Introduction
Desmoid tumors are benign proliferations of spindle
cells originating in fibro-aponeurotic tissue. They often occur in
the abdominal wall, mesentery, and retroperitoneum, and may cause
various symptoms such as gastrointestinal tract obstruction,
perforation, abscesses, and ureteral obstruction. No consensus has
been reached concerning the treatment of desmoid tumors in familial
adenomatous polyposis (FAP) patients. Pharmacotherapy, surgery, and
conservative treatment (follow-up) may be selected according to the
site and severity of the tumors (1). Many patients with FAP die from desmoid
tumors, which can arise spontaneously but often appear to be
surgically induced by prophylactic colectomy. The overall
prevalence of desmoid disease is 15% of 379 patients with
APC germline mutation (2).
Ishida et al reported that desmoid tumors accounted for
about 10% (n=71) of deaths due to FAP between 1990 and 2003,
approximately 3.0% (n=268) up to 1980, and approximately 4.8%
(n=171) between 1981 and 1990(1).
They are the second most common cause of death in patients with FAP
following colorectal cancers, and their incidence is increasing
(1,3). Although many patients can live a long
life with desmoid tumors without symptoms, when symptoms appear
(ranging from bowel or ureteric obstruction to bowel perforation
with abscess and fistula) or when there is a risk of functional
impairment, a wide spectrum of therapies (local and systemic) can
be useful in improving symptoms and controlling the disease
(4-6).
However, the tumors are often refractory and are reported to recur
in about 70% of patients following resection (4). Chemotherapy, surgery, and radiation
therapy have been reported as treatments for desmoid tumors, but no
consensus on the best treatment has been reached. Surgical
treatment is often required for large tumors (6-9).
Church et al retrospectively analyzed the treatment
strategies actually performed and suggested a staging system for
desmoid tumors. In this system, all stage-IV cases required some
