Introduction
Aurora kinases A and B (AURKA) and (AURKB) are
essential for mitosis, though overexpression produces centrosome
amplification, aneuploidy and resistance to apoptosis (1-3).
Across multiple cancer types, including localized bladder cancer
(4-11),
overexpression is associated with poor prognosis and may contribute
to cisplatin resistance (1,12).
We recently investigated the prognostic value of AURKA and AURKB
protein expression in a cohort of muscle-invasive bladder cancer
(MIBC) patients treated with neoadjuvant platinum-based
chemotherapy (NAC), based on the premise that aurora kinase
over-expression may contribute to chemoresistance and poor outcome.
In this cohort, we found that high baseline AURKA levels were
prognostic for inferior relapse-free (HR=3.88, P=0.008) and overall
(HR=6.10, P<0.001) survival in this study. Similar trends were
observed with AURKB expression (13).
A co-regulatory interplay between the tumor
suppressor p53 and the aurora kinases has been previously described
raising the question of whether co-expression patterns may have
prognostic value in cancer (14).
Mutations in the TP53 gene are the most commonly detected somatic
mutation in urothelial bladder cancer (15). Before the widespread use of
next-generation sequencing, detection of p53 nuclear protein
accumulation by immunohistochemistry (IHC) was used as a reliable
proxy for mutant TP53 gene status (16,17).
Multiple retrospective studies have suggested that p53 protein
over-expression may confer an adverse prognosis In localized
bladder cancer (18-20).
However, results from a prospective, randomized trial failed to
validate both the adverse prognostic value of baseline p53
over-expression and the potential predictive value in the context
of adjuvant chemotherapy use (21), so further efforts to utilize p53 as
a biomarker in bladder cancer have been largely abandoned.
Due to the potential co-regulation, the prognostic
value of p53 may be influenced by aurora kinase co-expression
status in bladder cancer and has not yet been reported. Both AURKA
and AURKB phosphorylate p53, which impairs transcriptional activity
leading to chemoresistance (1,12,22).
p53 can reciprocally inhibit AURKA directly through transcriptional
repression or indirectly through expression of p53 target genes
that inhibit AURKA activity (23,24).
The regulatory loop is supported by the observation that mutant p53
is associated with overexpression of AURKA in other tumor settings
(25). p53 target genes are also
implicated in regulation of AURKB stability, suggesting that p53
may be an important global regulator of the aurora kinase family of
proteins (26).
Additional homologues of the p53 gene have also been
discovered and share conserved DNA-binding domains which may
regulate expression of overlapping target genes (27). Little is known about the potential
contribution that p53 family members (p63 and p73) may also play in
regulation of the aurora kinases, but preliminary data suggest that
shared properties may exist (28).
Expression of deltaNp63 (dNp63), a dominant-negative p53 homologue
that can repress transcription of p53 pro-apoptotic target genes,
commonly occurs in urothelial carcinoma but not in benign
urothelium (29-31).
Given the potential for co-regulation between p53 and aurora kinase
family members, we hypothesized that tumor p53 status may impact
the prognostic value of baseline AURKA expression. Since an unmet
need exists for biomarkers to identify which MIBC patients are most
likely to respond to NAC, we conducted a pilot study to determine
whether p53 and p63 expression status may improve the prognostic
value of baseline tumor AURKA expression.
Materials and methods
Patients
Following institutional review board protocol
approval, a cohort of fifty consecutive patients with
muscle-invasive urothelial carcinoma of the bladder who received
neoadjuvant platinum-based chemotherapy followed by radical
cystectomy diagnosed between July 2009 and August 2016 were
retrospectively identified from an institutional tumor bank as
previously described (13).
Eligible patients included those with available archival
pretreatment specimens for study analysis who received neoadjuvant
platinum-based chemotherapy prior to radical cystectomy.
Hematoxylin and eosin stained slides from study cases were reviewed
by two tumor pathologists (CL, AH) to confirm adequate tumor
cellularity for analysis. Formal statistical methods to estimate
sample size were not utilized, because the co-expression rates of
aurora kinase and p53 family members has not been previously
reported and thus assumptions regarding expression rates and
potential clinical outcomes were considered exploratory.
