Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide and the second-leading cause of new cases of
cancer. Non-small cell lung cancer (NSCLC) is the most common type
(1). In drug therapy for lung
cancer, patients with stage IV NSCLC are treated with
molecular-targeted drugs (2-9),
immune checkpoint inhibitors (ICIs) (10-12)
and cytotoxic anticancer drugs (13). In particular, in patients
classified as driver gene mutation/rearrangement-positive,
molecular-targeted drugs (kinase inhibitors) against epidermal
growth factor receptor (EGFR) (2-4),
anaplastic lymphoma kinase (ALK) (5), c-ROS oncogene 1 (ROS1) (6), v-raf murine sarcoma viral oncogene
homologue B (BRAF) (7), and
proto-oncogene cMET (8,9) have shown high therapeutic efficacy.
In contrast, high efficacy with pembrolizumab monotherapy or
platinum combination chemotherapy plus ICI, such as programmed cell
death 1 (PD-1)/programmed cell death 1-ligand 1 (PD-L1) inhibitor,
has been seen in patients with driver gene mutation/rearrangement
negative disease (10-12).
The introduction of pembrolizumab as an ICI, has
changed the outcome of treatment drastically, extending
progression-free survival (PFS) and overall survival (OS) compared
to the conventional platinum-based therapy (10). The addition of pembrolizumab to
conventional platinum-based therapy has also been shown to be more
effective than platinum-based therapy alone (11,12).
While pembrolizumab monotherapy significantly extended PFS and OS
only in patients with PD-L1 expression on at least 50% of tumor
cells, pembrolizumab combined therapy showed efficacy even when
PD-L1 expression was below 50% (11,12).
Now, the main treatment of advanced NSCLC without a targetable
mutation with PD-L1 expression of more than 50% is pembrolizumab,
and pembrolizumab combined therapy when PD-L1 expression is less
than 50% (13).
The efficacy of pembrolizumab and pembrolizumab
combined therapy remains limited, however, and predictive markers
of ICIs are important (14).
Although tumor proportion score (TPS) is used to measure the
expression of PD-L1 in tumor cells, its validity in predicting the
effects of pembrolizumab and pembrolizumab combined therapy is also
insufficient (15). Other
biological features that predict high tumor expression include a
high tumor mutational burden and the presence of tumor infiltrating
CD8+ (16). Currently, however,
only PD-L1 expression is used in routine practice, despite being an
incomplete tool for prediction, as mentioned above, and new
biomarkers to maximize the response of tumor regression and
minimize immune-related adverse events (irAEs) are urgently
needed.
Cancer cachexia is a feature of cancer that reflects
the metabolic changes that occur with this condition (17). Cancer cachexia is defined as
progressive skeletal muscle loss with or without weight loss that
does not completely recover with conventional nutritional support
and which leads to functional disability (18). The main symptom of cancer cachexia
is involuntary weight loss. Cachexia is diagnosed when a weight
loss greater than 5% occurs, or a weight loss greater than 2%
occurs in individuals with a body mass index (BMI) below 20 or loss
of skeletal muscle mass (sarcopenia) (18).
Roch et al reported that cancer sarcopenia,
diagnosed by a decrease in the third lumbar vertebra skeletal
muscle index (mSMI), is a useful determinant of disease control
rate and survival in NSCLC patients receiving first- and
second-line treatment with ICIs (19). They also reported that a body
weight loss of 5% or more reduced disease control rate and OS.
However, 87% of their patient population received second-line
pembrolizumab monotherapy, with PD-L1 expression of 1% or more. It
therefore remains unclear whether cancer cachexia predicts the
efficacy of pembrolizumab in first-line treatment, in either mono-
or combination therapy.
Here, we conducted a retrospective study to evaluate
whether cancer cachexia is a determinant of treatment efficacy in
patients receiving first-line pembrolizumab monotherapy and
combined therapy.
Patients and methods
Patients
As a retrospective study, we collected data from
medical records of NSCLC patients receiving first-line
pembrolizumab treatment at our institution from April 2014 to June
2020. Eligibility was limited to patients treated with first-line
treatment with pembrolizumab either alone or in combination with
another agent.
