Introduction
Trifluridine/tipiracil (TAS-102) is an oral
anticancer agent that comprises trifluridine and tipiracil
hydrochloride. Trifluridine exhibits anticancer activity through
its ability to incorporate into the DNA by substitution for
thymidine (1), whereas tipiracil
hydrochloride works as a thymidine phosphorylase inhibitor and
prevents the degradation of trifluridine, which maintains the blood
concentration of trifluridine (1,2). A
global randomized controlled trial of TAS-102 (RECOUSE trial;
https://clinicaltrials.gov/ct2/show/NCT01607957) for
patients with refractory metastatic colorectal cancer (mCRC)
demonstrated that TAS-102 significantly prolonged overall survival
(OS) and progression-free survival (PFS) compared with a
placebo-based treatment (3). In a
phase II trial (C-TASK FORCE; https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000015039)
the combined treatment of TAS-102 with bevacizumab demonstrated
survival benefits for patients with mCRC in a refractory setting.
However, combined treatment of bevacizumab with TAS-102 increased
the frequency of neutropenia of grade 3 or higher up to 77% and
that of febrile neutropenia (FN) up to 16%, necessitating treatment
interruption (3). Thus, adequate
management of neutropenia is critical to ensure the effectiveness
of combined TAS-102 and bevacizumab treatment in prolonging the
survival in salvage lines.
Recombinant human granulocyte colony-stimulating
factors (G-CSFs), including pegfilgrastim and filgrastim, are
widely used to manage severe neutropenia. G-CSFs reduce the
incidence of infection in patients with non-myeloid malignancies
receiving myelosuppressive chemotherapy (4-6).
The National Comprehensive Cancer Network guidelines (7) (NCCN Clinical Practice Guidelines in
Oncology; NCCN Guidelines®; Myeloid Growth Factors
Version 2. 2017) recommend prophylactic use of G-CSFs in patients
with cancer, based on the chemotherapy regimen and patient-related
risk factors, particularly for the high- (>20%) and
intermediate-risk (10-20%) groups (8). In the C-TASK FORCE trial, 16% of
patients were reported to develop FN, which indicated that the
combined treatment of TAS-102 with bevacizumab harbors an
intermediate risk of FN (9).
Pegfilgrastim is a long-acting pegylated form of G-CSF with a
sustained duration of action, and a single dose is comparable to
daily injections of filgrastim (5 g/kg/day) for 10-11 days
(10). Furthermore, pegfilgrastim
reduced the incidence of FN in patients with advanced colorectal
cancer who received FOLFOX (or FOLFIRI) plus bevacizumab (11).
The present study aimed to further elucidate the
benefits of the prophylactic use of pegfilgrastim for severe
neutropenia, and verified the efficacy and safety of the combined
treatment of TAS-102 with bevacizumab.
Materials and methods
Patients
A total of 35 patients with mCRC, including 16 males
and 19 females, were recruited for the present retrospective
analysis. The median age of the patients was 69 years (range, 29-80
years) and their Eastern Cooperative Oncology Group (ECOG)
performance status (PS) was used for an indicator of general
condition. Patients with a PS of 0, 1 or 2 were recruited for the
present retrospective analysis. Numerous previous treatments,
including oxaliplatin, irinotecan, and 5-Fluorouracil were
acceptable. The patients were treated with TAS-102 plus bevacizumab
between April 2016 and December 2020 at Saitama Medical Center,
Jichi Medical University (Saitama, Japan). Patients did not receive
TAS-102 plus bevacizumab if they exhibited uncontrollable
hypertension, a history of thrombosis or embolism within the 6
months, or a history of gastrointestinal perforation or severe
hemorrhage.
The present study was approved by the Research
Ethics Committee of Jichi Medical University (approval no. R19-30;
Saitama, Japan) and conducted in accordance with the principles of
The Declaration of Helsinki. Written informed consent was obtained
from all participants before administering chemotherapy, in
accordance with the guidelines of the Jichi Medical University
Institutional Review Board.
Treatment schedule
The patients received treatment according to a
28-day regimen of the C-TASK FORCE, in which 35 mg/m2 of
TAS-102 was administered orally twice daily on days 1-5 and 8-12 in
the 28-day cycle. Moreover, 5 mg/kg of bevacizumab was administered
by intravenous infusion for 30 min every 2 weeks, on days 1 and 15.
The patients also received a single subcutaneous pegfilgrastim
injection of 3.6 mg on day 15 of every 28-day cycle. The median
number of treatment cycles were 11 (range, 2-32 cycles). Treatment
was continued until the disease progressed, levels of unacceptable
toxicity were reached, ECOG PS deteriorated to >2 or patient
consent was withdrawn.
Efficacy and safety assessment
The incidence of adverse events (AEs), disease
control rate (DCR), progression-free survival (PFS) and overall
survival (OS) were assessed. DCR was defined as the percentage of
patients who have achieved complete response (CR), partial response
(PR) and a stable disease status following therapeutic
intervention. PFS was defined as the length of time from the start
of TAS-102 plus bevacizumab treatment to either disease progression
or death. OS was defined as the interval from the start of TAS-102
plus bevacizumab treatment to death from any cause. Tumors were
evaluated every 2 or 3 months using computed tomography (CT)
scanning or positron emission tomography/CT imaging for initial
tumor staging. Tumor response and progression were evaluated
according to the Response Evaluation Criteria in Solid Tumors
(version 1.1) (12). AEs were
graded according to the Common Terminology Criteria for Adverse
Events (version 4.0) (13).