Immunohistochemistry
Formalin-fixed paraffin-embedded tumor blocks from
diagnostic transurethral resection specimens were sectioned at 4
microns on positively charged glass slides and stained with the
following immunohistochemical antibodies: aurora kinase A
(polyclonal ab12875, 1:250 dilution, Abcam), aurora kinase B
(polyclonal ab2254, 1:250 dilution, Abcam), p53 (D07, predilute,
Leica Biosystems), and p63 (4A4, 1:50, Biocare Medical). Control
tissue for all staining runs consisted of tissue microarray cores
of human tonsil and urothelial carcinoma (positive controls) and
normal thyroid (negative control). For both aurora kinase
antibodies, expression was scored based on the percentage of tumor
cells showing positive staining. For both p53 and p63, expression
was scored based on the percentage of tumor cells showing positive
nuclear staining. Tumor overexpression of AURKA and AURKB were
defined as the upper quartiles for this cohort. Overexpression of
p53 and p63 was defined as greater than 10 and 50% of tumor cells
showing positive nuclear staining, respectively. A prognostic
protein overexpression threshold previously defined was utilized
for p53 staining (20). All slides
were scored manually by an experienced surgical pathologist.
Statistics
Survival was calculated from the day of MIBC
diagnosis. Chi-square and phi coefficient tests were used to assess
the relationship between p53/p63 expression and AURKA and AURKB.
Logistic regression models were used to determine the impact of
pre-NAC expression on pathologic staging at cystectomy.
Kaplan-Meier and Cox proportional hazards models were used to
assess relationship with relapse-free and overall survival.
Statistical analyses were performed using SAS version 9.4 (SAS
Institute Inc., Cary, NC).
Results
Co-expression of p53 and aurora kinase
family proteins in MIBC
We have previously reported detailed clinical
patient characteristics and aurora kinase expression patterns for
this patient cohort (13).
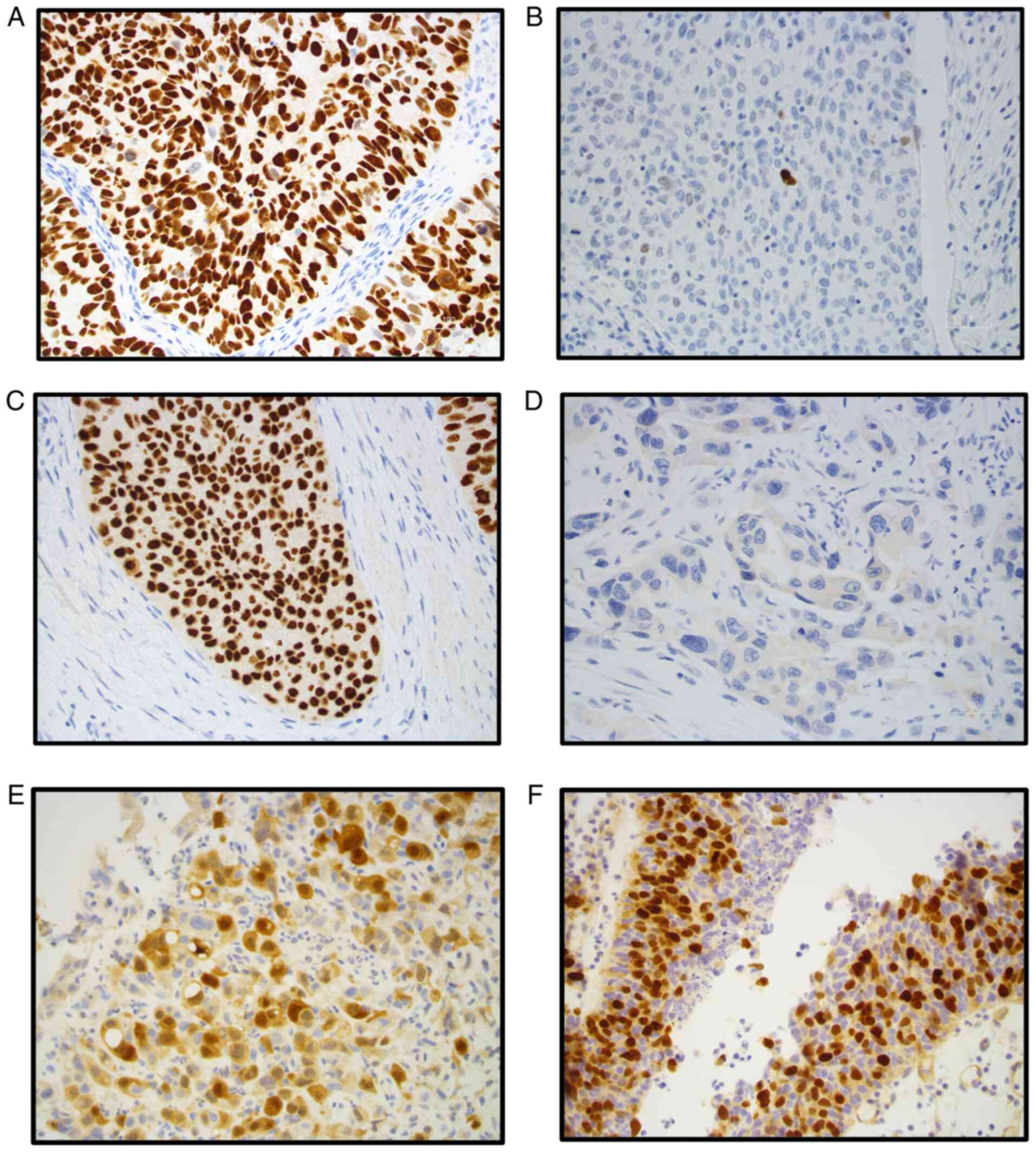
Representative examples of p53, p63, AURKA and AURKB protein
expression from treatment naïve tumor specimens are shown in
Fig. 1. High p53 protein
expression has been demonstrated to correlate with the presence of
an underlying TP53 gene mutation and separately to confer an
adverse prognosis regardless of mutation status (16-18).
