Introduction
Large-scaled randomized clinical studies of active
specific immunizations to prevent the recurrence of lung cancer
with melanoma-associated antigen 3 antigen (1), colon cancer with irradiated
autologous tumor cells (2), and
breast cancers with human epidermal growth factor receptor 2
(HER2)-derived peptides (3,4) have
been conducted since the 1980s. None of those studies identified
sufficient clinical benefits for the immunizations' approval,
although benefits were observed in some of the patients. In
addition, the immunological mechanisms involved in the
immunizations' insufficient clinical benefits were not fully
investigated. Although remarkable advances in cancer therapy were
achieved in the field of immune checkpoint inhibitors over the past
decade (5,6), the advances do not negate the need to
develop vaccines that could prevent the recurrence of cancer,
because active specific immunity is pivotal to cancer control.
The robust humoral immunity induced by prophylactic
vaccines against human papilloma virus is responsible for the
prevention of human papilloma virus-related cancers (7). Humoral immunity has also been
suggested to play an important role in the prevention of the
recurrence of several cancers (8-10).
It is thus worthwhile to investigate whether robust humoral
immunity is observed in cancer patients who have received a
peptide-based cancer vaccine, as such vaccines have been considered
a promising preventive or therapeutic option since the 1990s
(11,12). We conducted the present study to
investigate the immune kinetics of personalized peptide vaccination
(PPV)-induced immunity in patients with non-advanced patients and
no active tumor at their entry to the PPV. For the PPV examined
herein, four peptides chosen from a set of 31 warehouse peptides
were vaccinated to individual patients based on the patients' human
leukocyte antigen (HLA) type and their pre-existing immunity as
shown by their peptide-specific immunoglobulin G (IgG) levels
(13-17).
Patients and methods
Patients and protocols
From November 2008 to March 2019, phase II studies
of a PPV were conducted for 44 Japanese patients with cancers of
different histologies at the Kurume University Cancer Vaccine
Center or Kurume University Hospital. They were diagnosed as having
no active tumor at the time of their entry for the PPV: lung
adenocarcinoma (n=11), colon cancer (n=18), and breast cancer
(n=15). The eligibility criteria were a pathologically confirmed
diagnosis of lung adenocarcinoma, colon cancer, or breast cancer;
positive IgG responses [≥10 fluorescence intensity units (FIU)] for
≥2 of the 31 warehouse peptides in their prevaccination plasma
(Table SI); positive status for
HLA-A2, -A24, -A3 supertypes (HLA-A3, -A11, -A31, or -A33) or -A26;
age ≥20 years; Eastern Cooperative Oncology Group performance
status of 0-2; a life expectancy of ≥12 weeks; and adequate bone
marrow, hepatic, and renal functions.
The exclusion criteria were acute infection, a
history of severe allergic reactions, or other systemic diseases as
described (13-17).
The 31 warehouse peptides consisted of peptides matched for 12
HLA-A2-positive, 14 for HLA-A24-positive, and nine for HLA-A3
supertype-positive patients, and four peptides for HLA-A26-positive
cancer patients, respectively (Table
SI). The peptides were prepared under Good Manufacturing
Practice conditions with the use of a multiple peptide system
(Multiple Peptide System, Inc.). The patients were vaccinated in a
subcutaneous region with two to four peptides based on their HLA
type and on their pre-existing immunity represented by their
peptide-specific IgG levels as described (13-17).
The protocols were as follows: patients with lung
cancer (under the protocol with UMIN registration no. 00002984),
breast cancer (no. 000003081), or colon cancer (no. 000002987)
received vaccinations consisting of a 1.5-ml emulsion (3 mg/each
peptide) of 2-4 peptides across three cycles as follows: four
visits at 1-week intervals, followed by four visits at 2-week
intervals (the 1st cycle); four visits at 2-week intervals followed
by four visits at 4-week intervals (the 2nd cycle); and eight
visits at 4-week intervals (the 3rd cycle).
In addition, certain colon cancer patients from
northern Japan or outside of Japan were entered in the protocols
with UMIN registration nos. 000006927, 000011230 and 000001482.
