Introduction
Venous thromboembolism (VTE) including deep vein
thrombosis and pulmonary embolism is a well-recognized common
complication associated with malignant tumors. VTE is also a
leading reason for the morbidity and mortality of patients with
malignant tumors (1). The incidence
of VTE has increased during recent years (2). The incidence of VTE in the US is
estimated to be 1 to 2 patients per 1,000 people, with 50% of these
patients experiencing long-term symptoms of VTE (3). In Japan, Sakuma et al reported
that 7,864 new patients suffer from pulmonary embolism (PE) every
year and the number of new patients with deep vein thrombosis (DVT)
is 14,674 each year (4).
Patients presenting with malignancies have a higher
risk of VTE. It has been estimated that cancer is an independent
major risk factor for VTE with an overall 4- to 7-fold increased
risk than the general population. According to estimations,
approximately 30% of new VTE patients suffer from cancer (5,6). This
in part is due to the pathogenesis of VTE, as malignant tumors can
activate the coagulation system and make it a prothrombotic state
(7). The incidence of VTE in
patients with malignancies range between 3 and 25%. In fact, the
incidence is affected by a variety of variables including patient
basic information, tumor-related status, and factors associated
with treatment (8). The correlation
between cancer and VTE was reported for the first time by the
famous Parisian physician Armand Trousseau in 1865(9). Since then, many studies have found
that VTE is associated with numerous types of tumors, including
hematologic malignancies, brain tumors, ovarian cancer, lung cancer
and gastric cancer (10-13).
Cervical cancer is one of the most common
gynecologic malignancies worldwide, with an incidence of 26.2 per
100,000 women in Taiwan (14). In
the US, there are approximately 12,000 women diagnosed with
cervical cancer every year (15).
Matsuo et al found that the incidence of VTE was 12.3% in
American patients with cervical cancer (16). Moreover, Jacobson et al
utilized a retrospective chart review and reported that the
incidence of VTE in cervical cancer patients was 11.7% (17). As these statistics suggest, the risk
of VTE is high in patients with cervical cancer. Surgical treatment
is a first-line treatment to improve the survival of patients with
cervical cancer. However, VTE is a significant complication after
surgery in patients with gynecological malignancies, and cancer
patients undergoing surgery have twice the risk of developing
postoperative VTE (18). In
addition, 1 in 12 patients presenting with VTE associated with
cancer surgery die within 30 days of their surgery (19). Yet, the incidence of VTE after
surgery for cervical cancer remains undefined. The aim of this
case-control study was to clarify the incidence and major risk
factors of perioperative VTE for both preoperative and
postoperative VTE in women undergoing surgery for cervical
cancer.
Patients and methods
Patients
This retrospective study reviewed the medical
records and selected patients who met the criteria.
This was a retrospective analysis and thus no
informed consent was required. After this study was approved
[(2020)(S514), April 7, 2020] by the Research Ethics Board of