High (>10%) p53 protein expression was observed in 33 (66%)
tumors; whereas high (>50%) p63 protein expression was observed
in 40 (80%) tumors.
The relationship of protein co-expression in
pretreatment TURBT specimens was analyzed. Co-expression rates of
p53 and aurora kinase family members is shown in Table I. Most tumors (15/17) with low p53
protein expression demonstrated high p63 expression, (phi=-0.148)
due to the prevalence of p63 overexpression. However, low p53
protein expression levels were not associated with AURKA
(phi=0.190) or AURKB expression (phi=0.075).
 | Table ICo-expression of p53 and aurora kinase
family members. |
Table I
Co-expression of p53 and aurora kinase
family members.
| | p53 | p63 |
|---|
| Aurora kinase | High (n=33) | Low (n=17) | High (n=40) | Low (n=10) |
|---|
| Aurora kinase
A | | | | |
|
High | 6 | 6 | 11 | 1 |
|
Low | 27 | 11 | 29 | 9 |
| Aurora kinase
B | | | | |
|
High | 8 | 3 | 8 | 3 |
|
Low | 25 | 14 | 32 | 7 |
Baseline tumor p53 and aurora kinase
family expression and pathologic response rate
We previously reported that baseline AURKA and AURKB
expression did not predict for pathologic response to NAC; however,
both high baseline AURKA expression and ypT2 or greater tumor stage
predicted for inferior overall survival (OS) in this cohort
(13). In the current study, we
also assessed whether baseline tumor p53 and p63 protein status may
predict for pathologic response. Univariate analysis of baseline
low p53 expression did not predict for pathologic complete response
(pCR) [OR 0.82 (0.211-3.19), P=0.775]; however, baseline low p63
expression was associated with increased likelihood of pCR [OR 7.1,
(1.6-31.8), P=0.011]. Only two of ten patients with low p63
expression also had low p53 expression.
Since the aurora kinases may impair p53 function, we
next examined whether high AURKA or AURKB is associated with the
response to NAC in tumors with low p53 protein expression. High
AURKA or AURKB expression was observed in 6/17 (35%) tumors with
low p53 expression and was not associated with pCR (OR 1.0, 95% CI
0.2-5.1, P=1.0). The potential relationship between p63 and
AURKA/AURKB expression could not be evaluated due to our cohort
size and the high observed rate of p63 overexpression. Only 3/10
tumors with low p63 expression demonstrated high AURKA or AURKB
expression in this cohort.
Baseline tumor p53 and p63 expression
and survival
Prior studies have reported conflicting results
regarding the prognostic value of baseline p53 protein expression
in localized bladder cancer; however, these studies did not examine
the potential confounding impact of deltaNp63 co-expression. We
therefore investigated the prognostic impact of p53 and p63 protein
expression on relapse free survival (RFS) and OS using Cox
proportional hazards models.