Treatment was continued until the disease progressed, levels of
unacceptable toxicity were reached, ECOG PS deteriorated to >2
or patient consent was withdrawn. The median follow-up period was
13.1 months (range, 2.1-35.2 months).
Statistical analysis
Statistical analyses were performed using StatView
5.0.1 (SAS Institute Inc.). The OS and PFS curves were analyzed
using the Kaplan-Meier method, and intergroup differences were
compared using the log-rank test. Data are presented as the median
and range. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics and
treatment
The characteristics of the 35 patients, including 16
males and 19 females, are displayed in Table I. The median age of the patients
was 69 years (range, 29-80 years). ECOG PS 0 was observed in 16
patients, PS 1 was observed in 15 patients and PS 2 in 4 patients.
All patients were treated with at least one regimen before
receiving TAS-102 plus bevacizumab. All patients started at the
full dose of TAS-102 and received 3.6 mg pegfilgrastim for primary
prophylaxis. The median follow-up period was 13.1 months (range,
2.1-35.2 months). No dose modification was performed using
bevacizumab. A total of 23 patients received pegfilgrastim at day
15 of the first 28-day cycle, 8 patients received it in the second
cycle, 3 in the third cycle and 1 in the fifth cycle. The treatment
time course within 12 months in 35 patients is displayed in
Fig. 1, and includes the number of
leucocytes and neutrocytes before and during the treatment with
TAS-102 plus bevacizumab. The time course and use of pegfilgrastim
is displayed in Fig. 2.
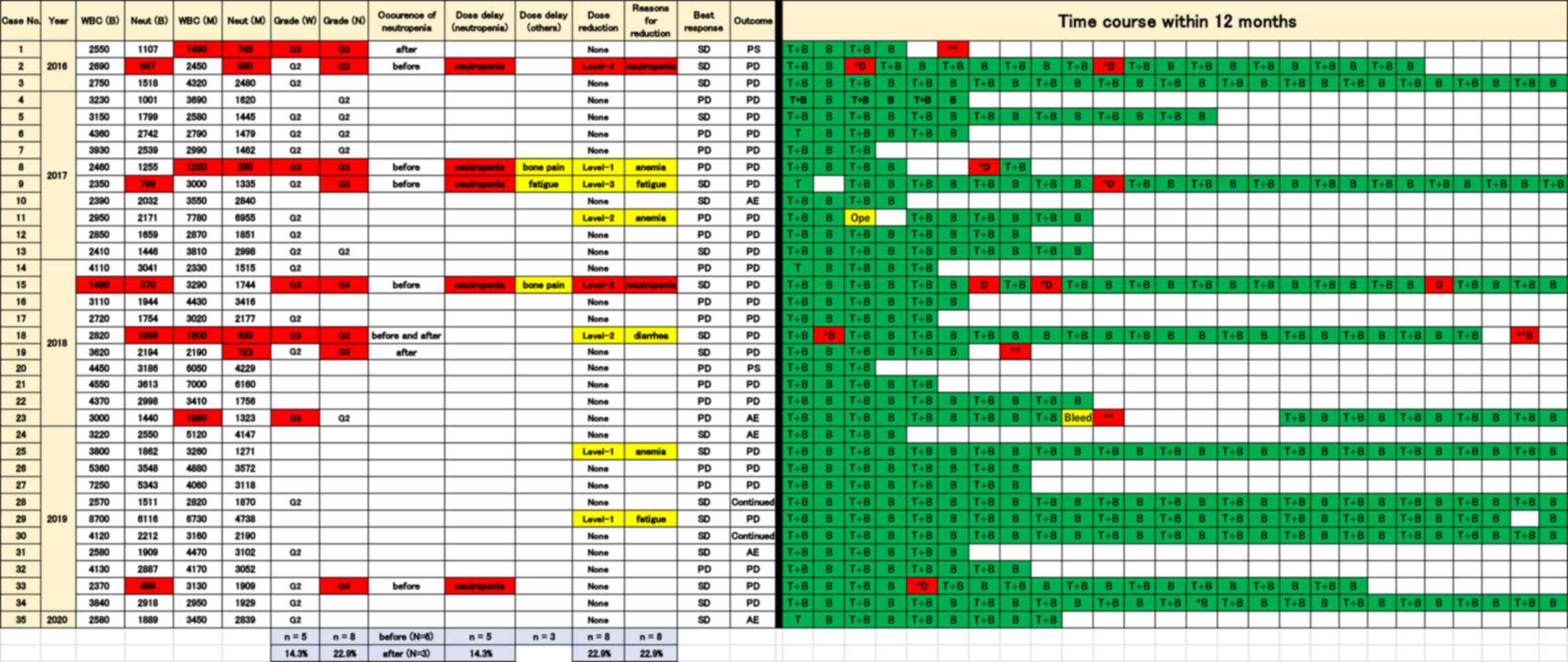 | Figure 1Time course of the treatments within
12 months in connection with safety, adverse events and efficacy in