They received vaccinations consisting of a 3.0-ml emulsion (6
mg/each peptide) of two to four peptides at each visit (half the
dose was injected into either side of the body) across three cycles
as follows: four visits at 4-week intervals (the 1st cycle),
followed by four visits at 4-to-8-week intervals (the 2nd cycle),
and finally four visits at 8- to 12-week intervals (the 3rd
cycle).
After the 3rd cycle, all patients who wished to
continue received the vaccination at 4- to 8-week intervals until
withdrawal of consent or unaccepTable toxicity. All protocols were
approved by the ethical committee of Kurume University and by the
regional ethical committee [Fukuoka Clinical Research Board (no.
718004)] and then registered in the UMIN Clinical Trials Registry
of the Japanese government. All of these studies were in accordance
with the Declaration of Helsinki and the International Conference
on Harmonization of Good Clinical Practice guidelines and were
conducted in an outpatient setting. Before their inclusion in the
study, all participants gave written informed consent to
participate in the clinical trial and to have their data used for
research and publication purposes.
Toxicity and general conditions were monitored at
the time of each visit. Toxicity was evaluated using the Common
Terminology Criteria for Adverse Events ver. 4.0.
Immune responses
The patients' peripheral blood was collected at
pre-treatment and at the end of the 1st, 2nd and 3rd cycles, and
then at each visit after ≥3 months (range 3-45 months). Their IgG
titers and cytotoxic T lymphocyte (CTL) activity specific to the
peptide in plasma or peripheral blood mononuclear cells (PBMCs)
were evaluated by a beadbased multiplexed Luminex assay (Luminex
Platform LHC6003M; Invitrogen; Thermo Fisher Scientific, Inc.) and
by an interferon (IFN)-γ ELISPOT (Immunocyte IFN-γ ELISPOT kit;
Medical and Biological Laboratories), respectively (17). Pre-vaccination peptide-specific IgG
levels with a cut-off level of 10 FIU were taken as detecTable
levels of IgG. Patients were considered to have a positive IgG
boost when the total sum of their post-vaccination IgG levels
against the vaccinated peptides (2-4 peptides) was >5,000 FIUs
compared to that of the pre-vaccination level.
The spreading of IgG boosting to non-vaccinated
peptides was quantified by measuring the total sum of FIU in
response to non-vaccinated peptides. The HLA-matched
peptide-specific CTL activity in PBMCs was evaluated by the IFN-γ
ELISPOT assay. Positive CTL boosting was defined as a >5-fold
increase in the total sum of HLA-matched peptide-specific IFN-γ
spots compared to the pre-vaccination level or >50 IFN-γ spots
if the pre-vaccination CTL activity was undetectable. The CEF
peptide pool (Mabtech, Cincinnati, OH) consisting of 23 HLA-class
I-restricted peptides from human influenza virus, cytomegalovirus,
and Epstein Barr virus was used as a control peptide.
The target cells used for the assay were T2 cells
for HLA-A2 or HMy2.CIR (CRL-1993; ATCC) cells transfected with the
HLA- A11, -A24, -A26, -A31, or -A33 gene as described (13-17).
We calculated the spreading of CTL activity to non-vaccinated
peptides by subtracting the sum of IFN-γ spots in response to all
31 peptide-mixtures from the sum of IFN-γ spots in response to the
vaccinated peptides. Pre-vaccination samples at the screening time
(14 days before the first vaccination) were provided for the
measurement of blood cell counts and C-reactive protein (CRP) as
described (17).
The corresponding author had full access to all of
the study data and had final responsibility for the decision to
submit this report for publication.
Statistical analyses
The Kaplan-Meier method was used for the statistical
analyses. Recurrence was determined by the new appearance of tumor
based on a radiological diagnosis or the increase of a tumor
marker. Recurrence-free survival (RFS) and overall survival (OS)
were calculated as the time in months from the day of the 1st
vaccination (for events) or to the date of last contact. Mixed
model analysis using Kenward-Roger F test was used for the
patients' IgG level values after peptide vaccination. All
statistical analyses were performed using JMP version 16.0 (SAS
Institute Inc., Cary, NC, USA).