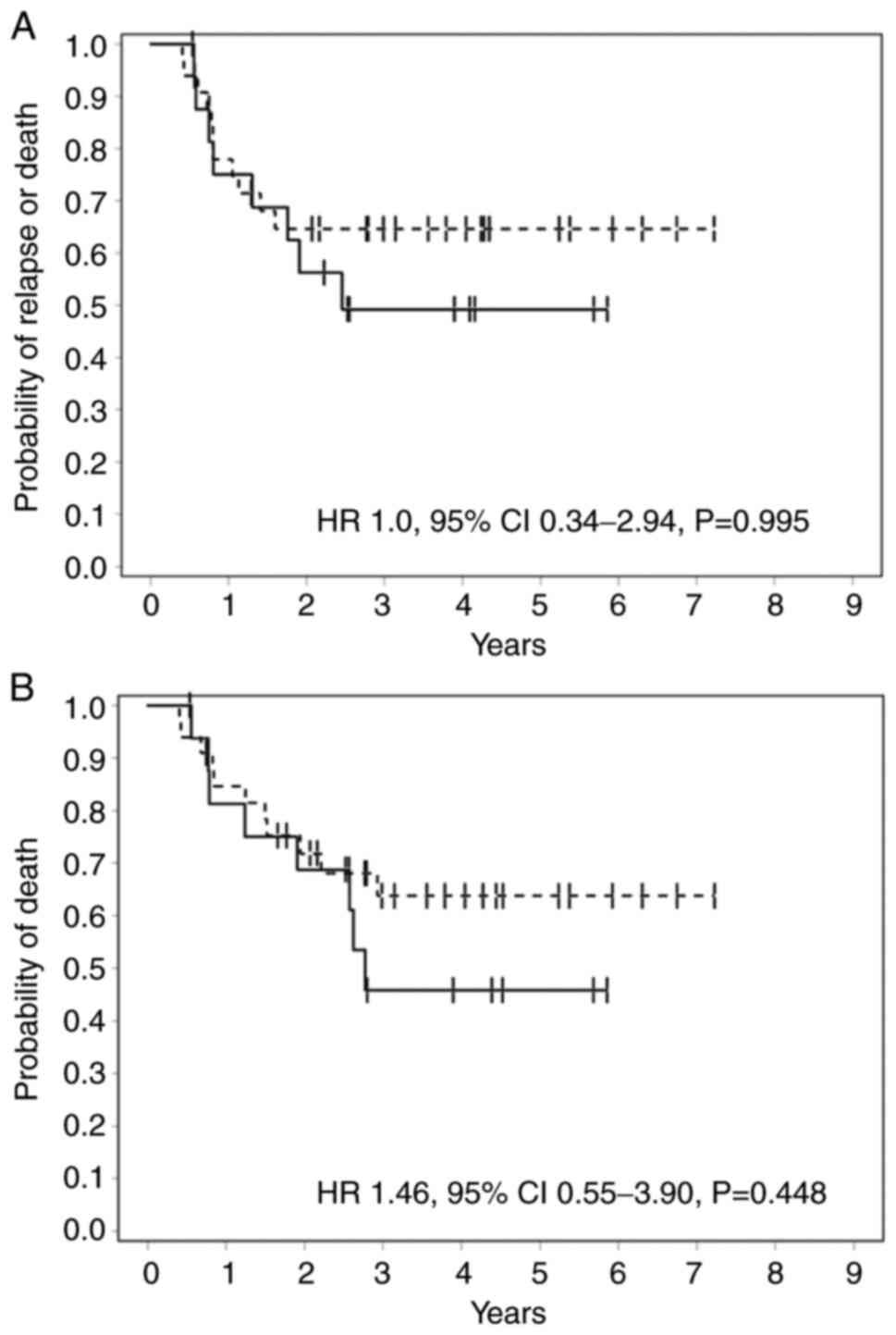
Baseline p53 expression level did not predict for
RFS (HR=1.0 95% CI 0.34-2.94, P=0.995) or OS (1.46 95% CI=0.55-3.9,
P=0.448) in this cohort (Fig. 2).
Low baseline p63 expression did correlate with inferior RFS (HR
4.32, 95% CI 1.3-14.4, P=0.042), though an inferior correlation
with OS was not statistically significant (HR 2.02 95% CI 0.51-8.1,
P=0.312). The association between p63 expression and survival
should be interpreted with caution based on the small cohort size,
since one of the ten patients with low p63 expression was lost to
follow up within two months after cystectomy.
Relationship of aurora kinase and p53
expression on survival
Although we did not observe a relationship between
p53 protein levels and AURKA or AURKB expression, we examined
whether aurora kinase expression may correlate with survival
stratified by p53 status. As anticipated, in the subset of tumors
with low p53 expression (n=17), the presence of either high AURKA
or AURKB expression predicted for an increased risk for relapse (HR
27.1 95% CI=2.7-270.1, P=0.005) and death (HR 14.9 95% CI=2.3-95.6,
P=0.004) compared to tumors with both low AURKA and AURKB. However,
in the subset of tumors with low AURKA expression (n=38), p53
status did not clearly predict for RFS (HR 0.472 95% CI 0.09-2.5,
P=0.373) or OS (HR 0.62, 95% CI 0.2-3.2, P=0.572). To investigate
the relationship further, we performed multivariable analysis, and
after controlling for high p53, high AURKA and AURKB and pathologic
complete response, only baseline high AURKA was an independent
predictor for inferior OS (HR 4.9, 95% CI 1.7-14.1, P=0.003).