35 patients treated with TAS-102 plus bevacizumab. Safety, adverse
events and efficacy in 35 patients are shown on the left side of
the figure, and the time course of the treatments within 12 months
is shown on the right. The Y-axis indicates patients ordered by
year of chemotherapy. The X-axis in the time course indicates the
treatment procedure during chemotherapy. WBC (B), the number of
leucocytes before the treatment of pegfilgrastim; Neut (B), the
number of neutrocytes before the treatment of pegfilgrastim; WBC
(M), minimum number of leucocytes before the treatment of
pegfilgrastim; Neut (M), minimum number of neutropenia before the
treatment of pegfilgrastim; Grade (W), grade of leukopenia; Grade
(N), grade of neutropenia; Continued; chemotherapy is continued; T
+ B, TAS-102 + bevacizumab; B, bevacizumab; *In red,
grade 3 or worse neutropenia [corresponding numbers of leucocytes
and neutrocytes are shown in the WBC (B), WBC (B), WBC (M) and Neut
(M) in red when these adverse events occurred]; **In
red, grade 3 or worse neutropenia occurred after discontinuation of
treatments with TAS-102 plus bevacizumab; B in red, treatment with
bevacizumab without dose delay. Ope, operation; Bleed, bleeding;
SD, stable disease; PD, progressive disease; PS, performance
status; AE, adverse event; D, dose delay; TAS-102,
trifluridine/tipiracil; WBC, white blood cell; Neut,
neutrocyte. |
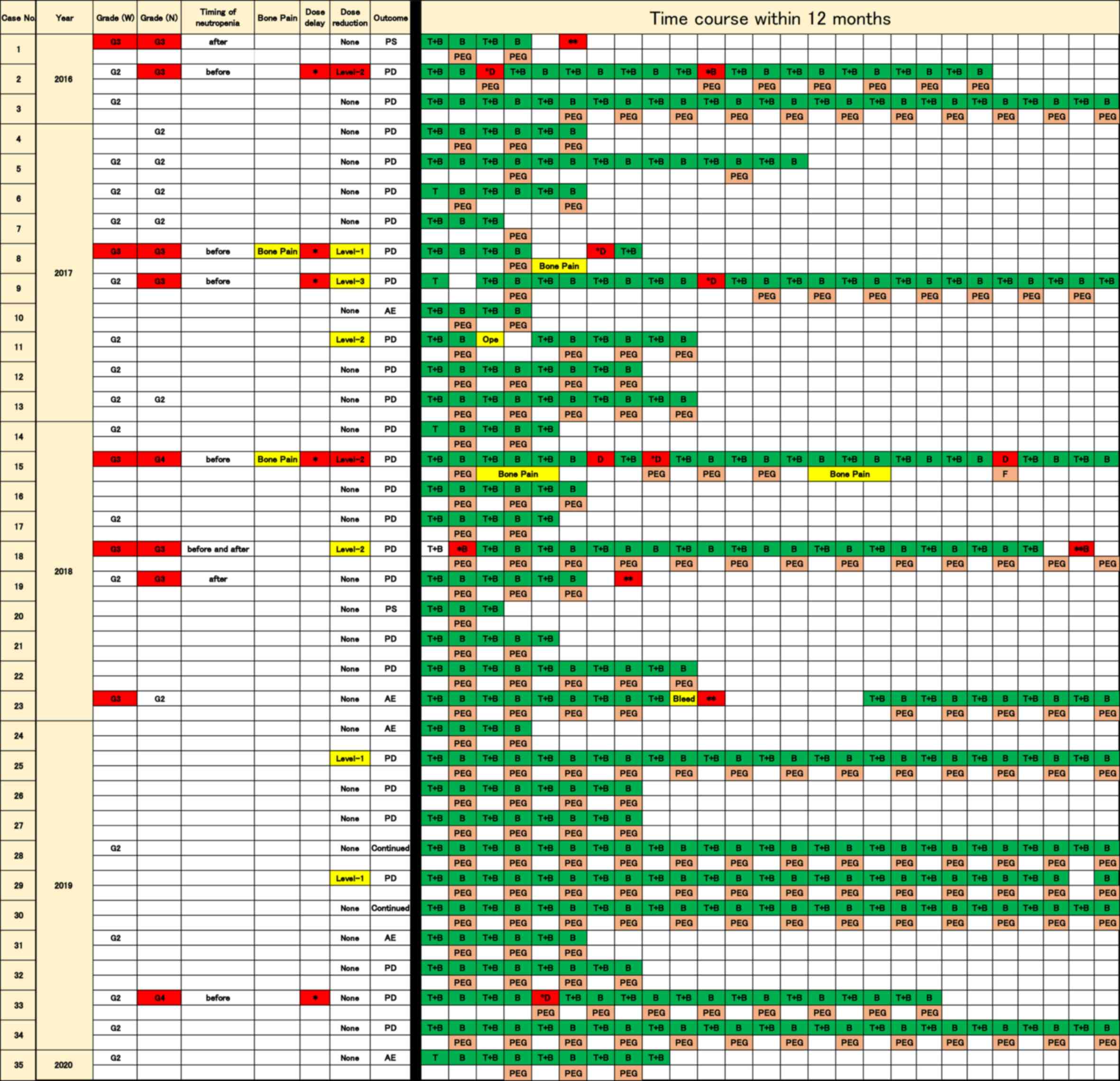 | Figure 2Time course and treatment of
pegfilgrastim within 12 months in 35 patients treated with TAS-102
plus bevacizumab. Safety, adverse events and efficacy in 35
patients are shown on the left side of the figure and the time
course of the treatments within 12 months is shown on the right.