Evaluation of cancer cachexia at the
start of pembrolizumab therapy
Cancer cachexia is defined as progressive skeletal
muscle loss with or without weight loss that does not completely
recover by conventional nutritional support and leads to functional
disability (18). Accordingly, we
defined cancer cachexia as any of the following: i) weight loss
greater than 5%; ii) weight loss greater than 2% in an individual
with a BMI below 20; and iii) loss of skeletal muscle mass
(sarcopenia) and weight loss greater than 2%. We compared weight
with that 6 months prior to the day of therapy initiation as
baseline. Sarcopenia was evaluated by tracing the outline of the
psoas major muscle at the L2-L3 position, performed by the same
single operator for all cases. The sum of the right and left areas
was calculated and a change rate in psoas major muscle area (PMMA)
of more than 10% was defined as sarcopenia. Change rate was defined
as follows: Change rate of PMMA (%)=(1-PMMA ICI initiation/PMMA
before 6 months of ICI initiation) x100
These criteria are consistent with a study by
Nishioka et al showing the association of sarcopenia and
efficacy of ICI therapy in NSCLC (20).
Evaluation of pembrolizumab therapy
efficacy
Time to treatment failure (TTF) was used as the
primary endpoint of efficacy for pembrolizumab. We defined TTF as
the time from the start of pembrolizumab therapy to the end of
pembrolizumab therapy. Secondary endpoints were OS, tumor response
and incidence rate of AEs. OS was defined from the start of
pembrolizumab therapy to death by any cause.
Tumor response was assessed in four criteria in
accordance with Response Evaluation Criteria in Solid Tumors
guideline version 1.1(21).
Response rate was defined as complete response (CR) plus partial
response (PR), and disease control rate as CR plus PR plus stable
disease (SD).
Assessment of AEs
AEs were classified as pneumonitis, colitis, adrenal
insufficiency, hypothyroidism, renal dysfunction, diabetes
mellitus, hepatitis, severe skin toxicity and infusion-related
reaction, and graded according to the Common Terminology Criteria
for Adverse Events version 4.0(22). Incidence rates of AEs were compared
between patients with and without cancer cachexia.
Statistical analysis
Patient characteristics were summarized as medians
with 25th and 75th percentiles for continuous variables, and
frequencies and percentages for categorical variables. Differences
in patient characteristics between the two groups were compared
using the χ2 test, Fisher's exact test or Mann-Whitney
U-test. For the primary analysis, a Kaplan-Meier estimate and
log-rank test were used to assess OS and TTF by development of
cancer cachexia. Cox proportional hazards regression was used to
evaluate the association between OS and cancer cachexia with
adjustment for covariates. Categorical variables such as the
incidence of AEs, tumor response and one-year survival were
compared between patients with and without cancer cachexia using
the χ2 test. All analyses were conducted using IBM SPSS
version 22 (IBM Japan Ltd.) and R software version 3.5.1
(www.r-project.org), with P<0.05 considered
significant.
Results
Patient demographics
A total of 53 NSCLC patients were eligible. Among
them, 55% (29/53) were diagnosed with adenocarcinoma and 32%
(17/53) with squamous cell carcinoma. 32 patients were treated with
pembrolizumab monotherapy and 21 with pembrolizumab combination
therapy. Of these 21 patients, 10 patients received carboplatin
plus pemetrexed, 9 received carboplatin plus nab-paclitaxel, and 2
received cisplatin plus pemetrexed other than pembrolizumab. There
were 23 and 30 patients with and without cancer cachexia,
respectively (Table I), giving an
overall incidence rate of cancer cachexia at the start of
pembrolizumab of 43% (23/53). As shown Table I, BMI, albumin, lymphocytes and
hemoglobin were significantly lower in patients with cachexia than
in those without cachexia. On the other hand, C-reactive protein
(CRP), neutrophils, white blood cells, platelets and
neutrophil-lymphocyte ratio (NLR) were significantly higher in
patients with cachexia than in those without. On evaluation for
newly arising cancer cachexia, 13 patients had a weight loss of
more than 5% and 10 with a BMI below 20 had a weight loss of more
than 2%, meaning 23 patients met the criteria for cancer
cachexia.
 | Table IPatient demographics and baseline
characteristics in patients receiving pembrolizumab with or without
cancer cachexia. |
Table I
Patient demographics and baseline
characteristics in patients receiving pembrolizumab with or without
cancer cachexia.