Results
Patient characteristics and clinical
outcome
Table I summarizes
the patients' characteristics and clinical outcomes. The 44
patients without an active tumor consisted of 11 patients with lung
adenocarcinoma, 18 with colon cancer (origin of primary cancers: 13
rectal, two sigmoid colon, one each of cecum, ascending, and
transverse colon); and 15 with breast cancer (eight luminal, two
HER2+, four triple-negative types, and one apocrine cancer).
Systemic therapies were combined with PPV in none of the patients
with lung cancer, six of the patients with colon cancer
(chemotherapy in six cases), and eight of the patients with breast
cancer (chemotherapy and target therapy in one case each, and
hormone therapy in six cases) (Table
I). The median number of vaccinations was 20. The median
follow-up time was 67.6 months (interquartile range (IQR):
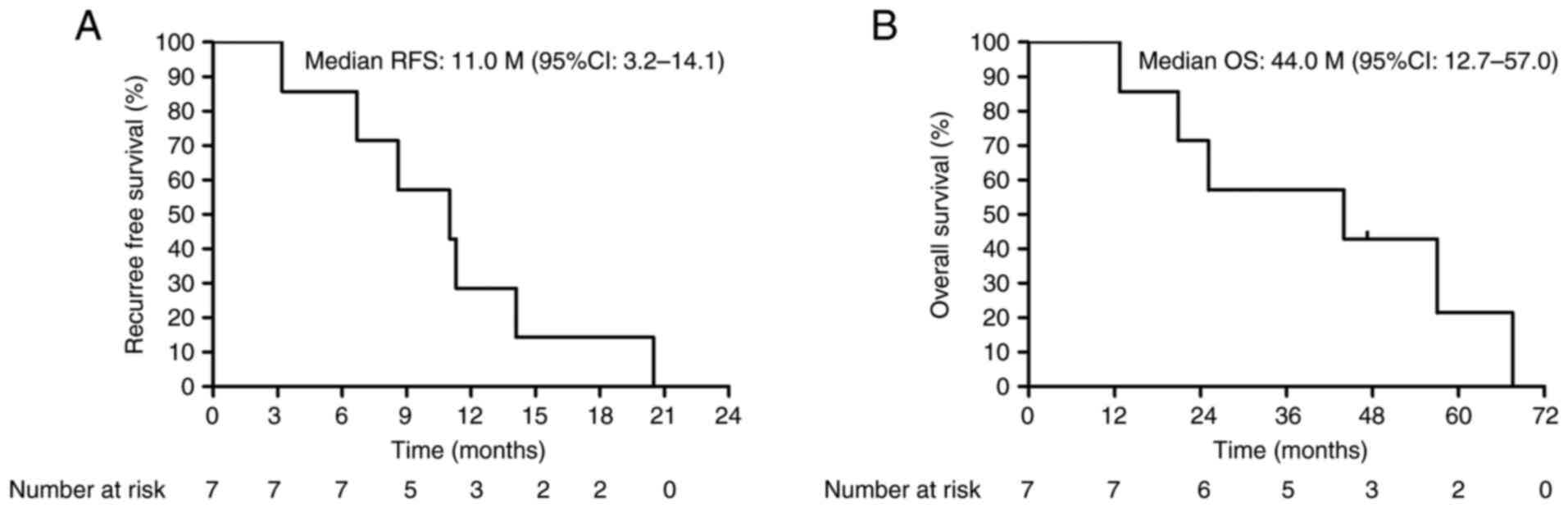
45.6-82.8). Under these circumstances, 37 of the 44 patients (84%)
had no recurrence. The remaining seven patients (one stage III lung
cancer, two stage IV and two recurrent colon cancers, and two stage
II breast cancers) had recurrence, and their median RFS and OS
rates were 11.0 months [95% confidence interval (CI): 3.2-14.1] and
44.0 months (95% CI: 12.7-57.0), respectively (Fig. 1A and B).