Qianfoshan Hospital & The First Affiliated Hospital of Shandong
First Medical University (Jinan, Shandong, China), we reviewed the
medical records of patients diagnosed with cervical cancer,
including general information and clinical pathology. A total of
338 consecutive patients who underwent surgery treatment at our
hospital from July 2014 to July 2017 with a clear pathological
diagnosis of cervical cancer were considered for admission to the
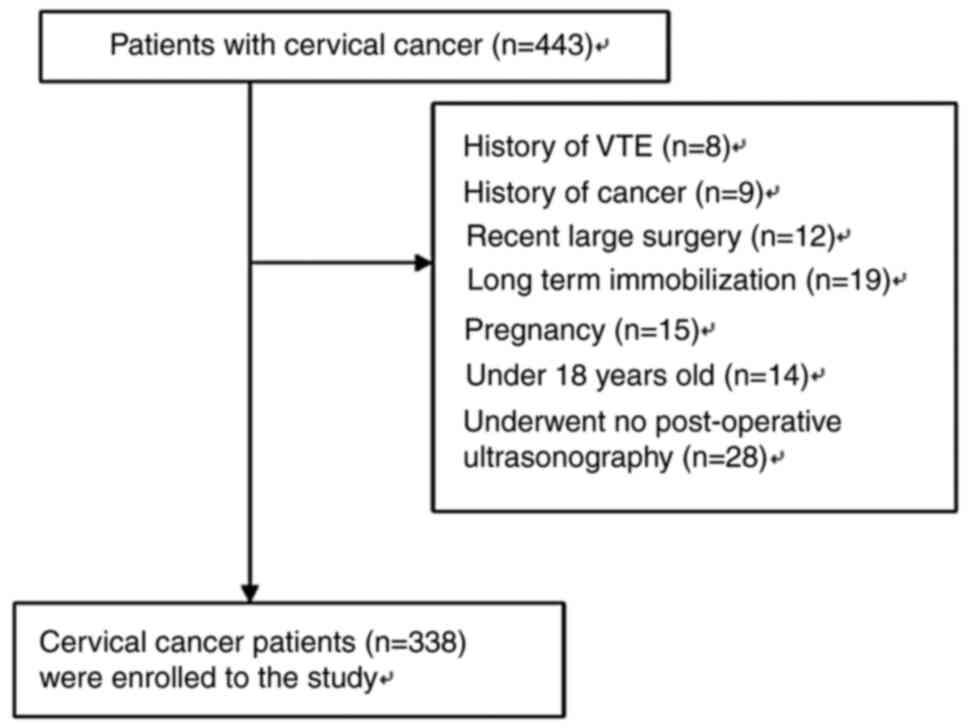
present study. In this analysis, the exclusion criteria included
patients with major risk factors such as history of VTE, history of
cancer, recent major surgery, long-term immobilization, pregnancy
or objectively confirmed deep venous thrombosis or pulmonary
thromboembolism and patients under 18 years of age and who
underwent no postoperative ultrasonography.
Preoperative parameters that were analyzed and
collected included age, body mass index (BMI) (kg/m2),
smoking history, hypertension, hypercholesterolemia, diabetes,
cardiovascular complications, Federation International of
Gynecology and Obstetrics (FIGO) stage, distant metastasis,
adjuvant chemotherapy, pathological type (squamous cell,
adenocarcinoma, adenosquamous or others), size of the cervical
tumor on MRI, laboratory tests such as platelets (109/l)
and levels of D-dimer (mg/ml). Data collected after surgery
included length of operation, surgical method (abdominal or
laparoscopic), blood transfusion and adjuvant chemotherapy
(adjuvant chemotherapy or radiation). Cancers were staged according
to the 2014 FIGO guidelines (20).
All cases were grouped into different types and graded according to
a revised standard of the WHO (21).
VTE prevention, detection, diagnosis
and treatment
In our institution, Doppler study of the
extremities, computed tomography (CT) pulmonary artery angiogram,
or ventilation-perfusion lung scan were performed by experienced
clinical laboratory technologists before and 7 days after surgery
to determine the incidence and specific location of VTE both before
and after the operation. The possible types of VTE were examined as
follows: deep venous thrombosis (DVT) alone, pulmonary embolism
(PE) alone, and DVT/PE combined. Mechanical thromboprophylaxis for
VTE is as follows: compression stockings and intermittent pneumatic
compression. The related medication treatment for VTE is as
follows: heparin, low-molecular weight heparin, warfarin, and
inferior vena cava filter.
Statistical analysis
General characteristics of the population were
described using mean ± standard deviation (SD). Independent-sample
t-test and the Chi-square test were used to compare the differences
between patients with and without VTE. Univariate and multivariate
analyses were performed with logistic analyses to identify the
significant risk factors for VTE. Statistical Package for Social
Science (SPSS) software (v17.0; SPSS, Inc.) was used to perform
statistical analysis. A probability value (P) <0.05 was
considered to indicate statistical significance.
Results
There were 443 patients identified who underwent
surgery for cervical cancer during the study period. Among the 443
cases of cervical cancer with available medical records, 105
(23.7%) cases were excluded due to various unsuitable factors.