| Characteristic | With cachexia
(n=23) | Without cachexia
(n=30) | P-value |
|---|
| Number of patients
with combination of cytotoxic agents | 10 (43.5%) | 11 (36.7%) | 0.615a |
| Sex, male/female | 18/5 | 24/6 | 1.000a |
| Age, years | 71.0
(67.5-76.5) | 71.0
(67.2-76.7) | 0.914b |
| Height, cm | 164.9
(157.4-169.6) | 162.2
(158.9-164.9) | 0.290b |
| Body weight,
kg | 49.4
(45.4-56.5) | 46.4
(43.4-58.3) | 0.061b |
| Body mass
index | 20.9
(18.5-22.6) | 22.2
(20.8-24.5) | 0.002b |
| Albumin, mg/dl | 3.5 (3.0-3.8) | 4.0 (3.6-4.3) | 0.007b |
| Aspartate
aminotransferase, IU/l | 24.0
(17.0-33.5) | 20.0
(16.3-24.8) | 0.254b |
| Alanine
aminotransferase, IU/l | 23.0
(12.0-40.5) | 16.0
(12.0-26.0) | 0.146b |
| Serum creatinine,
mg/dl | 0.64
(0.61-0.73) | 0.79
(0.60-0.90) | 0.068b |
| Total bilirubin,
mg/dl | 0.5 (0.5-0.65) | 0.6 (0.5-0.7) | 0.299b |
| C-reactive protein,
mg/dl | 3.1 (1.4-7.9) | 0.43
(0.11-3.98) | 0.004b |
| Neutrophils,
/l | 7,840
(5,342.5-9,185) | 4,630
(3,800-5,597.5) |
<0.001b |
| Lymphocytes,
/l | 1,210
(883.5-1,355.5) | 1,393.5
(1,115.2-1,821.2) | 0.032b |
| White blood cells,
/l | 9,880
(7,340-11,445) | 7,315
(5,970-8,320) | 0.006b |
| Hemoglobin,
g/dl | 11.6
(10.6-13.2) | 13.0
(11.93-14.05) | 0.042b |
| Platelets,
104/l | 32.9
(24.3-39.4) | 24.6
(19.9-28.8) | 0.023b |
| Modified Glasgow
prognostic score, 0/1/2 | 4/9/10 | 19/6/5 | 0.003a |
|
Neutrophil-lymphocyte ratio | 6.10
(5.01-8.23) | 3.35
(2.52-4.58) |
<0.001b |
| Carcinoembryonic
antigen, U/ml | 4.2 (2.1-19.9) | 5.35
(1.6-36.8) | 0.799b |
| Carbohydrate
antigen 19-9, U/ml | 6.0 (3.9-16.0) | 2.6 (0.8-9.85) | 0.095b |
| Squamous cell
carcinoma antigen, ng/ml | 2.2
(1.27-14.6) | 1.4 (1.1-2.75) | 0.274b |
| Number of
metastatic organs/sites, 0/1/≥2 | 8/9/6 | 11/14/5 | 0.371a |
| Squamous cell
carcinoma/Adenocarcinoma/Others | 8/10/5 | 9/19/2 | 0.942a |
Efficacy of treatment
The relative dose intensity (RDI) of pembrolizumab
in patients with and without cancer cachexia was 0.98 and 0.93,
respectively. Median follow up was 13.6 months (interquartile
range: 2.2-6.6). For all patients who received pembrolizumab,
median TTF and median OS were 6.6 months [95% confidence interval
(CI): 4.7-8.5] and 22.7 months (95% CI: 18-27).