 | Table ICharacteristics of the enrolled
patients without active tumor at entry. |
Table I
Characteristics of the enrolled
patients without active tumor at entry.
| Characteristics | Characteristics of
all patients (n=44) | Patients with lung
adenocarcinoma (n=11) | Patients with colon
cancer (n=18) | Patients with breast
cancer (n=15) |
|---|
| Median age, years
(range) | 58 (36-83) | 66 (54-80) | 56 (36-83) | 53 (37-69) |
| Male:Female | 17:27 | 7:4 | 10:8 | 0:15 |
| Performance status
(0/1) | 43/1 | 10/1 | 18/0 | 15/0 |
| HLA
(A24/A2/A3family/A26) | 30/16/26/9 | 7/4/2/6 | 15/5/12/1 | 8/7/12/2 |
| Stage at entry
(I/II/III/IV recurrence) | 9/10/12/8/5 | 4/2/3/1/1 | 0/2/6/6/4 | 5/6/3/1/0 |
| Chemotherapy/hormone
therapy/ | 7/6/1 | 0/0/0 | 6/0/0 | 1/6/1 |
| targeted therapy | | | | |
| Median number of
vaccinations (range) | 20 (4-65) | 24 (8-65) | 16 (4-47) | 19 (5-56) |
| Median follow-up,
months (range) | 68 (13-113) | 70 (13-113) | 51 (25-96) | 74 (22-96) |
| Recurrence free
patients | 37/44 | 10/11 | 14/18 | 13/15 |
| IgG boosted at end of
1st cycle | 36/43 | 10/11 | 15/18 | 11/14 |
| CTL boosted at end of
1st cycle | 23/30 | 1/1 | 11/15 | 11/14 |
The detailed information for each of the 44 enrolled
patients from their first cancer surgery to the end of the clinical
study (Table II) helps clarify
the intervals from the surgery to the vaccination effect to the
outcome of PPV-induced cancer prevention. The median period from
surgery to the first vaccination was 14.5 months (IQR: 6.4-27.5
months). The median periods of the seven patients with recurrence
from their surgery to their first vaccination were as follows: 1.5
months (stage IV colon cancer), 2.5 months (stage II
triple-negative breast cancer), 6.5 months (stage III colon
cancer), 13 months (stage III lung cancer), 17.5 months (stage II
luminal type breast cancer), 17.5 months (stage II colon cancer),
and 90 months (stage I colon cancer). These results indicate that
the intervals from surgery to the first vaccination in the entire
series of 44 patients were not very different from the intervals of
the seven recurrent patients.
 | Table IIDetailed information of 44 enrolled
patients: From first operation to end of following-up
observation. |
Table II
Detailed information of 44 enrolled
patients: From first operation to end of following-up
observation.