type of treatment, such as surgery, chemotherapy, or radiation
(10). There is one report that
palliative surgery was effective for a large symptomatic desmoid
tumor (11).
The aim of this case study was to present our
experience of resecting a giant mesenteric desmoid tumor in stage
IV, resulting in a good postoperative course and improvement of
quality of life and prognosis of the patient.
Case report
A 41-year-old half-Japanese half-Caucasian male who
had been diagnosed with intra-abdominal desmoid tumors associated
with FAP at age 13 visited our hospital with fever, abdominal pain,
vomiting, and oral uptake disorder. His Caucasian father died from
FAP. An abdominal mass was detected at the age of 35, but the
patient was diagnosed as not eligible for surgery. He had been
treated with abdominal wall incision for decompression and
chemotherapy (tamoxifen, imatinib, doxorubicin, and dacarbazine)
since he was 38 years of age. The tumors exposed on the abdominal
wall were treated using Mohs paste for more than one year. The
therapeutic response was progressive disease, based on Modified
Response Evaluation Criteria in Solid Tumors (mRECIST). There was
no history of prophylactic colectomy.
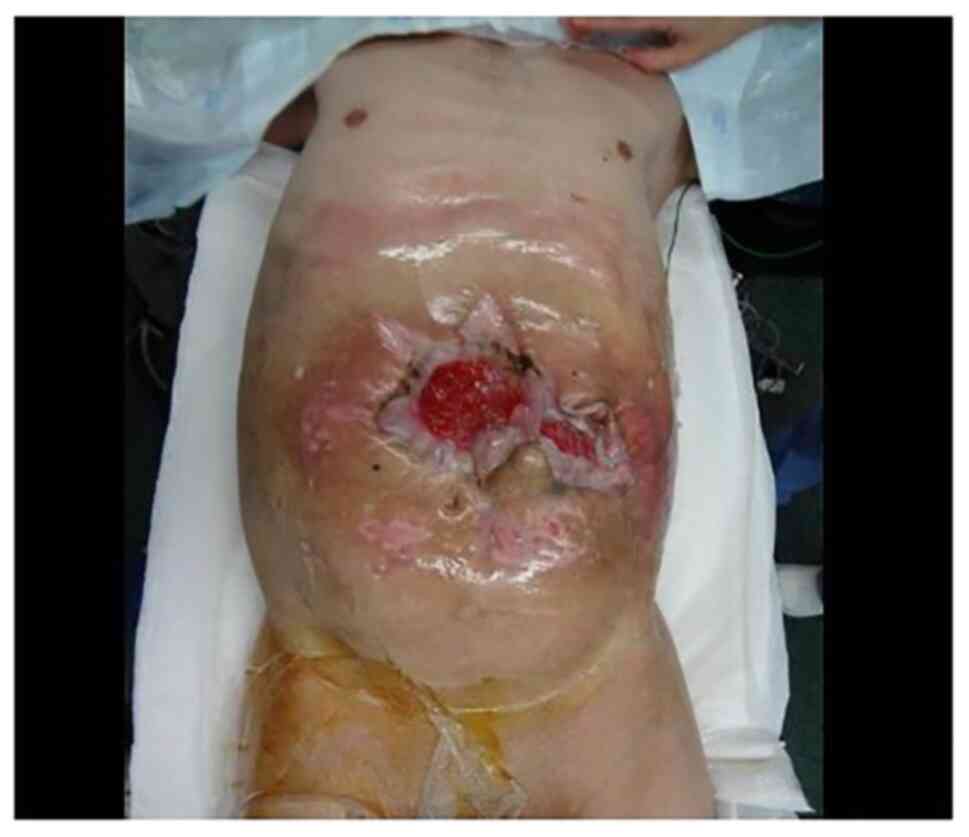
A desmoid tumor was exposed on the body surface and
the tumor invaded the abdominal wall and small intestine; thus, pus
and digestive juice from the tumor were observed (Fig. 1). A blood test showed a mild
increase in neutrophilia (15,300/µl) and C-reactive protein (CRP)
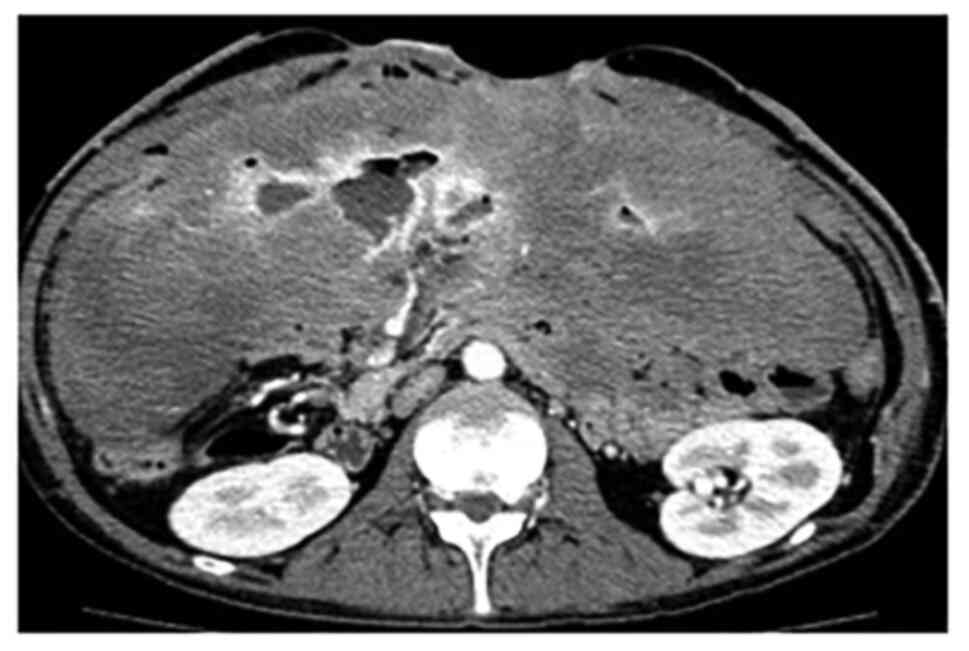
(8.47 mg/dl). Serum albumin was 2.7 g/dl. Enhanced computed
tomography (CT) examination revealed a large tumor throughout the
abdominal cavity. A branch of the superior mesenteric artery (SMA)
penetrated the tumor. An abscess was also found in the upper
abdomen (Fig. 2).
Although it was unlikely that the tumor could be
completely removed by surgery, and the risk of postoperative
complications (short bowel syndrome, massive bleeding) was high,
the medical team decided to perform surgery for the purpose of
symptom improvement.