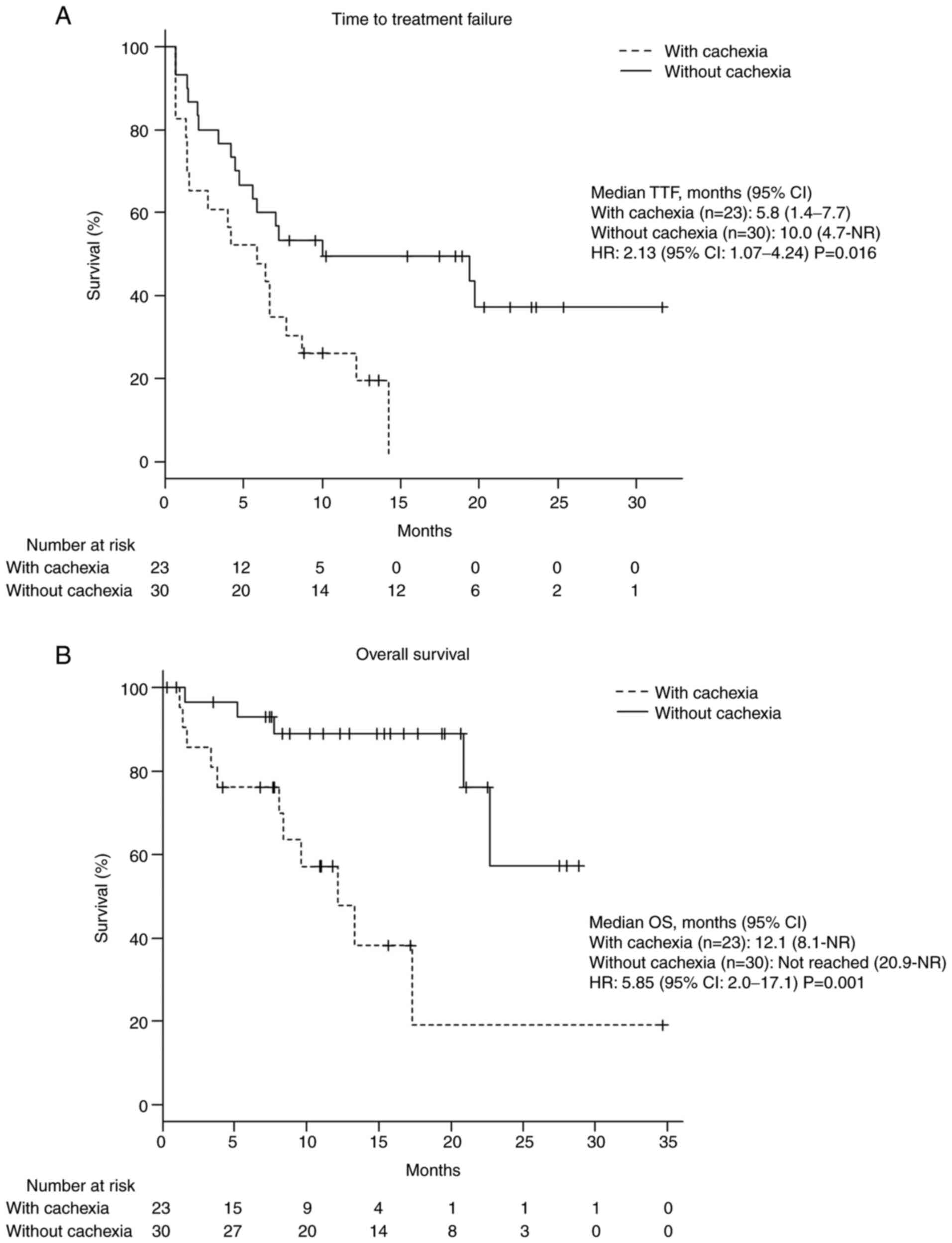
Median TTF and OS were significantly shorter in
patients with cancer cachexia than in those without [TTF: 5.8 vs.
10 months; hazard ratio (HR): 2.13; 95% CI: 1.07-4.24; P=0.016; OS:
12.1 months vs. not reached months; HR: 5.85; 95% CI: 2.0-17.1;
P=0.001; Fig. 1].