Finally, there were 338 patients who met the inclusion criteria for
the study. The flow diagram of the patients included in this study
is provided in Fig. 1. Among the
338 patients (patients with VTE, 28; patients without VTE, 310),
the mean age of the patients was 52.90±12.57 years. The histologic
types included in this study included squamous cell (n=272),
adenocarcinoma (n=50), adenosquamous (n=14), and other (n=2) types.
The patients were divided into two subgroups according to the stage
of tumors: stage I+II and stage III+IV. The number of patients with
stage I+II disease was 133, and the number of patients with stage
III+IV disease was 205. Table I
shows the clinical characteristics of the patients with and without
VTE. Age, BMI, smoking history, FIGO stage, distant metastasis,
pathological type (squamous cell, adenocarcinoma, adenosquamous or
others), laboratory data such as platelet count and complications
including hypertension, hypercholesterolemia, diabetes,
cardiovascular complications were not significant factors for
VTE.
 | Table IClinical characteristics of the VTE
and non-VTE groups. |
Table I
Clinical characteristics of the VTE
and non-VTE groups.
| Preoperative risk
factors | VTE group (N=10) | Non-VTE group
(N=310) | t/χ2 | P-value |
|---|
| Agea,b, years, mean ± SD (range) | 54.40±12.73
(33-78) | 52.87±12.57
(7-80) | 0.383 | 0.859 |
| BMIa, kg/m2 (range) | 24.63±3.87
(19.1-33) | 23.29±3.69
(14-34) | 1.131 | 0.893 |
| Smoking
historyb, n (%) | 2 (20%) | 30 (9.1%) | 1.334 | 0.248 |
|
Hypertensionb, n (%) | 3 (30.0%) | 81 (24.7%) | 0.146 | 0.702 |
|
Hypercholesterolemiab, n (%) | 3 (30%) | 76 (23.2%) | 0.253 | 0.615 |
| Diabetesb, n (%) | 2 (20.0%) | 58 (17.7%) | 0.036 | 0.850 |
| Cardiovascular
complicationsb, n
(%) | 2 (20.0%) | 64 (17.9%) | 0.001 | 0.969 |
| FIGO
stageb, n (%) | | | 0.490 | 0.484 |
|
I +II | 5 (50.0%) | 128 (39.0%) | | |
|
III+IV | 5 (50.0%) | 200 (61.0%) | | |
| Distant
metastasisb, n
(%) | 2 (20.0%) | 50 (15.2%) | 0.169 | 0.681 |
| Adjuvant
chemotherapy, n (%) | 5 (50.0%) | 57 (17.4%) | 6.895 | 0.009 |
| Pathological
typeb, n (%) | | | 1.244 | 0.742 |
|
Squamous
cell | 7 (70.0%) | 265 (80.8%) | | |
|
Adenocarcinoma | 2 (20.0%) | 48 (14.6%) | | |
|
Adenosquamous | 1 (10.0%) | 13 (4.0%) | | |
|
Other | 0 | 2 (0.6%) | | |
| Cervical tumor
sizeb, n (%) | | | 8.941 | 0.003 |
|
≥50 mm | 7 (70.0%) | 79 (25.5%) | | |
|
<50
mm | 3 (30.0%) | 249 (80.3%) | | |
| Platelet count
(109/l)a,
mean ± SD (range) | 290.3±29.94
(230-355) | 279.5±32.31
(225-386) | 1.040 | 0.935 |
| D-dimer
(mg/ml)a, mean ± SD
(range) | 1.33±0.31
(0.4-1.5) | 0.67±0.34
(0.1-1.7) | 6.054 |
<0.001 |
| Postoperative risk
factors | VTE group
(n=18) | Non-VTE group
(n=310) | | |
| Length of surgery
>3 hb, n (%) | 10 (55.6%) | 69 (22.3%) | 0.316 | 0.001 |
| Blood
transfusionb, n
(%) | 4 (22.2%) | 66 (21.3%) | 0.009 | 0.925 |
| Surgical
methodb, n (%) | | | 0.040 | 0.842 |
|
Abdominal, n
(%) | 16 (88.9%) | 280 (90.3%) | | |
|
Laparoscopic,
n (%) | 2 (27.8%) | 30 (20.7%) | | |
| Adjuvant
chemotherapyb, n
(%) | 9 (50.0%) | 86 (27.7%) | 4.096 | 0.043 |
Perioperative VTE was detected in 28 (8.3%) of the
338 patients. Ten events (3.0%) occurred in preoperative patients
whereas 18 patients (5.5%) had postoperative events. Six of these
28 patients were also diagnosed with pulmonary embolism (PE), 1
with DVT and PE and no patient was deceased. All patients had
non-lethal PE. Nine patients had a VTE in the left leg among the 28
patients, 10 in the right leg, and 9 in both legs. Three patients
had proximal DVT and the remaining 15 patients had distal DVT. All
VTE cases were asymptomatic.