The Y-axis indicates patients ordered by year of chemotherapy. The
X-axis in the time course indicates the treatment procedure during
chemotherapy. Grade (W), grade of leukopenia; Grade (N), grade of
neutropenia; Continued; chemotherapy is continued; T + B, TAS-102 +
bevacizumab; B, bevacizumab; *In red, grade 3 or higher
neutropenia; **In red, grade 3 or higher neutropenia
occurred after discontinuation of treatment with TAS-102 plus
bevacizumab; B in red, treatment with bevacizumab without dose
delay. Ope, operation; F, filgrastim; Bleed, bleeding; PD,
progressive disease; PS, performance status; AE, adverse event;
PEG, pegfilgrastim; D, dose delay; TAS-102,
trifluridine/tipiracil. |
 | Table ICharacteristics of patients. |
Table I
Characteristics of patients.
| Characteristic | Value |
|---|
| Median age (range),
years | 69 (29-80) |
| Sex, n | |
|
Male | 16 |
|
Female | 19 |
| ECOG PS, n | |
|
0 | 16 |
|
1 | 15 |
|
2 | 4 |
| Primary site of
tumor, n | |
|
Right-sided
colon | 7 |
|
Left-sided
colorectum | 28 |
| Primary lesion
resection, n | |
|
Yes | 28 |
|
No | 7 |
| Metastatic organs,
n | |
|
1 | 18 |
|
2 | 16 |
|
≥3 | 1 |
| KRAS mutation,
n | |
|
No,
wild-type | 25 |
|
Yes,
mutant | 10 |
| Number of previous
therapies, n | |
|
1 | 4 |
|
2 | 15 |
|
3 | 8 |
|
4 | 3 |
|
5 | 5 |
| Prior systemic
anticancer agents, n | |
|
Fluoropyrimidine | 35 |
|
Irinotecan | 33 |
|
Oxaliplatin | 34 |
|
Bevacizumab | 31 |
|
Anti-EGFR
monoclonal antibody | 15 |
|
Regorafenib | 2 |
Safety and AEs
AEs are summarized in Table II. The most common AEs
(experienced in ≥30% patients) of any grade were leukopenia,
neutropenia, anemia, fatigue, hypertension, anorexia, nausea and
diarrhea. The most frequent AEs of grade 3 or worse were leukopenia
(n=5, 14.3%), neutropenia (n=8, 22.9%), anemia (n=5, 14.3%) and
bleeding (n=1, 2.9%). FN was seen in 1 patient (2.9%), but it
occurred before the patient received pegfilgrastim. A total of 2
patients (5.7%) with grade 3 neutropenia required an antiemetic
drug. No treatment-related deaths occurred.
 | Table IIAdverse events affecting patients
during the study treatment period. |
Table II
Adverse events affecting patients
during the study treatment period.
| A, Hematological
adverse events |
|---|
| Adverse event | Any grade, n
(%) | Grade 3/4, n
(%) |
|---|
| Leucopenia | 26 (74.3) | 5 (14.3) |
| Neutropenia | 22 (62.9) | 8 (22.9) |
| Anemia | 23 (65.7) | 5 (14.3) |
|
Thrombocytopenia | 15 (42.9) | 0 (0.0) |
| B,
Non-hematological adverse events |
| Adverse event | Any grade, n
(%) | Grade 3/4, n
(%) |
| Fever | 4 (11.4) | 1 (2.9) |
| Febrile
neutropenia | 1 (2.9) | 1 (2.9) |
| Fatigue | 31 (88.6) | 3 (8.6) |
| Hypertension | 11 (31.4) | 0 (0.0) |
| Anorexia | 16 (45.7) | 1 (2.9) |
| Nausea | 24 (68.6) | 2 (5.7) |
| Vomiting | 7 (20.0) | 0 (0.0) |
| Diarrhea | 24 (68.6) | 3 (8.6) |
| Constipation | 9 (25.7) | 1 (2.9) |
| Proteinuria | 29 (82.9) | 1 (2.9) |
| Bone pain | 4 (11.4) | 2 (5.7) |
| Bleeding | 0 (0.0) | 1 (2.9) |
Neutropenia at grade 3 or worse was identified in 8
patients (22.9%) prior to receiving pegfilgrastim or following
discontinuation of pegfilgrastim administration. A total of 6
patients (17.1%) exhibited symptoms before receiving pegfilgrastim
(case 2, 8, 9, 15, 18 and 33; ‘before’ in occurrence of neutropenia
of Fig. 1). Each case is indicated
by a red asterisk (*) of time course in Figs. 1 and 2. A total of 3 patients (8.6%) displayed
symptoms after discontinuing pegfilgrastim. Case 1 and 19 exhibited
grade 3 neutropenia following the discontinuation of treatment, due
to worsened PS and progression in disease, respectively. Case 18
demonstrated grade 3 neutropenia both prior to receiving
pegfilgrastim and after discontinuation of pegfilgrastim. Moreover,
case 23 displayed grade 3 leukopenia after discontinuation of
treatment due to bleeding. Each case is delineated by a red double
asterisk (**) of time course in Figs. 1 and 2. Patients who received pegfilgrastim for
primary prophylaxis did not exhibit severe neutropenia.
Dose delays due to neutropenia during the treatment
period were required in 5 patients [14.3%; neutropenia in dose
delay (neutropenia) in Fig. 1].