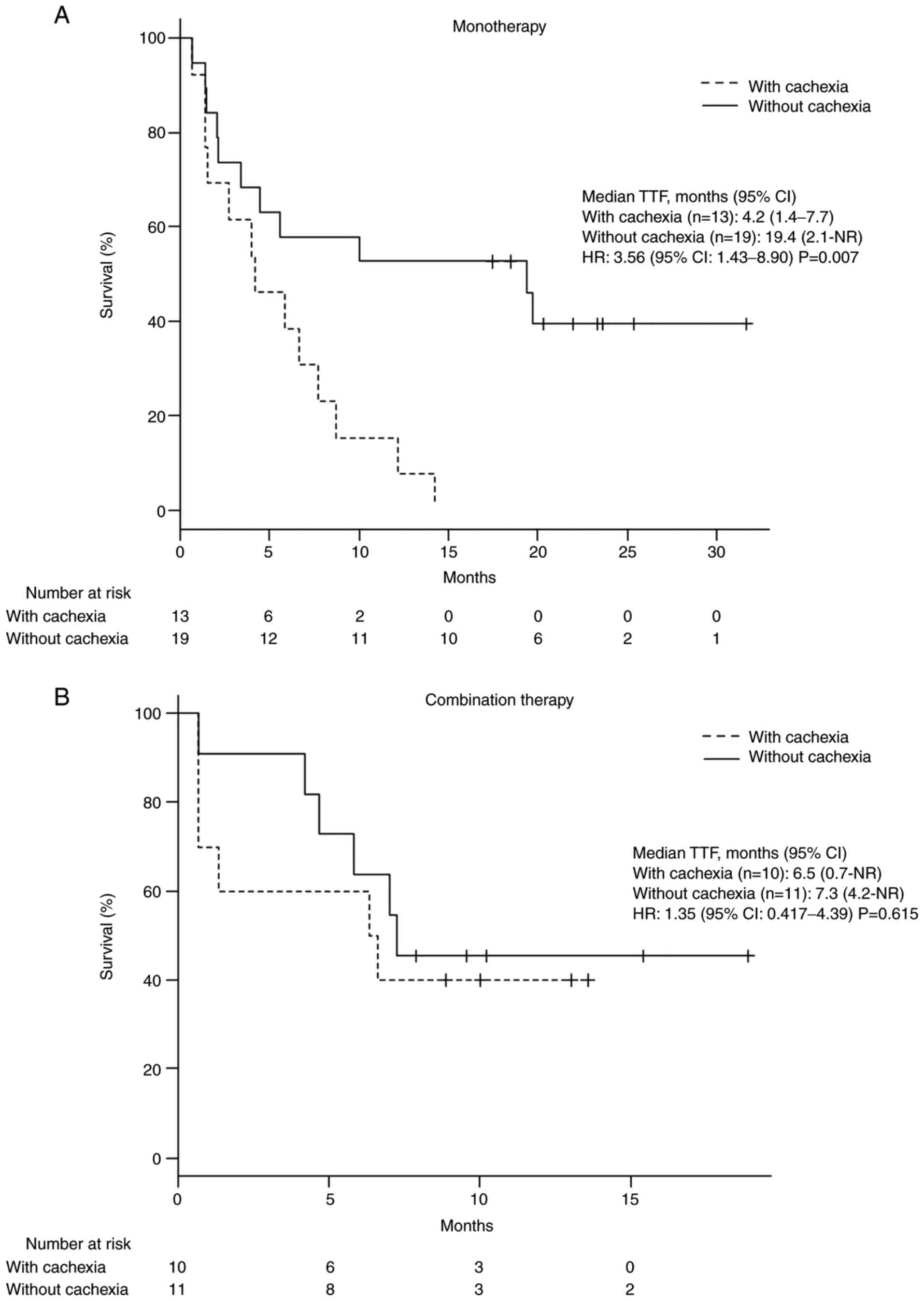
In patients receiving pembrolizumab monotherapy,
median TTF was shorter in patients with cancer cachexia than in
those without. This result was not seen in patients receiving
combination therapy including pembrolizumab (monotherapy: 4.2 vs.
19.4 months; HR: 3.56; 95% CI: 1.43-8.90; P=0.007; combination
therapy: 6.5 vs. 7.3 months; HR: 1.35; 95% CI: 0.417-4.39; P=0.615;
Fig. 2).
There was no significant difference between patients
with and without cachexia in tumor response rate including response
rate and disease control rate. One-year survival rate was lower in
patients with cachexia than in those without (1-year survival: 26
vs. 60%; P=0.029) (Table II).
 | Table IIComparison of median time to
treatment failure and disease control rate in patients with
non-small cell lung cancer with or without cachexia. |
Table II
Comparison of median time to
treatment failure and disease control rate in patients with
non-small cell lung cancer with or without cachexia.
| Effect | With cachexia
(n=23) | Without cachexia
(n=30) | P-value |
|---|
| Tumor response rate
(%) | | | |
|
Response
rate (CR + PR) | 6 (26.1) | 10 (33.3) | 0.789a |
|
Disease
control rate (CR + PR + SD) | 19 (82.6) | 26 (86.7) | 0.715b |
| One-year survival
(%) | 6 (26.1) | 18 (60.0) | 0.029a |
Incidence of AEs
Rates of pneumonitis, colitis, adrenal
insufficiency, renal dysfunction, diabetes mellitus, hepatitis,
severe skin toxicity and infusion-related reaction did not
significantly differ between patients with and without cancer
cachexia (Table III). In
contrast, the rate of hypothyroidism was significantly lower in
patients with cancer cachexia than in those without (P=0.048).