Factors associated with VTE
development
The results of the univariate analysis of
perioperative risk factors for VTE are listed in Table I. Adjuvant chemotherapy (P=0.009),
larger size of the cervical tumor (P=0.003), and high D-dimer
levels (P<0.001) were independent risk factors for preoperative
VTE. Length of surgery (P=0.001) and adjuvant chemotherapy
(P=0.043) were significantly related to postoperative VTE on
univariate analysis, but there was no significant difference in
regards to type of surgery, and need for blood transfusion.
In the multivariate analysis, larger size of the
cervical tumor [odds ratio (OR)=0.118; 95% confidence interval
(CI), 0.02-0.56; P=0.007] and high levels of D-dimer (OR=15.092;
95% CI, 8.281-31.353; P<0.001) were independently associated
with VTE development within 30 days before surgery (Table II). In the multivariate analysis,
length of surgery (OR=19.021; 95% CI, 6.523-55.464; P<0.001) and
use of chemotherapy (OR=3.152; 95% CI, 1.08-9.18; P=0.035) were
independently associated with VTE development within 30 days after
surgery (Table III).
 | Table IIMultivariate analysis of the
preoperative risk factors for VTE. |
Table II
Multivariate analysis of the
preoperative risk factors for VTE.
| Risk factors | OR | 95% CI | P-value |
|---|
| Cervical tumor
size | 0.118 | 0.025-0.564 | 0.007 |
| D-dimer (mg/l) | 15.092 | 8.281-31.353 |
<0.001 |
 | Table IIIMultivariate analysis of the
postoperative risk factors for VTE. |
Table III
Multivariate analysis of the
postoperative risk factors for VTE.
| Risk factors | OR | 95% CI | P-value |
|---|
| Length of surgery
>3 h | 19.021 | 6.523-55.464 |
<0.001 |
| Use of
chemotherapy | 3.152 | 1.083-9.178 | 0.035 |
Discussion
Virchow's triad is a well-known factor that
contributes to thrombosis and consists of vessel wall injury,
hypercoagulability and abnormal venous flow (22). A large number of pathological,
experimental and clinical studies have shown that
hypercoagulability exists locally or systemically in most cancer
patients, for the following reasons. i) Postoperative bed rest and
venous compression by the tumor itself cause blood circulation
stasis in patients with malignant tumors. ii) Chemotherapeutic
drugs and tumors themselves can induce inflammatory mediators that
damage the intima of veins. iii) Levels of fibrinogen are higher in
cancer patients than in non-cancer patients, and the tumors
themselves can release various coagulant substances. The above
reasons lead to increased blood coagulation and deep venous
thrombosis (DVT).
The incidence of malignant tumors in China is
increasing year by year, while the incidence of venous thrombosis
caused by malignant tumors is 2.4-12%, which is at least 6 times
higher than that of non-cancer patients. Therefore, it is
particularly critical to study the risk factors and therapeutic
efficacy of DVT in cervical cancer patients undergoing surgery and
to prevent its occurrence as early as possible.