| Patients |
Origin/histology/stage | Operation to first
vaccine, months | Age, years | Stage at first
vaccine | Number of
vaccinations | OS from first
vaccine, months |
|---|
| AMA-001 | BC/Lum/II | 2.0 | 69 | Ⅱ | 8 | 94.6 |
| AMA-003 | BC/Lum/I | 39.5 | 41 | I | 5 | 78.5 |
| AMA-004 | BC/apocrine
Ca/II | 6.0 | 64 | Ⅳ | 16 | 95.6 |
| AMA-005 | BC/TN/I | 4.5 | 61 | Ⅰ | 48 | 91.3 |
|
AMA-006a | BC/Lum/II | 17.5 | 42 | II | 19 | 66.7 |
| AMA-007 | BC/TN/III | 16.0 | 37 | Ⅲ | 24 | 87.6 |
| AMA-010 | BC/Her2/I | 6.8 | 63 | Ⅰ | 49 | 89.1 |
| AMA-012 | BC/Lum/III | 12.0 | 48 | Ⅲ | 9 | 70.2 |
| AMA-013 | BC/Lum/II | 150.0 | 51 | Ⅱ | 56 | 82.9 |
|
AMA-014a | BC/TN/II | 2.5 | 55 | II | 16 | 24.7 |
| AMA-015 | BC/Her2/I | 20.0 | 65 | Ⅰ | 43 | 79.1 |
| AMA-025 | BC/Lum/III | 76.0 | 52 | Ⅲ | 24 | 41.4 |
| AMA-026 | BC/TN/IIA | 16.5 | 38 | Ⅱ | 11 | 80.2 |
| AMA-042 | BC/Lum/II | 26.0 | 54 | Ⅱ | 16 | 36.0 |
| AMA-053 | BC/Lum/I | 7.6 | 50 | Ⅰ | 24 | 49.0 |
| ALU-001 | LC/Ad/II | 11.5 | 66 | Ⅱ | 18 | 98.5 |
| ALU-004 | LC/Ad/II | 6.0 | 65 | Ⅱ | 24 | 95.7 |
| ALU-005 | LC/Ad/I | 6.3 | 54 | Ⅰ | 65 | 113.0 |
| ALU-006 | LC/Ad/III | 6.2 | 61 | Ⅲ | 8 | 93.9 |
| ALU-014 | LC/Ad/II | 33.0 | 58 | Recurrence | 24 | 86.4 |
| ALU-047 | LC/Ad/I | 114.0 | 68 | Ⅰ | 23 | 62.1 |
| ALU-059 | LC/Ad/IV | 11.0 | 70 | Ⅳ | 23 | 67.1 |
| ALU-080 | LC/Ad/III | 4.0 | 67 | Ⅲ | 24 | 52.7 |
| ALU-082 | LC/Ad/I | 11.6 | 72 | Ⅰ | 52 | 69.8 |
| ALU-112 | LC/Ad/I | 64.0 | 65 | I | 20 | 27.8 |
|
ALU-119a | LC/Ad/III | 13.0 | 80 | Ⅲ | 8 | 12.6 |
| F2-037 | CC/II | 3.5 | 52 | Ⅱ | 6 | 81.3 |
| F2-047 | CC/III | 45.0 | 83 | Ⅲ | 8 | 72.5 |
| F-141 | CC/III | 12.5 | 50 | Ⅳ | 47 | 95.7 |
| F2-GAS-048 | CC/III | 2.0 | 72 | Ⅲ | 4 | 45.6 |
| F2-GAS-052 | CC/III | 9.0 | 36 | Ⅲ | 4 | 35.0 |
| F2-GAS-062 | CC/III | 19.0 | 42 | Ⅲ | 4 | 25.0 |
|
ACO-002a | CC/IV | 1.5 | 51 | Ⅳ | 45 | 43.4 |
| ACO-040 | CC/III | 10.5 | 59 | Ⅳ | 24 | 75.5 |
| ACO-054 | CC/III | 26.5 | 48 | Ⅲ | 22 | 72.4 |
|
ACO-086a | CC/I | 90.0 | 72 | II | 21 | 20.6 |
| ACO-091 | CC/IV | 55.0 | 40 | Ⅳ | 40 | 64.7 |
| ACO-093 | CC/III | 22.0 | 65 | IV | 8 | 61.3 |
|
ACO-094a | CC/III | 6.5 | 60 | III | 11 | 7.0 |
|
ACO-106a | CC/II | 17.5 | 53 | IV | 11 | 56.2 |
| ACO-134 | CC/III | 19.5 | 62 | Recurrence | 16 | 46.7 |
| ACO-137 | CC/II | 27.6 | 73 | Recurrence | 16 | 45.1 |
| ACO-145 | CC/IV | 30.5 | 43 | Recurrence | 27 | 43.5 |
| ACO-149 | CC/IV | 27.0 | 50 | IV | 30 | 41.5 |
Adverse events
The majority of patients had grade I or II skin
reactions at the injection sites. There were no PPV-related severe
adverse events other than grade III injection site reactions.
Details are given in Table
SII.