These neutropenia-induced drug delays appeared in patients before
they received pegfilgrastim or after discontinuation of
pegfilgrastim administration. The time course of 5 patients who
required dose delays before taking pegfilgrastim are shown in cases
2, 8, 9, 15 and 33. Each case is shown as ‘D’ in red of time course
in Figs. 1 and 2. Among these patients, cases 2 and 15
required dose delays due to the discontinuation of pegfilgrastim.
Dose delays due to non-hematological AEs during the treatment
period were required in 3 patients [8.6%; dose delay (others) in
Figs. 1 and 2]. A total of 2 patients (5.7%)
demonstrated grade 3 bone pain (cases 8 and 15), which resulted in
the discontinuation of pegfilgrastim (Figs. 1 and 2). Grade 3 bone pain prevented
sustainable administration of pegfilgrastim in case 15. The time
course with the treatment of pegfilgrastim is displayed in Fig. 2. Grade 3 neutropenia occurred when
the patient was not undergoing pegfilgrastim treatment, which
resulted in dose delays and administration of filgrastim (‘F’ in
time course in case 15 of Fig. 2).
A total of 1 patient displayed grade 3 fatigue, resulting in a dose
delay (case 9). Patients who received regular administration of
pegfilgrastim for primary prophylaxis did not exhibit dose delays.
Although sustainable administration of pegfilgrastim was not
sufficiently achieved in the years after the introduction of
pegfilgrastim, improvements have been observed since the middle of
2018 (Fig. 2).
A total of 8 patients (22.8%) required a dose
reduction due to adverse events; namely, anemia (3 patients),
neutropenia (2 patients), fatigue (2 patients) and diarrhea (1
patient; reasons for dose reduction in Fig. 1). Although 2 patients required a
dose reduction of TAS-102 due to neutropenia while they were not
taking pegfilgrastim, no further dose reduction was required after
taking pegfilgrastim (cases 2 and 15 in Fig. 2). A total of 2 patients required a
dose reduction due to anemia, as shown in cases 8 and 11 (‘anemia’
in reasons for dose reduction in Figs.
1 and 2). The relative dose
intensity was 96.8% (65.0-100.0%). A total of 24 patients (77.2%)
received 1-3 more subsequential chemotherapy regimens, whereas 8
patients (22.8.%) were treated with the best supportive care to
improve quality of life without chemotherapy.
Efficacy
No patients exhibited CR or PR; however, 19 patients
(54.3%) exhibited a stable disease state. The DCR was 54.3%. A
total of 16 patients (45.7%) exhibited progressive disease (PD).
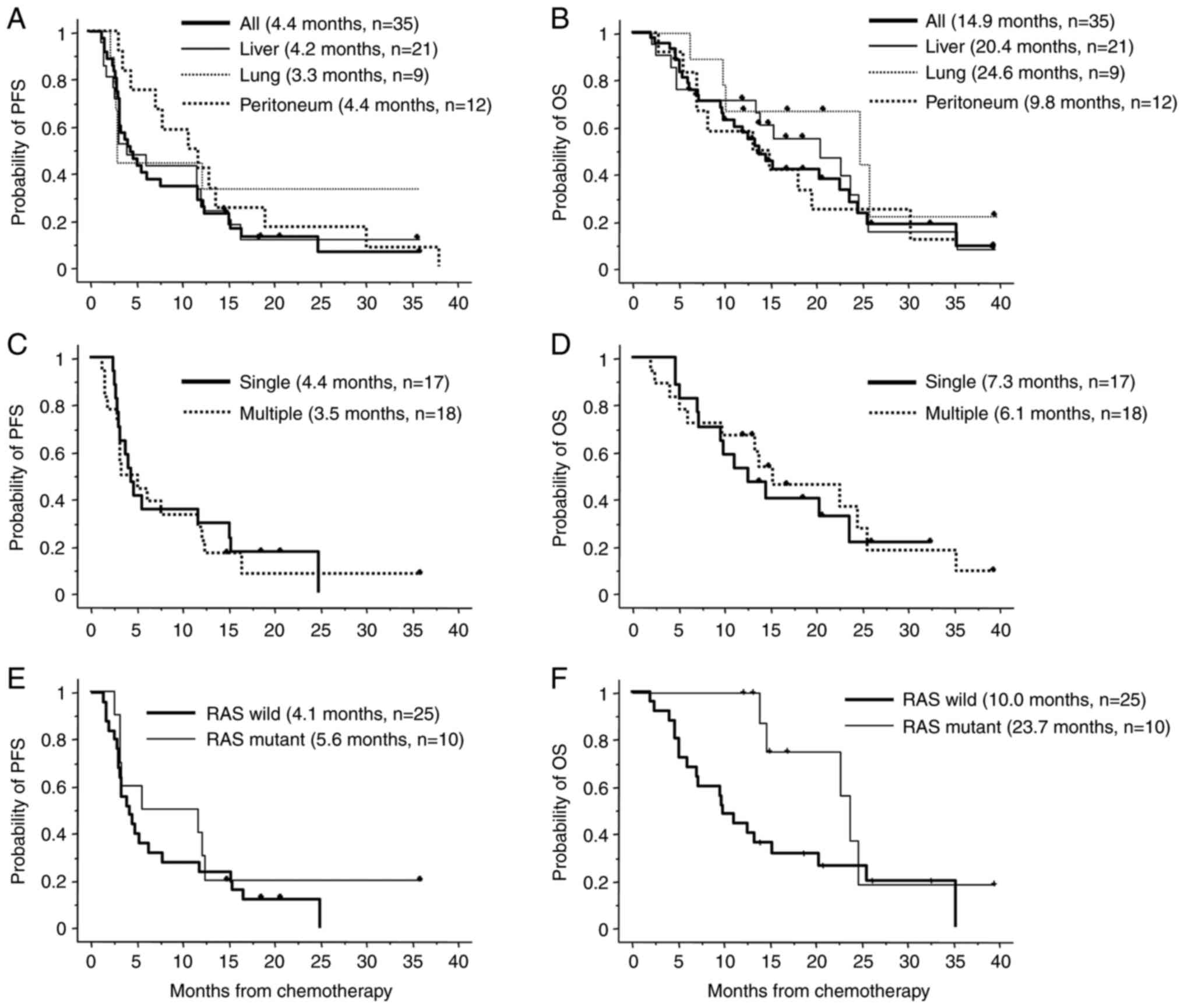
The median PFS period was 4.4 months (3.3-7.1 months; Fig. 3A). The median OS was 14.9 months
(9.9-24.0 months; Fig. 3B). The
association between metastatic sites and PFS revealed no
significant difference in patients with liver (n=21), lung (n=9) or
peritoneum metastasis (n=12; 4.2, 3.3 and 4.4 months, respectively;
Fig. 3A). With regards to OS,
patients with peritoneum metastasis exhibited poor outcomes, but
there was no significant difference between OS and patients with
liver, lung or peritoneum metastasis (20.4, 24.6 and 9.8 months,
respectively; Fig. 3B). In
addition, a significant difference was not identified between PFS
(4.4 and 3.5 months) or OS (7.3 and 6.1 months) and patients with
single or multiple metastases, respectively (Fig. 3C and D).
Regarding the kirsten rat sarcoma virus oncogene
homolog (KRAS) status and its association with treatment
outcomes, the median PFS was 4.1 months (1.4-24.9 months) in 25