In the present study, we found that the incidence of
venous thromboembolism (VTE) was 3.0% during hospitalization and
5.5% during postoperative day 30 in female Chinese patients
undergoing surgery for cervical cancer when perioperative
mechanical thromboprophylaxis was performed, which was similar to
previous studies. Satoh et al (8) investigated the incidence of VTE before
treatment in 272 consecutive patients with cervical cancer and the
incidence of VTE was 4.8%. Tsai et al analyzed data
deposited between 2003 and 2008 in the National Health Insurance
Research Database and reported that the 5-year cumulative risk for
VTE was 3.3% in the cervical cancer group (23).
Studies have reported that the risk factors of
thrombosis associated with gynecological tumors include pelvic
surgery, age, race, previous leg edema, presence of venous
varicosities, a history of VTE, longer duration of surgery, receipt
of adjuvant chemotherapy or radiation therapy, and immobility
(24). In the present study, we
found that high D-dimer and larger size of the cervical tumor were
independent risk factors for preoperative VTE whereas operative
time longer than 3 h and postoperative adjuvant chemotherapy were
independent risk factors for postoperative VTE.
D-dimer is one of the products of the degradation of
crosslinked fibrin. The increase in plasma levels indicates the
hypercoagulation state or the activation of fibrinolytic system
in vivo. Some scholars believe that the value of D-dimer
alone in the diagnosis of postoperative DVT of malignant tumors is
limited (25). The factors leading
to the fluctuation of D-dimer level include disease factors,
physical factors and examination methods, thus its specificity is
low. Researchers believe that the high negative predictive value of
D-dimer makes it unable to be used as a diagnostic tool and is only
suitable for the early screening of VTE (26). However, some scholars believe that
dynamic detection of D-dimer level may have a certain early warning
effect on postoperative VTE of gynecological malignant tumors
(27). It has the advantages of
rapid, economic, noninvasive and dynamic monitoring for the
diagnosis of VTE, and can be popularized and applied in the clinic
(28). D-dimer was introduced into
the risk assessment system of VTE after malignant tumor surgery,
which improved the accuracy of prediction to a certain extent.
Research has confirmed that the level of D-dimer is correlated with
the prognosis of acute pulmonary thromboembolism (29), and can be used as a predictor of
venous thromboembolism, and the cutoff value of D-dimer for
predictive VTE is still controversial. Ay et al (28) suggested the cutoff for elevated
D-dimer was 1.44 mg/ml; however, Kawaguchi et al recommended
the cutoff value could be more than 1.5 mg/ml in ovarian cancer
(30). In the present study, it was
found that the median value of D-dimer was 1.33 mg/ml. The results
are similar to the above mentioned studies. The occurrence of VTE
adverse events in patients with cervical cancer with significantly
increased dimer levels should be actively intervened as soon as
possible. D-dimer can be used as a primary screening tool for
postoperative VTE of gynecological tumors.
Large cervical tumors may compress pelvic
vasculature impacting pelvic and lower limb blood circulation,
thereby increasing blood viscosity and subsequent thrombus
formation. In addition, larger cervical tumors may invade the
pelvic wall and damage endothelial cells (31). Satoh et al (8) suggested that tumor size >50 mm was
an independent risk factors of VTE, which was in keeping with our
study. Therefore, chemotherapy drugs may be necessary for patients
with larger tumors.
With the use of new chemotherapeutic drugs, the
incidence of venous thrombosis in patients with malignant tumors
has increased. Studies have reported that the incidence of venous
thrombosis can reach 6-30%. Chemotherapeutic drugs lead to injury
of vascular endothelial cells, release of procoagulants and
destruction of endogenous anticoagulants. Jacobson et al
reported that the incidence of thrombosis in cervical cancer
patients treated with chemotherapy and radiotherapy was
approximately 16.7% (32). And in
another study, the incidence of thrombosis of 11.7% in patients
with the same type of cancer (17).