Discussion
We present results from the first known analysis of
aurora kinase and p53 family member protein co-expression from a
substantial cohort of patients with MIBC that received standardized
neoadjuvant platinum-based chemotherapy. Independently, aberrant
function of p53 and the aurora kinases have been associated with
resistance to platinum-based chemotherapy (32,33).
Since a co-regulatory feedback loop between wild type p53 and
aurora kinase family members has been established in preclinical
models, we tested whether the expression status of p53 and
homologue p63 could influence the established prognostic value of
AURKA or AURKB. Multivariable analysis confirmed the previously
described adverse prognostic value for baseline high AURKA tumor
expression, independent from p53 protein status [OS (HR 4.9, 95% CI
1.7-14.1, P=0.003)]. In the more favorable prognosis tumors with
low aurora kinase expression, no relationship with p53 status was
observed [OS (HR 0.62, 95% CI 0.2-3.2, P=0.572)] in this cohort.
Our findings support prior observations that univariate analysis of
baseline p53 protein expression does not predict for poor survival
in patients with MIBC treated with peri-operative platinum-based
chemotherapy. Furthermore, the prognostic value of baseline AURKA
expression does not appear to be impacted by p53 status. Regarding
the potential impact of p63 overexpression, formal analysis of the
relationship between p63 and aurora kinase expression could not be
performed due to the censor rate and small patient number with low
p63 expression in this cohort.
While the total number of cases mandates a need for
cautious interpretation of our findings showing lack of prognostic
correlation, the strong positive correlation with baseline AURKA
expression that we report reflects significant underlying biology.
A potential limitation of our study is lack of concurrent analysis
of the alternate p53 homologue, p73(27). Little is known about p73 protein
expression in bladder cancer since widely validated diagnostic
antibodies are not available. However, preliminary studies of RNA
expression suggest a pattern similar to p63, characterized by loss
of the tumor suppressive TAp73 isotypes with increased local tumor
stage and poor outcome (34,35).
We also did not assess TP53 gene mutation
status in these specimens. Since concordance between p53 protein
expression and gene mutation status has been previously reported,
we presumed that low protein expression correlated with wild type
gene status in our study. However, we also chose to analyze p53
protein expression by IHC to mirror the techniques utilized by
earlier studies that implicated a prognostic role for p53
expression analysis in local bladder cancer.
Since we did not observe an obvious correlation
between p53 and aurora kinase expression levels in this study, it
is worth noting that the co-regulatory interplay between the aurora
kinases is best characterized with wild type p53 and may be altered
in the setting of TP53 gene mutations (14). The potential interaction between
the aurora kinases and mutant p53 proteins may vary depending on
the specific TP53 mutation site and resulting impact on the
expressed mutant protein and was not investigated in this study.
The retrospective nature of this study and cohort size may have
confounded results, though consecutive, eligible patients were
selected in effort to mitigate potential selection bias. We did not
perform molecular subtyping to exclude unintentional enrichment of
a particular subtype that may have influenced patient outcomes
(36).
Although we did not detect a prognostic relationship
of p53 and aurora kinase family co-expression in this cohort of
patients who received platinum-based chemotherapy, it remains
unknown whether p53 status may have prognostic value in the setting
of therapeutic aurora kinase inhibition. As monotherapy, the
selective AURKA inhibitor alisertib showed limited efficacy in
advanced urothelial carcinoma (37). However, preclinical studies suggest
tumor cell apoptosis in response to AURKA inhibition may be
dependent upon intact p53 or p73 function (38,39).
Continued study of p53 as a potential predictive biomarker should
be considered to accompany future development of aurora kinase
inhibitors.