 | Table IIIComparison of incidence of adverse
events between patients with non-small cell lung cancer with or
without cachexia. |
Table III
Comparison of incidence of adverse
events between patients with non-small cell lung cancer with or
without cachexia.
| | With cachexia
(n=23) | Without cachexia
(n=30) | |
|---|
| Adverse event | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Overall | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Overall | P-value |
|---|
| Pneumonitis | 0.0 | 0.0 | 0.0 | 0/23 | 0.0 | 0.0 | 0.0 | 0/30 | - |
| Colitis | 4.3 | 0.0 | 0.0 | 1/23 | 0.0 | 0.0 | 0.0 | 0/30 | 0.434 |
| Hypothyroidism | 8.7 | 0.0 | 0.0 | 2/23 | 23.3 | 13.3 | 0.0 | 11/30 | 0.048 |
| Adrenal
insufficiency | 0.0 | 0.0 | 0.0 | 0/23 | 0.0 | 0.0 | 0.0 | 0/30 | - |
| Renal
dysfunction | 4.3 | 0.0 | 0.0 | 1/23 | 26.7 | 0.0 | 0.0 | 8/30 | 0.061 |
| Pancreatitis | 4.3 | 0.0 | 0.0 | 1/23 | 3.3 | 3.3 | 0.0 | 2/30 | 0.667 |
| Hepatitis | 26.1 | 4.3 | 4.3 | 8/23 | 40.0 | 3.3 | 0.0 | 13/30 | 0.524 |
| Severe skin
toxicity | 21.7 | 21.7 | 0.0 | 10/23 | 33.3 | 20.0 | 0.0 | 16/30 | 0.642 |
| Infusion-related
reaction | 4.3 | 0.0 | 0.0 | 1/23 | 0.0 | 0.0 | 0.0 | 0/30 | 0.434 |
Discussion
In this study, we evaluated the impact of cancer
cachexia in NSCLC patients receiving first-line treatment with
pembrolizumab. Cancer cachexia was found to be predictive in these
patients, and was associated with significantly shortened TTF, OS,
and 1-year survival. However, no association was seen between the
first-line treatment effect of pembrolizumab combined with
cytotoxic anticancer agents and cancer cachexia. These findings
suggest that avoidance of cachexia will not result in a weakening
of the therapeutic effect of pembrolizumab monotherapy in patients
with NSCLC.
In our study, TTF in patients receiving
pembrolizumab was 6.6 months. This finding is inconsistent with the
KEYNOTE-024 trial of Reck et al (10), who reported a PFS of 10.3 months in
305 patients with advanced NSCLC receiving pembrolizumab. It is
also inconsistent with the KEYNOTE-189 trial of Gandhi et al
(11), who reported a PFS of 8.8
months in 410 patients with advanced NSCLC receiving pembrolizumab
in combination with pemetrexed and a platinum-based drug.
This difference in TTF might be ascribable to
recruitment: The KEYNOTE-024 and KEYNOTE-189 trials were Phase 3
clinical trials which limited recruitment to patients having
adequate organ function (10). In
contrast, our present study recruited all patients who received
pembrolizumab in real-world clinical practice, including those in
poor general condition. In addition, we considered cachexia as a
factor in some patients with poor condition, whereas these are
typically excluded from clinical trials. Indeed, 43.4% of our
patients had cachexia. Of note, the TTF of patients who did not
have cachexia (10.0 months) was generally similar to that of the
pembrolizumab group (10.3 months) in the KEYNOTE-024 trial
(10).
In this study, significant differences were found in
BMI, albumin, CRP, neutrophil count, white blood cell count, HGB,
platelets, mGPS, and NLR. Since systemic inflammation is present in
cachexia patients (18), CRP,
neutrophil count, white blood cell count, platelet count and NLR
may have been higher in cachexia patients. Low BMI, albumin,
lymphocytes, and hemoglobin in patients with cancer cachexia may
also be due to reduced nutritional status.
Our finding that cancer cachexia is a predictor of
worse clinical outcome is consistent with previous findings by Roch
et al that evolving cancer sarcopenia as determined by third
lumbar vertebra skeletal muscle index is associated with a
shortened OS (19). It is also
consistent with the finding of Shiroyama et al that
sarcopenia determined by PMI can be used to predict a poor outcome
of therapy (23).