Surgical findings
General anesthesia was initiated after placing a
balloon catheter in the SMA under fluoroscopy to prepare for
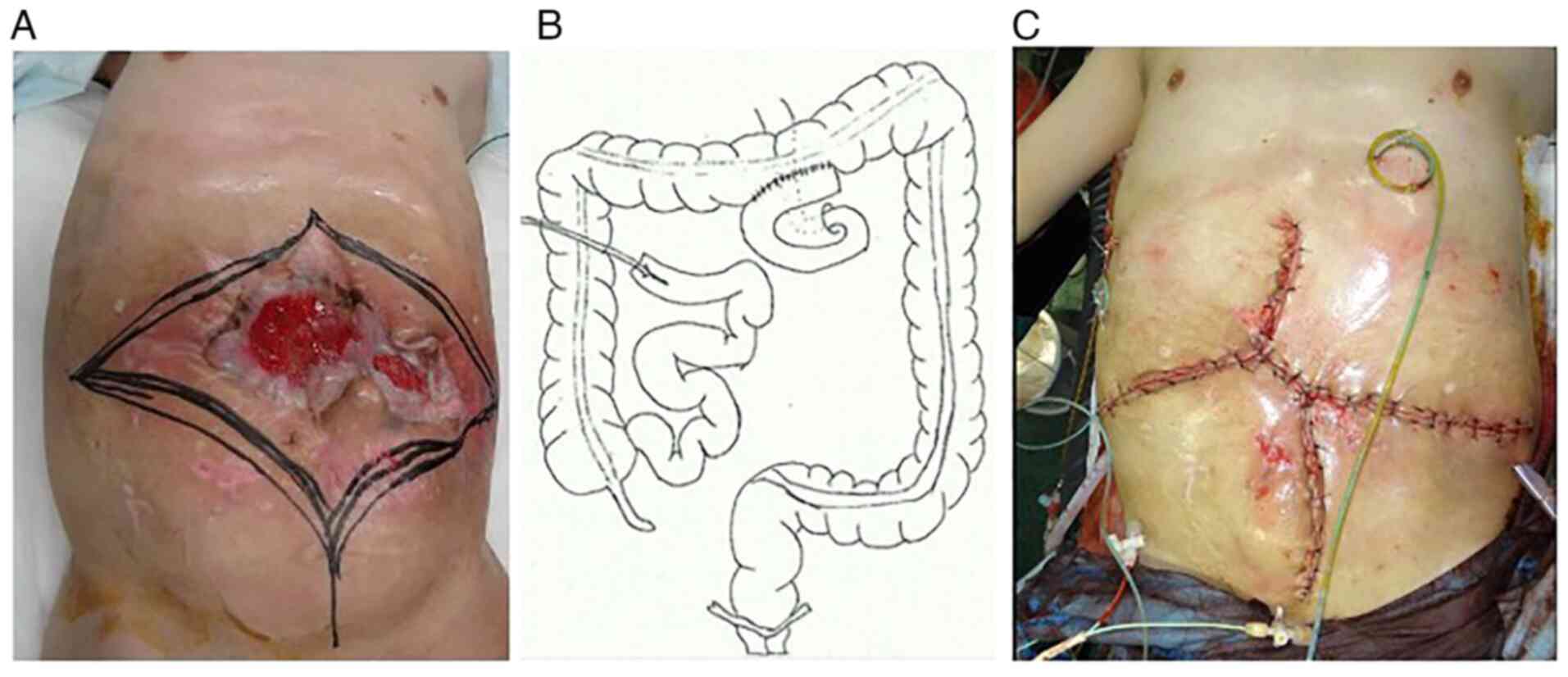
bleeding during surgery. The skin incision was designed to remove
the desmoid tumor that formed a mass with the abdominal wall
(Fig. 3A). In regards to the small
intestine, only 30 cm on the anal side from the Treitz ligament and
100 cm on the oral side from the terminal ileum were able to be
preserved. The other small tumors and the remainder of the small
intestine were removed. When bleeding occurred, hemostasis was
performed while dilating the SMA balloon to reduce blood flow
appropriately. SMA occlusion occurred within 10 min. A side-to-side
anastomosis between the oral jejunum and the transverse colon was
performed, and an intestinal fistula was constructed from the oral
side of the remaining ileum on the anal side (Fig. 3B). Reconstruction of the abdominal
wall was performed by removing the remaining anterior sheath of the
rectus abdominis and the peritoneum to bring them closer together
(Fig. 3C). The operation time was 9
h and 20 min, and the blood loss was 3,430 ml.