patients with wild-type KRAS and 5.6 months (2.8-36.1
months) in 10 patients with mutant RAS (Fig. 3E). In 25 patients with wild-type
KRAS, the median OS was 10.0 months (2.1-35.4 months) and in
patients with mutant KRAS, the median OS was 23.7 months
(12.3-36.1 months; Fig. 3F). There
was no significant difference between PFS or OS and patients with
or without KRAS mutations.
Discussion
The present study demonstrated the benefits of
pegfilgrastim in patients receiving TAS-102 plus bevacizumab for
the management of neutropenia. Prophylactic use of pegfilgrastim
prevented dose delays or dose reductions of TAS-102, resulting in
improved survival time in the salvage line. To the best of our
knowledge, the present retrospective analysis is the first to
demonstrate the potential prophylactic use of pegfilgrastim for
appropriate management of neutropenia in patients receiving TAS-102
plus bevacizumab.
Neutropenia is a major AE associated with
chemotherapy in patients with advanced CRC (14,15).
The additional use of bevacizumab in chemotherapy increases the
risk of all and high-grade neutropenia (16). Results of previous studies
demonstrated that among patients receiving TAS-102, grade 3 or
higher neutropenia was reported to be the most frequent AE
experienced (3,9). TAS-102 monotherapy induced grade 3 or
higher neutropenia in 38% of patients in the RECOUSE trial
(3), and TAS-102 plus bevacizumab
induced grade 3 or higher neutropenia in 72% of the patients in the
C-TASK FORCE trial (9). Fujii
et al (17) demonstrated
that TAS-102 plus bevacizumab treatment was associated with a
higher risk of neutropenia when compared with TAS-102 monotherapy,
suggesting that TAS-102 plus bevacizumab is required for adequate
management of neutropenia. Results of the present study
demonstrated that grade 3 or higher neutropenia was most frequently
observed in 22.9% of patients, which was lower than the 72%
incidence rate in patients receiving TAS-102 plus bevacizumab in
the C-TASK FORCE trial, and comparable to the 38% incidence rate in
those receiving TAS-102 monotherapy in the RECOUSE trial (3). Moreover, neutropenia was not observed
in patients treated with pegfilgrastim. The present study
identified 3 patients with grade 3 neutropenia or leucopenia after
discontinuing pegfilgrastim, due to worsening PS, PD and AE;
therefore, close management of neutropenia is required following
the discontinuation of treatment.
The incidence of FN has been reported to increase
with regimens containing bevacizumab (18). The pegfilgrastim and anti-vascular
endothelial growth factor Evaluation Study trial (the PAVES trial;
https://clinicaltrials.gov/ct2/show/NCT00911170) was
conducted to evaluate the effect of pegfilgrastim on the incidence
of grade 3/4 FN in patients with locally advanced CRC or mCRC
receiving bevacizumab combined with first-line FOLFOX or FOLFIRI
(11). Grade 3/4 FN was observed
in 2.4% of the patients who received pegfilgrastim and in 5.7% of
those who received a placebo. Thus, the incidence of grade 3/4 FN
declined by >50% following administration of pegfilgrastim. The
odds ratio of 0.41 calculated in the PAVES trial indicated that the
risk of FN was reduced to 59%. In comparison, TAS-102 plus
bevacizumab increased the risk of FN by up to 16% in the C-TASK
FORCE trial. In the present study, 1 patient (2.9%) experienced FN
following treatment with TAS-102 plus bevacizumab, which occurred
prior to administration of pegfilgrastim.