In the present study, the surgical time of the VTE
group was significantly longer than that of the non-VTE patients,
which was in keeping with other studies (33). Zhang et al recommended that
gynecological surgery, unlike general surgery, requires lymph node
dissection, which results in prolonged operation time and increased
incidence of VTE (34).
In the present study, age, body mass index (BMI),
and smoking history, were not associated with the development of
VTE, which is consistent with the results of previous studies. In
tumor-related factors, FIGO stage and pathological type were not
associated with the development of VTE. However, Satoh et al
recommended that pathological type was not a risk factor for VTE
but age >60 years and FIGO stage IV were risk factors for VTE
before treatment in patients with cervical cancer (8). Perhaps the reason is that his subjects
were patients before treatment and in this study, the subjects were
patients undergoing surgery with cervical cancer. In the present
study, patient complications were not a risk factor for VTE, and
may be less severe in these patients.
In a study by Chen et al, the authors
investigated the incidence, clinical and pathological
characteristics of thrombosis in excised tissues after
pneumonectomy, and its association with survival rate in patients
with non-small cell lung cancer but not cervical cancer (22). Satoh et al investigated the
incidence of VTE before treatment in 272 consecutive patients with
cervical cancer, and the impact of management on prevention of VTE
during and after treatment (8).
Tsai et al analyzed data deposited between 2003 and 2008 in
the NHIRD, provided by the National Health Research Institutes in
Taiwan. Strategies to reduce these risks need to be examined
(23). Ailawadi and Del Priore
found that heparin should not be used with sequential compression
devices (SCDs) unless an additional benefit can be demonstrated in
a randomized controlled trial (24). D-dimer has a negative predictive
value of ≥93% for excluding DVT in symptomatic outpatients and it
can be a useful test in the diagnostic work-up of suspected upper
extremity DVT. To improve prediction of VTE in cancer patients, Ay
et al performed a prospective and observational cohort study
of patients with newly diagnosed cancer or progression of disease
after remission (28). In a review
by Barbera and Thomas, the authors focused on the incidence of VTE,
patient, tumor, and treatment-related risk factors for VTE, and
treatment and prevention of VTE in the setting of cervical cancer
(31). Jacobson et al noted
a high incidence of thromboembolic events (TE) (16.7%) in patients
treated at UIHC (University of Iowa Hospitals and Clinics) with
chemoradiation for invasive cervical cancer. They did not find a
statistical association between age, stage, smoking history, or BMI
and risk of TE in this group. Jacobson et al found that
there was a clear and significant difference in survival between
patients with and without TE (32).
Kim et al found that an increase in surgical duration was
directly associated with an increase in the risk for VTE (33). The aim of a study by Zhang et
al was to assess the major risk factors for venous
thromboembolism in Chinese patients with ovarian cancer and to
explore optimal methods of prophylaxis and treatment (34).
This study has some limitations including a small
sample size, and the fact that it was a single-center,
retrospective and non-protocolized study. In particular, the small
sample size of the study might have led to false statistical
conclusions, and therefore a large-scale, prospective, randomized
controlled trial is needed to confirm the results. In addition, all
patients enrolled in the current study were Chinese, and there were
differences in some basic risk factors, such as BMI.
In conclusion, the present study screened out
significant risk factors for prevention of VTE in patients with
cervical cancer undergoing surgery. First of all, for patients with
cervical cancer, we should choose reasonable treatment and reduce
surgical intervention. Secondly, we should evaluate the risk
factors of cervical cancer patients with VTE in time and administer
early anticoagulation treatment in order to reduce the incidence of
VTE.
Acknowledgements
I would like to give my heartfelt thanks to all the
people who have ever helped me in this paper.
Funding
Funding: The present study was funded by the Key Technology
Research and Development Program of Shandong (2017RKB14047).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and SZ made substantial contributions to the
conception and design of the work; and the acquisition, analysis,
and interpretation of data. YP, ML and YS drafted the manuscript
and revised it critically for important intellectual content. All
authors gave final approval of the version to be published and
agreement to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work (including the provided data) are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This was a retrospective analysis and thus no
informed consent was required from the patients. This study was
approved by the Research Ethics Board of Qianfoshan Hospital &
The First Affiliated Hospital of Shandong First Medical University
(Jinan, Shandong, China). The approval number is [2020](S514), and
the date of approval was April 7, 2020.