Specimen
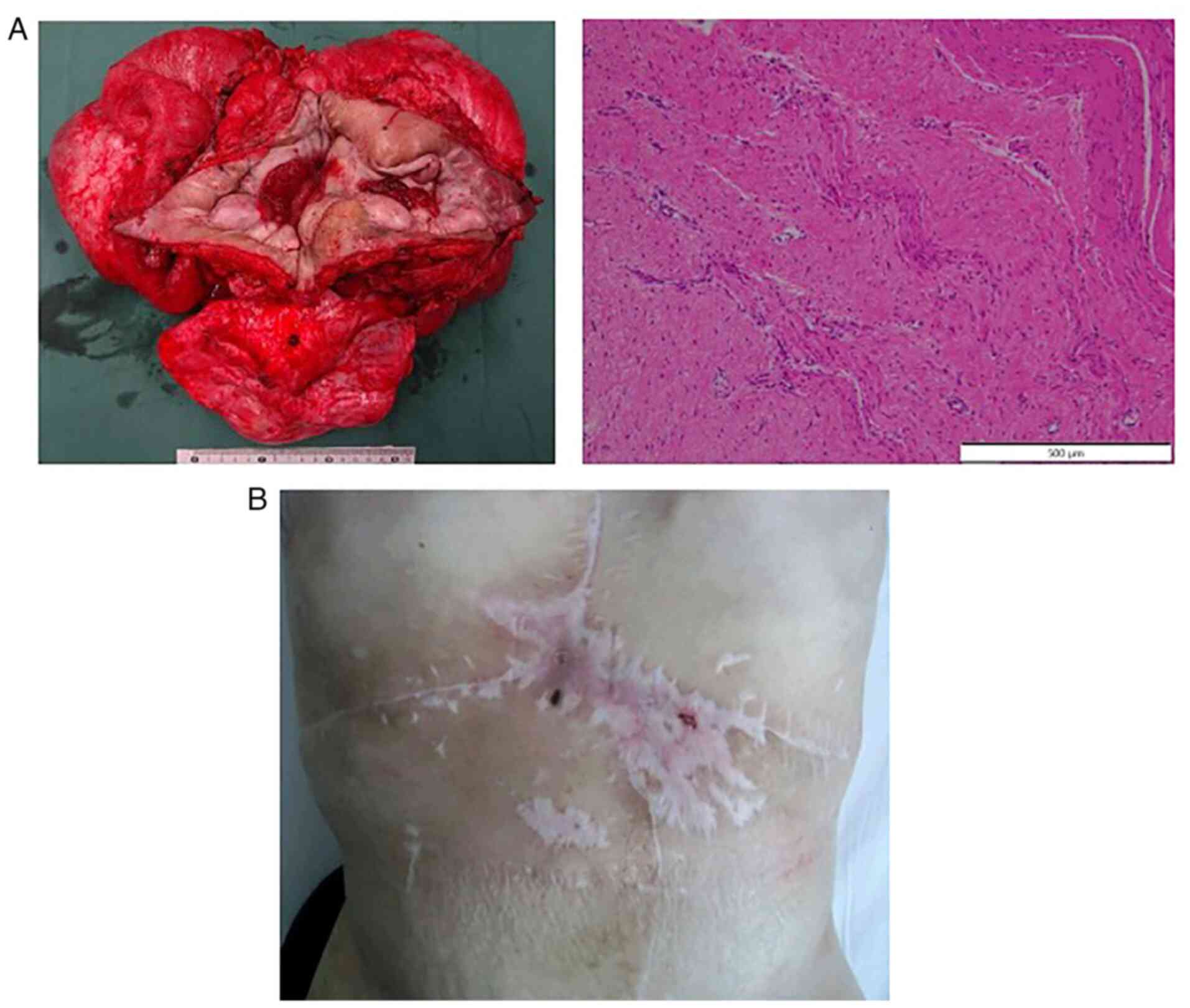
The tumor dimensions were 38x32 cm and it weighed
approximately 6,000 g. Histologically, there were proliferating
spindle cells with collagen fascicle formation continuing from the
dermis to the muscular layer of the small intestine (Fig. 4A). In the tumor there was no
necrosis, the cell density was low, and there were no malignant
findings such as mitoses or atypical nuclei. The pathologic
diagnosis was desmoid tumor, matching the preexisting
diagnosis.
Since this case was severely symptomatic and the
tumor diameter exceeded 20 cm, it was considered to be of stage IV
in the desmoid tumor staging system.