Neoadjuvant cisplatin-based chemotherapy prior to
cystectomy remains standard of care in eligible MIBC patients
(40). No predictive biomarker to
identify which patients are most likely to benefit from NAC has
been validated for routine clinical use. We previously showed that
AURKA overexpression correlates with a poor prognosis in this
setting (13), but whether high
AURKA expression is also a predictive marker that identifies
patients unlikely to benefit from NAC is unknown. The aurora
kinases contribute to chemoresistance though regulation of p53
family members in preclinical models. Although we did not find a
prognostic relationship of co-expression of p53/aurora kinase
family members in the present study, the adverse prognostic value
of AURKA was again demonstrated independent from p53 status. These
results underscore the potential importance of AURKA as a possible
biomarker in MIBC patients receiving NAC and support future study
to determine if high baseline AURKA may predict which patients are
likely not to benefit from NAC and thus may be spared from
potential treatment related toxicities in the absence of
anticipated therapeutic benefit.
In summary, our results are consistent with prior
studies that failed to identify prognostic value associated with
p53 protein expression in patients with MIBC who received
peri-operative chemotherapy (21).
Although co-regulatory feedback loops may exist between the aurora
kinase and p53 family members, p53 protein status did not impact
AURKA prognosis in our analysis. These results are contrary to the
relationship hypothesized by preclinical models and suggests that
wild type TP53 gene status does not mitigate the adverse
prognostic value of AURKA expression in MIBC patients (23). However, our results do not rule out
the possibility that expression of the p53 family members may be
relevant biomarkers in the context of therapeutic aurora kinase
inhibition, which may be of greater interest as aurora kinase
inhibitors are tested in the clinical setting. Importantly, these
results support the continued investigation of the potential
predictive value of baseline tumor AURKA expression in patients
with MIBC.
Acknowledgements
Not applicable.
Funding
Funding: Funding for this work was gratefully provided by Mr and
Mrs Don and Betty Anderson, the 5M Power Foundation, the Leon
Levine Foundation through the Atrium Health Foundation (grant no.
2862).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EFB, CL, ST and DR conceived and designed the
present study. HFO provided administrative support. EFB, CL and AH
provided study materials. EFB, CL, ST, HFO and AH collected data
and created the figures and tables. EFB, ST, JZ, PEC, CG and DR
analyzed and interpreted the data. EFB and ST confirm the
authenticity of all the raw data. All authors wrote the manuscript.
All authors have read and approved the final manuscript. The
authors are accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki. The study was approved by the
Genitourinary Scientific Review Committee of the Levine Cancer
Institute of Atrium Health and Advarra IRB (approval No.
Pro00013882), and individual consent for this retrospective
analysis was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Gully CP, Velazquez-Torres G, Shin JH,
Fuentes-Mattei E, Wang E, Carlock C, Chen J, Rothenberg D, Adams
HP, Choi HH, et al: Aurora B kinase phosphorylates and instigates
degradation of p53. Proc Natl Acad Sci USA. 109:E1513–E1522.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nikonova AS, Astsaturov I, Serebriiskii
IG, Dunbrack RL Jr and Golemis EA: Aurora A kinase (AURKA) in
normal and pathological cell division. Cell Mol Life Sci.
70:661–687. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
González-Loyola A, Fernández-Miranda G,
Trakala M, Partida D, Samejima K, Ogawa H, Cañamero M, de Martino
A, Martínez-Ramírez Á, de Cárcer G, et al: Aurora B overexpression
causes aneuploidy and p21Cip1 repression during tumor development.
Mol Cell Biol. 35:3566–3578. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lin ZZ, Jeng YM, Hu FC, Pan HW, Tsao HW,
Lai PL, Lee PH, Cheng AL and Hsu HC: Significance of aurora B
overexpression in hepatocellular carcinoma. Aurora B overexpression
in HCC. BMC Cancer. 10(461)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Compérat E, Bièche I, Dargère D,
Laurendeau I, Vieillefond A, Benoit G, Vidaud M, Camparo P, Capron
F, Verret C, et al: Gene expression study of Aurora-A reveals
implication during bladder carcinogenesis and increasing values in
invasive urothelial cancer. Urology. 72:873–877. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lei Y, Yan S, Ming-De L, Na L and Rui-Fa
H: Prognostic significance of Aurora-A expression in human bladder
cancer. Acta Histochem. 113:514–518. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang J, Li B, Yang Q, Zhang P and Wang H:
Prognostic value of Aurora kinase A (AURKA) expression among solid
tumor patients: A systematic review and meta-analysis. Jpn J Clin
Oncol. 45:629–636. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang D, Huang Y, Huang Z, Weng J, Zhang S
and Gu W: Relation of AURKB over-expression to low survival rate in
BCRA and reversine-modulated aurora B kinase in breast cancer cell
lines. Cancer Cell Int. 19(166)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sen S, Zhou H, Zhang RD, Yoon DS,
Vakar-Lopez F, Ito S, Jiang F, Johnston D, Grossman HB, Ruifrok AC,
et al: Amplification/overexpression of a mitotic kinase gene in
human bladder cancer. J Natl Cancer Inst. 94:1320–1329.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mobley A, Zhang S, Bondaruk J, Wang Y,
Majewski T, Caraway NP, Huang L, Shoshan E, Velazquez-Torres G,
Nitti G, et al: Aurora kinase A is a biomarker for bladder cancer
detection and contributes to its aggressive behavior. Sci Rep.