Dose intensity has been reported to be an important
factor influencing treatment outcomes. A high relative dose
intensity (RDI) with a threshold of 85% has been identified as an
independent factor for improving outcomes in different cancers,
including breast cancer, lymphoma (19) and CRC (20,21).
G-CSF supports the maintenance of a high RDI of myelosuppressive
chemotherapy. The patients in the present study exhibited a high
RDI of 96.8%, owing to the prophylactic use of pegfilgrastim. A
total of 8 patients (22.8%) required at least one dose reduction of
TAS-102 in the present study. This was similar to the findings
observed in the C-TASK FORCE trial (24%), although the RDI of 96.8%
observed in the present study was higher than that in the C-TASK
FORCE trial (81.3%). Neutropenia-induced dose delays were observed
during the treatment period in 84% of the patients in the C-TASK
FORCE trial, whereas 5 patients (14.3%) required dose delays during
the treatment period, owing to the prophylactic use of
pegfilgrastim in the present study. Furthermore, no dose delays
were observed when pegfilgrastim was administered.
An improvement of treatment outcomes was expected in
the present study due to the intensification of dose intensity and
prevention of dose delays. Results of the present study
demonstrated a longer OS (14.9 months) compared with that in the
C-TASK FORCE trial (11.4 months), whereas the PFS of 4.4 months was
lower than that in the C-TASK FORCE trial (5.7 months). Moreover,
the present study included 4 patients with a PS of 2, and these
patients exhibited a shorter PFS. The exclusion of these 4 patients
resulted in a PFS of 5.3 months (data not shown), suggesting that
PS was an important factor that influenced the selection of
patients more likely to benefit from TAS-102 plus bevacizumab.
However, stable conditions without severe neutropenia and drug
interruption may have contributed to the subsequential treatments
of TAS-102 plus bevacizumab, which would have resulted in the
prolonged OS (14.9 months) in the present study.
Bone pain is a pegfilgrastim-induced clinical problem
that may result in discontinuation of pegfilgrastim and lead to less
effective chemotherapy dosing (22). Kirshner et al (22) reported an overall pain incidence of
59%, with 24% of the patients experiencing severe bone pain. In the
present study, two patients (5.7%) experienced grade 3 bone pain,
which resulted in the discontinuation of pegfilgrastim, suggesting
that interventions for pegfilgrastim-induced bone pain are required.
Non-steroidal anti-inflammatory drugs (NSAIDs) have been reported to
be effective in preventing or decreasing the incidence and/or
severity of this pegfilgrastim-induced bone pain (22); therefore, NSAIDs were administered
to those who experienced bone pain. However, after severe bone pain
occurs, patients may refuse to continue taking pegfilgrastim;
therefore, detailed information should be provided to patients to
facilitate management of pegfilgrastim-induced bone pain with
NSAIDs. As an alternative to NSAIDs, loratadine (an antihistamine)
should be considered to help prevent bone pain in patients
receiving chemotherapy and pegfilgrastim. This is due to high
levels of tolerability, ease of administration and other potential
benefits (23).
The present study demonstrated promising results
with the use of pegfilgrastim for adequate management of
neutropenia in patients undergoing treatment with TAS-102 plus
bevacizumab. The absence of treatment interruptions may preserve
the patients' stable condition, facilitating subsequent treatment
and improving OS.
Several limitations of the present analysis must be
acknowledged. Namely, the study was conducted with a retrospective
design at a single center. Moreover, all enrolled patients with
mCRC were Japanese, and the sample size was small; thus, patient
diversity was lacking. In addition, pegfilgrastim was not
administered to all patients during the first 28-day cycle, and
24.3% of patients received pegfilgrastim during the second cycle or
later. Considering these limitations, the findings of the present
study may require further verification in a large-scale prospective
study.
Prophylactic use of pegfilgrastim enabled the
management of severe neutropenia without causing dose delays in
patients with mCRC treated with TAS-102 plus bevacizumab. The
appropriate management of neutropenia contributed to an improved
survival time in the salvage line. Although future studies are
required to draw definitive conclusions, the present study may
provide a novel theoretical basis for the use of further strategies
to circumvent severe neutropenia in patients receiving combination
treatment of TAS-102 with bevacizumab. Thus, this may act as a
potential treatment option to prolong survival in salvage line
therapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology (grant no. JP 16K10514) and the JKA
Foundation through its promotion funds from the Keirin Race (grant
no. 27-1-068).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. ST and KS confirm the authenticity of all the raw
data.
Authors' contributions
All the authors contributed to the study design. ST
and KS drafted the manuscript and analyzed the data. ST and HI
performed the experiments. All other authors (YK, RM, IA, YE, NK,
FW, KF, MS, ST, YM and TR) contributed to sample collection, data
collection and interpretation and manuscript review. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Jichi Medical University (approval no. R19-30;
Saitama, Japan) and conducted in accordance with the principles of
The Declaration of Helsinki. Written informed consent was obtained
from all participants before administering chemotherapy, in
accordance with the guidelines of the Jichi Medical University
Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tanaka N, Sakamoto K, Okabe H, Fujioka A,
Yamamura K, Nakagawa F, Nagase H, Yokogawa T, Oguchi K, Ishida K,
et al: Repeated oral dosing of TAS-102 confers high trifluridine
incorporation into DNA and sustained antitumor activity in mouse
models. Oncol Rep. 32:2319–2326. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fukushima M, Suzuki N, Emura T, Yano S,
Kazuno H, Tada Y, Yamada Y and Asao T: Structure and activity of
specific inhibitors of thymidine phosphorylase to potentiate the
function of antitumor 2'-deoxyribonucleosides. Biochem Pharmacol.