Postoperative course
Enteral nutrition was started on the 11th
postoperative day (POD), and oral intake was started on the 15th
POD. There were no perioperative complications, oral intake
gradually increased, and the patient was discharged on his own on
the 135th POD. At the time of discharge, tube feeding was not
necessary, and the number of defecations was 3 times a day with
only oral intake. No tumor exposure was observed from the abdominal
wall (Fig. 4B). After the
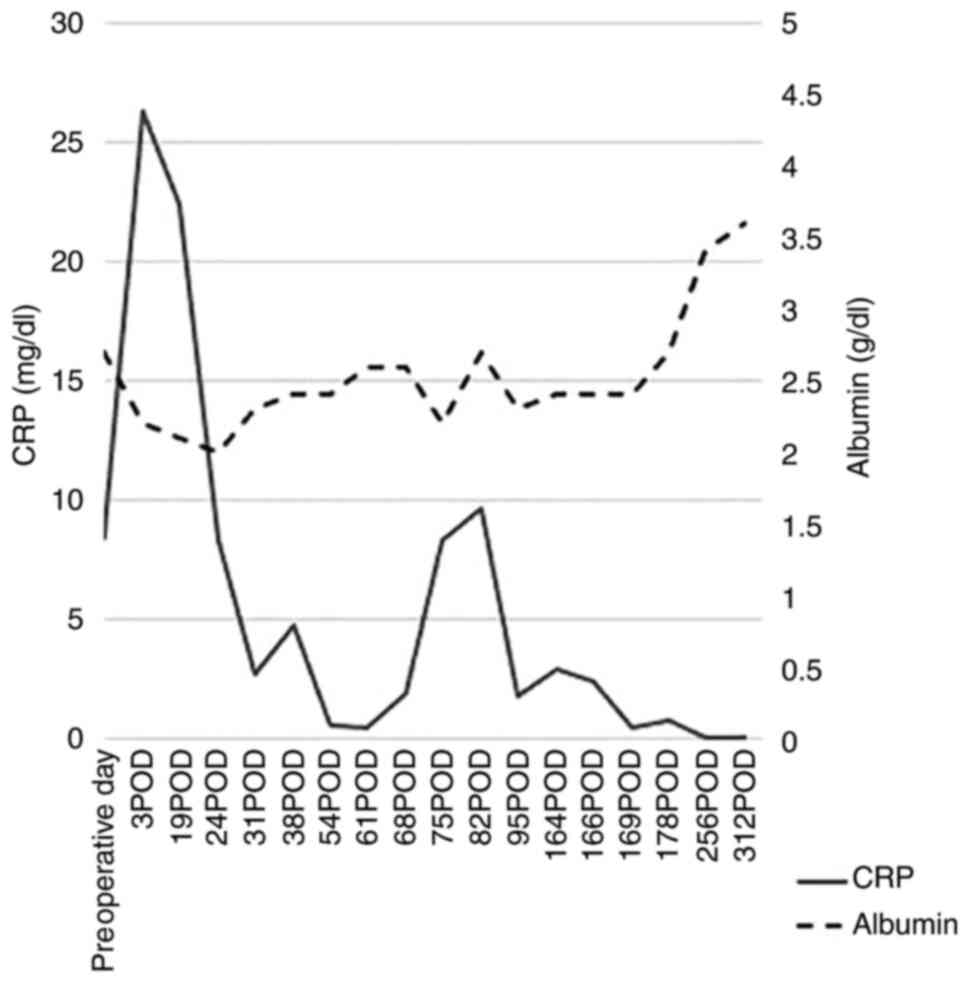
operation, inflammation (CRP levels) was reduced and nutrition
(albumin) was improved (Fig. 5).
One year and 8 months after the operation, intestinal obstruction
occurred due to an increase in intraperitoneal desmoid tumors.
Reoperation was judged difficult, and the patient died 1 year and
10 months after the operation.
Discussion
Desmoid tumors are benign monoclonal fibroblastic
proliferations arising in musculoaponeurotic structures. They often
cause various symptoms such as gastrointestinal tract obstruction,
perforation, abscesses, and ureteral obstruction. There are
currently no national or international guidelines for the
management of desmoid tumors. The strategy of therapy which may be
selected consists of surgery, pharmacotherapy or conservative
treatment (follow-up). In some cases, the mass is considered
unresectable and pharmacotherapy is selected (12).