7(40714)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu J, Zhou J, Xu F, Bai W and Zhang W:
High expression of aurora-B is correlated with poor prognosis and
drug resistance in non-small cell lung cancer. Int J Biol Markers.
33:215–221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Q, Kaneko S, Yang L, Feldman RI,
Nicosia SV, Chen J and Cheng JQ: Aurora-A abrogation of p53 DNA
binding and transactivation activity by phosphorylation of serine
215. J Biol Chem. 279:52175–52182. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Burgess EF, Livasy C, Trufan S, Hartman A,
Guerreri R, Naso C, Clark PE, Grigg C, Symanowski J and Raghavan D:
High aurora kinase expression identifies patients with
muscle-invasive bladder cancer who have poor survival after
neoadjuvant chemotherapy. Urol Oncol. 37:900–906. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sasai K, Treekitkarnmongkol W, Kai K,
Katayama H and Sen S: Functional significance of aurora kinases-p53
protein family interactions in cancer. Front Oncol.
6(247)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Robertson AG, Kim J, Al-Ahmadie H,
Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA,
Akbani R, et al: Comprehensive molecular characterization of
muscle-invasive bladder cancer. Cell. 171:540–556.e25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Esrig D, Spruck CH III, Nichols PW,
Chaiwun B, Steven K, Groshen S, Chen SC, Skinner DG, Jones PA and
Cote RJ: p53 nuclear protein accumulation correlates with mutations
in the p53 gene, tumor grade, and stage in bladder cancer. Am J
Pathol. 143:1389–1397. 1993.PubMed/NCBI
|
|
17
|
George B, Datar RH, Wu L, Cai J, Patten N,
Beil SJ, Groshen S, Stein J, Skinner D, Jones PA and Cote RJ: p53
gene and protein status: The role of p53 alterations in predicting
outcome in patients with bladder cancer. J Clin Oncol.
25:5352–5358. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Esrig D, Elmajian D, Groshen S, Freeman
JA, Stein JP, Chen SC, Nichols PW, Skinner DG, Jones PA and Cote
RJ: Accumulation of nuclear p53 and tumor progression in bladder
cancer. N Engl J Med. 331:1259–1264. 1994.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Garcia del Muro X, Condom E, Vigués F,
Castellsagué X, Figueras A, Muñoz J, Solá J, Soler T, Capellà G and
Germà JR: p53 and p21 expression levels predict organ preservation
and survival in invasive bladder carcinoma treated with a
combined-modality approach. Cancer. 100:1859–1867. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shariat SF, Tokunaga H, Zhou J, Kim J,
Ayala GE, Benedict WF and Lerner SP: p53, p21, pRB, and p16
expression predict clinical outcome in cystectomy with bladder
cancer. J Clin Oncol. 22:1014–1024. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stadler WM, Lerner SP, Groshen S, Stein
JP, Shi SR, Raghavan D, Esrig D, Steinberg G, Wood D, Klotz L, et
al: Phase III study of molecularly targeted adjuvant therapy in
locally advanced urothelial cancer of the bladder based on p53
status. J Clin Oncol. 29:3443–3449. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Katayama H, Sasai K, Kawai H, Yuan ZM,
Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA and Sen
S: Phosphorylation by aurora kinase A induces Mdm2-mediated
destabilization and inhibition of p53. Nat Genet. 36:55–62.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Yang TY, Teng CJ, Lin TC, Chen KC, Hsu SL
and Wu CC: Transcriptional repression of aurora-A gene by wild-type
p53 through directly binding to its promoter with histone
deacetylase 1 and mSin3a. Int J Cancer. 142:92–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shao S, Wang Y, Jin S, Song Y, Wang X, Fan
W, Zhao Z, Fu M, Tong T, Dong L, et al: Gadd45a interacts with
aurora-A and inhibits its kinase activity. J Biol Chem.