59:1227–1236. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Crawford J, Dale DC and Lyman GH:
Chemotherapy-induced neutropenia: Risks, consequences, and new
directions for its management. Cancer. 100:228–237. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lyman GH, Kuderer N, Greene J and Balducci
L: The economics of febrile neutropenia: Implications for the use
of colony-stimulating factors. Eur J Cancer. 34:1857–1864.
1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lyman GH and Kuderer NM: Filgrastim in
patients with neutropenia: Potential effects on quality of life.
Drugs. 62 (Suppl 1):S65–S78. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Network NCC: National Comprehensive Cancer
Network guidelines. 2022.
|
|
8
|
Crawford J, Becker PS, Armitage JO,
Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I,
Griffiths EA, et al: Myeloid growth factors, version 2.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:1520–1541. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kuboki Y, Nishina T, Shinozaki E, Yamazaki
K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T,
Mochizuki N, et al: TAS-102 plus bevacizumab for patients with
metastatic colorectal cancer refractory to standard therapies
(C-TASK FORCE): An investigator-initiated, open-label, single-arm,
multicentre, phase 1/2 study. Lancet Oncol. 18:1172–1181.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morrison VA, Wong M, Hershman D, Campos
LT, Ding B and Malin J: Observational study of the prevalence of
febrile neutropenia in patients who received filgrastim or
pegfilgrastim associated with 3-4 week chemotherapy regimens in
community oncology practices. J Manag Care Pharm. 13:337–348.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pinter T, Klippel Z, Cesas A, Croitoru A,
Decaestecker J, Gibbs P, Hotko Y, Jassem J, Kurteva G, Novotny J,
et al: A phase III, randomized, double-blind, placebo-controlled
trial of pegfilgrastim in patients receiving first-line
FOLFOX/bevacizumab or FOLFIRI/bevacizumab for locally advanced or
metastatic colorectal cancer: Final results of the pegfilgrastim
and anti-VEGF evaluation study (PAVES). Clin Colorectal Cancer.
16:103–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Institute NC: Common Terminology Criteria
for Adverse Events (CTCAE) Version 4.0. 2010.
|
|
14
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al: Phase III trial of infusional fluorouracil,
leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with
infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as
first-line treatment for metastatic colorectal cancer: The gruppo
oncologico nord ovest. J Clin Oncol. 25:1670–1676. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hecht JR, Pillai M, Gollard R, Heim W,
Swan F, Patel R, Dreiling L, Mo M and Malik I: A randomized,
placebo-controlled phase ii study evaluating the reduction of
neutropenia and febrile neutropenia in patients with colorectal
cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin
Colorectal Cancer. 9:95–101. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schutz FA, Jardim DL, Je Y and Choueiri
TK: Haematologic toxicities associated with the addition of
bevacizumab in cancer patients. Eur J Cancer. 47:1161–1174.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fujii H, Matsuhashi N, Kitahora M,
Takahashi T, Hirose C, Iihara H, Yamada Y, Watanabe D, Ishihara T,
Suzuki A and Yoshida K: Bevacizumab in combination with TAS-102
improves clinical outcomes in patients with refractory metastatic
colorectal cancer: A retrospective study. Oncologist. 25:e469–e476.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wildiers H and Reiser M: Relative dose
intensity of chemotherapy and its impact on outcomes in patients
with early breast cancer or aggressive lymphoma. Crit Rev Oncol
Hematol. 77:221–240. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nakayama G, Tanaka C, Uehara K, Mashita N,
Hayashi N, Kobayashi D, Kanda M, Yamada S, Fujii T, Sugimoto H, et
al: The impact of dose/time modification in irinotecan- and
oxaliplatin-based chemotherapies on outcomes in metastatic
colorectal cancer. Cancer Chemother Pharmacol. 73:847–855.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aspinall SL, Good CB, Zhao X, Cunningham
FE, Heron BB, Geraci M, Passero V, Stone RA, Smith KJ, Rogers R, et
al: Adjuvant chemotherapy for stage III colon cancer: Relative dose
intensity and survival among veterans. BMC Cancer.
15(62)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kirshner JJ, Heckler CE, Janelsins MC,
Dakhil SR, Hopkins JO, Coles C and Morrow GR: Prevention of
pegfilgrastim-induced bone pain: A phase III double-blind
placebo-controlled randomized clinical trial of the university of
rochester cancer center clinical community oncology program
research base. J Clin Oncol. 30:1974–1979. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kirshner JJ, McDonald MC III, Kruter F,
Guinigundo AS, Vanni L, Maxwell CL, Reiner M, Upchurch TE, Garcia J
and Morrow PK: NOLAN: A randomized, phase 2 study to estimate the
effect of prophylactic naproxen or loratadine vs no prophylactic
treatment on bone pain in patients with early-stage breast cancer
receiving chemotherapy and pegfilgrastim. Support Care Cancer.
26:1323–1334. 2018.PubMed/NCBI View Article : Google Scholar
|