In conclusion, in this case, the desmoid tumor
prevented oral intake of food by the patient. To improve his
symptoms, we performed a palliative operation which required some
contrivances such as a balloon catheter in the SMA to avoid
bleeding and reconstruction of the abdominal wall. Despite massive
resection of the desmoid tumor and the small intestine, the patient
was able to maintain his nutritional status by oral intake alone
without tube feeding. His quality of life improved for the 20
months following the operation until an intestinal obstruction
occurred 2 months prior to his death.
Acknowledgements
Not applicable.
Funding
Funding: No financial support was received.
Availability of data and materials
Further information regarding this case study is
available from the corresponding author upon reasonable
request.
Authors' contributions
YI drafted the manuscript. TM and HO supervised the
writing of the case study. YI, KL, GM, KM, YF, and HO were the
surgeons who operated on or attended the present patient. TY, SW,
and DY prepared the histological micrographs and assisted in
drafting the manuscript. All authors read and approved the final
manuscript for publication.
Ethics approval and consent to
participate
This retrospective study was performed according to
the Declaration of Helsinki. The study has been approved by an
Internal Board Committee of Moriguchi Keijinkai Hospital (Japan)
and written informed consent was obtained from the patient.
Patient consent for publication
The patient provided written informed consent for
publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ishida H, Yamaguchi T, Tanakaya K, Akagi
K, Inoue Y, Kumamoto K, Shimodaira H, Sekine S, Tanaka T, Chino A,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2016 for the clinical practice of hereditary colorectal
cancer (Translated Version). J Anus Rectum Colon. 2 (Suppl
I):S1–S51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sturt NJ, Gallagher MC, Bassett P, Philp
CR, Neale KF, Tomlinson IP, Silver AR and Phillips RK: Evidence for
genetic predisposition to desmoid tumours in familial adenomatous
polyposis independent of the germline APC mutation. Gut.
53:1832–1836. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iwama T, Tamura K, Morita T, Hirai T,
Hasegawa H, Koizumi K, Shirouzu K, Sugihara K, Yamamura T, Muto T,
et al: A clinical overview of familial adenomatous polyposis
derived from the database of the polyposis registry of Japan. Int J
Clin Oncol. 9:308–316. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Walter T, Zhenzhen Wang C, Guillaud O,
Cotte E, Pasquer A, Vinet O, Poncet G, Ponchon T and Saurin JC:
Management of desmoid tumours: A large national database of
familial adenomatous patients shows a link to colectomy modalities
and low efficacy of medical treatments. United European
Gastroenterol J. 5:735–741. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Clark SK, Neale KF, Landgrebe JC and
Phillips RK: Desmoid tumours complicating familial adenomatous
polyposis. Br J Surg. 86:1185–1189. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Inoue Y, Ishida H, Ueno H, Kobayashi H,
Yamaguchi T, Konishi T, Tomita N, Matsubara N, Ishida F, Hinoi T,
et al: The treatment of desmoid tumors associated with familial
adenomatous polyposis: The results of a Japanese multicenter
observational study. Surg Today. 47:1259–1267. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Santos M, Rocha A, Martins V and Santos M:
Desmoid tumours in familial adenomatous polyposis: Review of 17
patients from a portuguese tertiary center. J Clin Diagn Res.
10:PC01–PC05. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nagata T, Demizu Y, Okumura T, Sekine S,
Hashimoto N, Fuwa N, Okimoto T and Shimada Y: Carbon ion
radiotherapy for desmoid tumor of the abdominal wall: A case
report. World J Surg Oncol. 14(245)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nałęcz A, Dębski K, Dobosz M and Krauze
Ml: Desmoid tumor of the mesentery in a patient after restorative
proctocolectomy as a result of familial adenomatous polyposis-case
reports. Pol Przegl Chir. 90:53–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Church J, Lynch C, Neary P, LaGuardia L
and Elayi E: A desmoid tumor-staging system separates patients with
intra-abdominal, familial adenomatous polyposis-associated desmoid
disease by behavior and prognosis. Dis Colon Rectum. 51:897–901.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sugrue JJ, Cohen SB, Marshall RM and
Rilker AI: Palliative resection of a giant mesenteric desmoid
tumor. Ochsner J. 15:468–472. 2015.PubMed/NCBI
|
|
12
|
Xuereb S, Xuereb R, Buhagiar C, Gauci J
and Magri C: A case report of desmoid tumour-a forgotten aspect of
FAP? Int J Surg Rep. 30:122–125. 2017.PubMed/NCBI View Article : Google Scholar
|