Pathol. 42:927–935.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Solomon JP, Linkov I, Rosado A, Mullaney
K, Rosen EY, Frosina D, Jungbluth AA, Zehir A, Benayed R, Drilon A,
et al: NTRK fusion detection across multiple assays and 33,997
cases: Diagnostic implications and pitfalls. Mod Pathol. 33:38–46.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Drilon A, Leatsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele R, Farago AF, Pappo AS,
et al: Efficacy of larotrectinib in TRK fusion-positive cancers in
adults and children. N Engl J Med. 378:731–739. 2018.PubMed/NCBI View Article : Google Scholar
|