Patient consent for publication
The patients consented to the publication of their
data.
Competing interests
The authors declare no competing interests.
References
|
1
|
Lyman GH, Culakova E, Poniewierski MS and
Kuderer NM: Morbidity, mortality and costs associated with venous
thromboembolism in hospitalized patients with cancer. Thromb Res.
164 (Suppl 1):S112–S118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Timp JF, Braekkan SK, Versteeg HH and
Cannegieter SC: Epidemiology of cancer-associated venous
thrombosis. Blood. 122:1712–1723. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tsai AW, Cushman M, Rosamond WD, Heckbert
SR, Polak JF and Folsom AR: Cardiovascular risk factors and venous
thromboembolism incidence: The longitudinal investigation of
thromboembolism etiology. Arch Intern Med. 162:1182–1189.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sakuma M, Nakamura M, Yamada N, Ota S,
Shirato K, Nakano T, Ito M and Kobayashi T: Venous thromboembolism:
Deep vein thrombosis with pulmonary embolism, deep vein thrombosis
alone, and pulmonary embolism alone. Circ J. 73:305–309.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Walker AJ, Card TR, West J, Crooks C and
Grainge MJ: Incidence of venous thromboembolism in patients with
cancer-a cohort study using linked United Kingdom databases. Eur J
Cancer. 49:1404–1413. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ashrani AA, Gullerud RE, Petterson TM,
Marks RS, Bailey KR and Heit JA: Risk factors for incident venous
thromboembolism in active cancer patients: A population based
case-control study. Thromb Res. 139:29–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Furie B and Furie BC: Mechanisms of
thrombus formation. N Engl J Med. 359:938–949. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Satoh T, Matsumoto K, Tanaka YO, Akiyama
A, Nakao S, Sakurai M, Ochi H, Onuki M, Minaguchi T, Sakurai H and
Yoshikawa H: Incidence of venous thromboembolism before treatment
in cervical cancer and the impact of management on venous
thromboembolism after commencement of treatment. Thromb Res.
131:e127–e132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Trousseau A: Phlegmasia alba dolens. Clin
Med Hotel-Dieu Paris. 3:654–712. 1865.
|
|
10
|
Nakano F, Matsubara T, Ishigaki T,
Hatazaki S, Mouri G, Nakatsuka Y and Suzuki H: Incidence and risk
factor of deep venous thrombosis in patients undergoing craniotomy
for brain tumors: A Japanese single-center, retrospective study.
Thromb Res. 165:95–100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kekre N and Connors JM: Venous
thromboembolism incidence in hematologic malignancies. Blood Rev.
33:24–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Du H, Zhao H, Li M, Ji H, Ren F, Wang P,
Li X, Dong M, Dawar R, Chen G and Chen J: Analysis of the incidence
of lower extremity venous thrombosis and its related risk factors
in admitted patients with lung cancer. Zhongguo Fei Ai Za Zhi.
21:761–766. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
13
|
Osaki T, Saito H, Fukumoto Y, Kono Y,
Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Sato K, Hirooka Y
and Fujiwara Y: Risk and incidence of perioperative deep vein
thrombosis in patients undergoing gastric cancer surgery. Surg
Today. 48:525–533. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen YY, You SL, Chen CA, Shih LY, Koong
SL, Chao KY, Hsiao ML, Hsieh CY and Chen CJ: Taiwan Cervical Cancer
Screening Task Force. Effectiveness of national cervical cancer
screening programme in Taiwan: 12-Year experiences. Br J Cancer.
101:174–177. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matsuo K, Moeini A, Machida H, Fullerton
ME, Shabalova A, Brunette LL and Roman LD: Significance of venous
thromboembolism in women with cervical cancer. Gynecol Oncol.