Cancer cachexia also significantly shortened TTF in
patients who received pembrolizumab monotherapy. In contrast, in
patients who received combination therapy which included
pembrolizumab, TTF did not significantly differ between patients
with and without cachexia. It is widely known that the presence of
cancer cachexia shortens OS (24).
This corresponds to the finding of Sanders et al that NSCLC
patients with early weight loss during chemoradiotherapy had
shorter OS (25). Nevertheless,
Ross et al reported that NSCLC patients with weight loss
receiving chemotherapy did not have significantly shorter PFS than
those without weight loss (26).
This raises the possibility that cytotoxic treatment failure is not
associated with weight loss. Further investigation of the
association between weight loss and chemotherapy failure is
warranted.
Our findings indicate that cancer cachexia is
strongly associated with pembrolizumab monotherapy failure. This
may be the result of metabolic changes induced by cancer cachexia.
The mechanism of weight loss is multifactorial, including decreased
food intake, metabolic dysfunction and increased energy use
(27). TNFα and IL-6 have been
shown to cause weight loss (26).
IL-1 causes protein breakdown in skeletal muscle (27). Flint et al reported that
tumor-induced IL-6 causes hypoketonemia, which in turn triggers
glucocorticoids and results in immune suppression (28). These inflammatory cytokines may
downregulate the efficacy of pembrolizumab. Currently, the only
pharmacological treatment showing promise against cancer cachexia
is anamorelin (29). Further
investigation of immunotherapy downregulation may reveal the
pathophysiology of cancer cachexia and lead the way to promising
treatments.
The incidence of hypothyroidism was significantly
higher in patients without cancer cachexia. Osorio et al
reported that median OS was significantly longer in those with
thyroid dysfunction than in those without in patients with NSCLC
who received pembrolizumab treatment (30). Median duration to onset of
hypothyroidism was 63 and 167 days in patients with and without
cancer cachexia. It was considered that the incidence rate was
lower in patients who did not have cancer cachexia due to a longer
treatment period of chemotherapy including pembrolizumab.
Several limitations of our study warrant mention. It
was conducted under a retrospective design at a single center.
Further, the sample size was too small to allow precise
consideration of confounding factors.
In conclusion, pembrolizumab monotherapy was
associated with poor TTF and OS outcomes in NSCLC patients with
cachexia compared to those without cachexia. Nevertheless, cachexia
did not affect the clinical outcome in NSCLC patients receiving
pembrolizumab plus cytotoxic anticancer agents. Improvement in
cancer cachexia may improve clinical outcomes in patients with
NSCLC treated with pembrolizumab monotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All the datasets generated or analyzed during the
present study are included in this published article.
Authors' contributions
HF and HI conceptualized this study. HF, AA and DKai
acquired the clinical data. HF, AA and HI analyzed data. CH, MK,
MY, JE, TI, KY, YS, TG, CS, DKaw, YK, MF, RK, YO and AS
interpretated the data. YO and AS confirmed the authenticity of all
the raw data. HF, AA and HI drafted the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was carried out in accordance with the
guidelines for human studies adopted by the Ethics Committee of
Gifu University Graduate School of Medicine and the Japanese
Government, and approved by the Medical Review Board of Gifu
University Graduate School of Medicine (approval no. 2021-B050
Institutional Review Board). Informed consent was not obtained
because this was a retrospective observational study. We posted
information about the study and how patients could opt out on the
website of the hospital.
Patient consent for publication
In view of the retrospective nature of the study,
the need for informed consent from subjects was not mandated.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al:
Phase Ⅲ study of afatinib or cisplatin plus pemetrexed in patients
with metastatic lung adenocarcinoma with EGFR mutations. J Clin
Oncol. 31:3327–3334. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shaw AT, Ou SH, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Planchard D, Smit EF, Groen HJM, Mazieres
J, Besse B, Helland Å, Giannone V, D'Amelio AM Jr, Zhang P,
Mookerjee B and Johnson BE: Dabrafenib plus trametinib in patients
with previously treated BRAF (V600E)-mutant metastatic non-small
cell lung cancer: An open-label, multicentre phase 2 trial. Lancet
Oncol. 18:1307–1316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Paik PK, Felip E, Veillon R, Sakai H,
Cortot AB, Garassino MC, Mazieres J, Viteri S, Senellart H, Van
Meerbeeck J, et al: Tepotinib in non-small-cell lung cancer with
MET exon 14 skipping mutations. N Engl J Med. 383:931–943.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wolf J, Seto T, Han JY, Reguart N, Garon
EB, Groen HJM, Tan DSW, Hida T, de Jonge M, Orlov SV, et al:
Capmatinib (INC280) in METΔex14-mutated advanced non-small
cell lung cancer (NSCLC): Efficacy data from the phase Ⅱ GEOMETRY
mono-1 study. J Clin Oncol. 37 (Suppl 15)(9004)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-Positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
National Comprehensive Cancer Network
(NCCN): Clinical Practice Guidelines in Oncology (NCCN
Guidelines®). Non-Small Cell Lung Cancer. Version
5.2021. NCCN, Plymouth Meeting, PA, 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
|
|
14
|
Nakamura Y: Biomarkers for immune
checkpoint inhibitor-mediated tumor response and adverse events.