281:28943–28950. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Z, Sun Y, Chen X, Squires J,
Nowroozizadeh B, Liang C and Huang J: p53 mutation directs AURKA
overexpression via miR-25 and FBXW7 in prostatic small cell
neuroendocrine carcinoma. Mol Cancer Res. 13:584–591.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Teng CL, Hsieh YC, Phan L, Shin J, Gully
C, Velazquez-Torres G, Skerl S, Yeung SC, Hsu SL and Lee MH: FBXW7
is involved in aurora B degradation. Cell Cycle. 11:4059–4068.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Yang A and McKeon F: P63 and P73: P53
mimics, menaces and more. Nat Rev Mol Cell Biol. 1:199–207.
2000.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Katayama H, Wang J, Treekitkarnmongkol W,
Kawai H, Sasai K, Zhang H, Wang H, Adams HP, Jiang S, Chakraborty
SN, et al: Aurora kinase-A inactivates DNA damage-induced apoptosis
and spindle assembly checkpoint response functions of p73. Cancer
Cell. 21:196–211. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gailey MP and Bellizzi AM:
Immunohistochemistry for the novel markers glypican 3, PAX8, and
p40 (ΔNp63) in squamous cell and urothelial carcinoma. Am J Clin
Pathol. 140:872–880. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karni-Schmidt O, Castillo-Martin M, Shen
TH, Gladoun N, Domingo-Domenech J, Sanchez-Carbayo M, Li Y, Lowe S,
Prives C and Cordon-Cardo C: Distinct expression profiles of p63
variants during urothelial development and bladder cancer
progression. Am J Pathol. 178:1350–1360. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Park BJ, Lee SJ, Kim JI, Lee SJ, Lee CH,
Chang SG, Park JH and Chi SG: Frequent alteration of p63 expression
in human primary bladder carcinomas. Cancer Res. 60:3370–3374.
2000.PubMed/NCBI
|
|
32
|
Cao X, Hou J, An Q, Assaraf YG and Wang X:
Towards the overcoming of anticancer drug resistance mediated by
p53 mutations. Drug Resist Updat. 49(100671)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He S, Feng M, Liu M, Yang S, Yan S, Zhang
W, Wang Z, Hu C, Xu Q, Chen L, et al: P21-activated kinase 7
mediates cisplatin-resistance of esophageal squamous carcinoma
cells with aurora-A overexpression. PLoS One.
9(e113989)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bunch B, Krishnan N, Greenspan RD,
Ramakrishnan S, Attwood K, Yan L, Qi Q, Wang D, Morrison C, Omilian
A, et al: TAp73 expression and P1 promoter methylation, a potential
marker for chemoresponsiveness to cisplatin therapy and survival in
muscle-invasive bladder cancer (MIBC). Cell Cycle. 18:2055–2066.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Puig P, Capodieci P, Drobnjak M, Verbel D,
Prives C, Cordon-Cardo C and Di Como CJ: p73 expression in human
normal and tumor tissues: loss of p73alpha expression is associated
with tumor progression in bladder cancer. Clin Cancer Res.
9:5642–5651. 2003.PubMed/NCBI
|
|
36
|
Seiler R, Ashab HAD, Erho N, van Rhijn
BWG, Winters B, Douglas J, Van Kessel KE, Fransen van de Putte EE,
Sommerlad M, Wang NQ, et al: Impact of molecular subtypes in
muscle-invasive bladder cancer on predicting response and survival
after neoadjuvant chemotherapy. Eur Urol. 72:544–554.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Necchi A, Lo Vullo S, Mariani L, Raggi D,
Giannatempo P, Calareso G, Togliardi E, Crippa F, Di Genova N,
Perrone F, et al: An open-label, single-arm, phase 2 study of the
aurora kinase A inhibitor alisertib in patients with advanced
urothelial cancer. Invest New Drugs. 34:236–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dar AA, Belkhiri A, Ecsedy J, Zaika A and
El-Rifai W: Aurora kinase A inhibition leads to p73-dependent
apoptosis in p53-deficient cancer cells. Cancer Res. 68:8998–9004.
2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tentler JJ, Ionkina AA, Tan AC, Newton TP,
Pitts TM, Glogowska MJ, Kabos P, Sartorius CA, Sullivan KD,
Espinosa JM, et al: p53 family members regulate phenotypic response
to aurora kinase A inhibition in triple-negative breast cancer. Mol
Cancer Ther. 14:1117–1129. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Grossman HB, Natale RB, Tangen CM,
Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF,
Wood DP Jr, Raghavan D and Crawford ED: Neoadjuvant chemotherapy
plus cystectomy compared with cystectomy alone for locally advanced
bladder cancer. N Engl J Med. 349:859–866. 2003.PubMed/NCBI View Article : Google Scholar
|