142:405–412. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jacobson G, Lammli J, Zamba G, Hua L and
Goodheart MJ: Thromboembolic events in patients with cervical
carcinoma: Incidence and effect on survival. Gynecol Oncol.
113:240–244. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mandalà M, Falanga A and Roila F: ESMO
Guidelines Working Group. Management of venous thromboembolism
(VTE) in cancer patients: ESMO clinical practice guidelines. Ann
Oncol. 22 (Suppl 6):vi85–vi92. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Agnelli G, Bolis G, Capussotti L, Scarpa
RM, Tonelli F, Bonizzoni E, Moia M, Parazzini F, Rossi R, Sonaglia
F, et al: A clinical outcome-based prospective study on venous
thromboembolism after cancer surgery: The @RISTOS project. Ann
Surg. 243:89–95. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
FIGO Committee on Gynecologic Oncology.
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
World Health Organization (WHO): WHO
international classification of diseases for oncology, 3rd Edition
(ICD-O-3). WHO, Geneva, 2000. https://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology.
|
|
22
|
Chen W, Zhang Y, Yang Y, Zhai Z and Wang
C: Prognostic significance of arterial and venous thrombosis in
resected specimens for non-small cell lung cancer. Thromb Res.
136:451–455. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsai SJ, Ruan YX, Lee CC, Lee MS, Chiou
WY, Lin HY, Hsu FC, Su YC and Hung SK: The incidence of venous
thromboembolism in cervical cancer: A nationwide population-based
study. BMC Res Notes. 5(316)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ailawadi M and Del Priore G: A comparison
of thromboembolic prophylaxis in gynecologic oncology patients. Int
J Gynecol Cancer. 11:354–358. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aschwanden M, Labs KH, Jeanneret C, Gehrig
A and Jaeger KA: The value of rapid D-dimer testing combined with
structured clinical evaluation for the diagnosis of deep vein
thrombosis. J Vasc Surg. 30:929–935. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Adam SS, Key NS and Greenberg CS: D-dimer
antigen: Current concepts and future prospects. Blood.
113:2878–2887. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Takach Lapner S, Julian JA, Linkins LA,
Bates SM and Kearon C: Questioning the use of an age-adjusted
D-dimer threshold to exclude venous thromboembolism: Analysis of
individual patient data from two diagnostic studies. J Thromb
Haemost. 14:1953–1959. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ay C, Dunkler D, Marosi C, Chiriac AL,
Vormittag R, Simanek R, Quehenberger P, Zielinski C and Pabinger I:
Prediction of venous thromboembolism in cancer patients. Blood.
116:5377–5382. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sartori M, Migliaccio L, Favaretto E, Cini
M, Legnani C, Palareti G and Cosmi B: D-dimer for the diagnosis of
upper extremity deep and superficial venous thrombosis. Thromb Res.
135:673–678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kawaguchi R, Furukawa N and Kobayashi H:
Cut-off value of D-dimer for prediction of deep venous thrombosis
before treatment in ovarian cancer. J Gynecol Oncol. 23:98–102.
2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barbera L and Thomas G: Venous
thromboembolism in cervical cancer. Lancet Oncol. 9:54–60.
2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jacobson GM, Kamath RS, Smith BJ and
Goodheart MJ: Thromboembolic events in patients treated with
definitive chemotherapy and radiation therapy for invasive cervical
cancer. Gynecol Oncol. 96:470–474. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim JY, Khavanin N, Rambachan A, McCarthy
RJ, Mlodinow AS, De Oliveria GS Jr, Stock MC, Gust MJ and Mahvi DM:
Surgical duration and risk of venous thromboembolism. JAMA Surg.
150:110–117. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang W, Liu X, Cheng H, Yang Z and Zhang
G: Risk factors and treatment of venous thromboembolism in
perioperative patients with ovarian cancer in China. Medicine
(Baltimore). 97(e11754)2018.PubMed/NCBI View Article : Google Scholar
|