Front Med (Lausanne). 6(119)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schildhaus HU: Predictive value of PD-L1
diagnostics. Pathologe. 39:498–519. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lagos GG, Izar B and Rizvi NA: Beyond
tumor PD-L1: Beyond tumor PD-L1: Emerging genomic biomarkers for
checkpoint inhibitor immunotherapy. Am Soc Clin Oncol Educ Book.
40:1–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chindaprasirt J: Sarcopenia in cancer
patients. Asian Pac J Cancer Prev. 16:8075–8077. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Roch B, Coffy A, Jean-Baptiste S, Palaysi
E, Daures JP, Pujol JL and Bommart S: Cachexia-sarcopenia as a
determinant of disease control rate and survival in non-small lung
cancer patients receiving immune-checkpoint inhibitors. Lung
Cancer. 143:19–26. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nishioka N, Uchino J, Hirai S, Katayama Y,
Yoshimura A, Okura N, Tanimura K, Harita S, Imabayashi T, Chihara
Y, et al: Association of sarcopenia with and efficacy of
anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. J Clin Med.
8(450)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
U.S. Department of Health and Human
Services, National Institutes of Health National Cancer Institute:
Common Terminology Criteria for Adverse Events (CTCAE). Version
4.0. https://www.eortc.be/services/doc/ctc/. Accessed
September 1, 2018.
|
|
23
|
Shiroyama T, Nagatomo I, Koyama S, Hirata
H, Nishida S, Miyake K, Fukushima K, Shirai Y, Mitsui Y, Takata S,
et al: Impact of sarcopenia in patients with advanced non-small
cell lung cancer treated with PD-1 inhibitors: A preliminary
retrospective study. Sci Rep. 9(2447)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Peixoto da Silva S, Santos JMO, Costa E
Silva MP, Gil da Costa RM and Medeiros R: Cancer cachexia and its
pathophysiology: Links with sarcopenia, anorexia and asthenia. J
Cachexia Sarcopenia Muscle. 11:619–635. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sanders KJ, Hendriks LE, Troost EG,
Bootsma GP, Houben RM, Schols AM and Dingemans AM: Early weight
loss during chemoradiotherapy has a detrimental impact on outcome
in NSCLC. J Thorac Oncol. 11:873–879. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ross PJ, Ashley S, Norton A, Priest K,
Waters JS, Eisen T, Smith IE and O'Brien ME: Do patients with
weight loss have a worse outcome when undergoing chemotherapy for
lung cancers? Br J Cancer. 90:1905–1911. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Petruzzelli M and Wagner EF: Mechanisms of
metabolic dysfunction in cancer-associated cachexia. Genes Dev.
30:489–501. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Flint TR, Janowitz T, Connell CM, Roberts
EW, Denton AE, Coll AP, Jodrell DI and Fearon DT: Tumor-Induced
IL-6 reprograms host metabolism to suppress anti-tumor immunity.
Cell Metab. 24:672–684. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Katakami N, Uchino J, Yokoyama T, Naito T,
Kondo M, Yamada K, Kitajima H, Yoshimori K, Sato K, Saito H, et al:
Anamorelin (ONO-7643) for the treatment of patients with non-small
cell lung cancer and cachexia: Results from a randomized,
double-blind, placebo-controlled, multicenter study of Japanese
patients (ONO-7643-04). Cancer. 124:606–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Osorio JC, Ni A, Chaft JE, Pollina R,
Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok
JD, et al: Antibody-mediated thyroid dysfunction during T-cell
checkpoint blockade in patients with non-small-cell lung cancer.
Ann Oncol. 28:583–589. 2017.PubMed/NCBI View Article : Google Scholar
|