IgG responses
The kinetics throughout the study for the patients
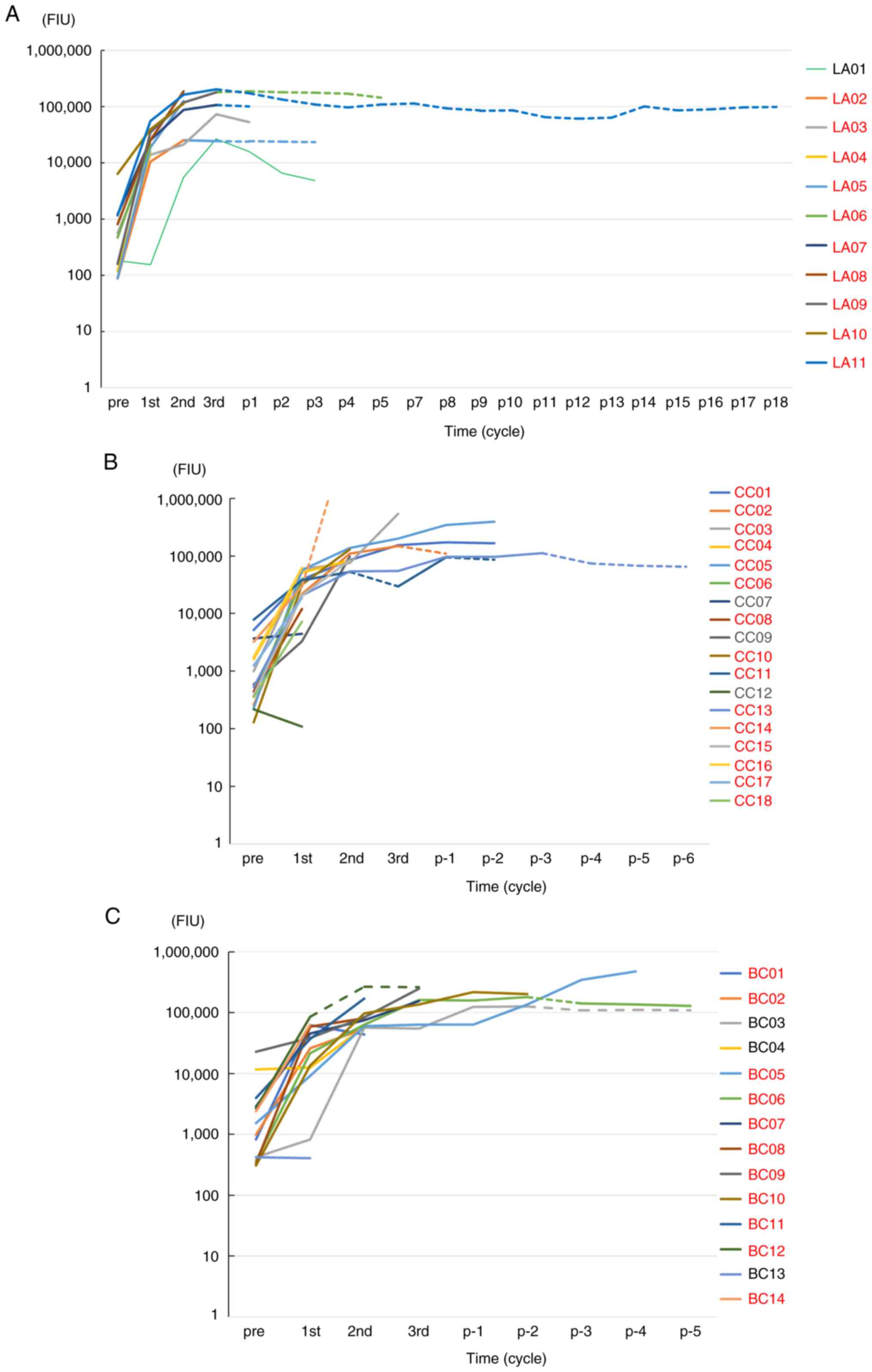
without an active tumor are depicted in Fig. 2 (lung adenocarcinoma, n=11), (colon
cancer, n=18), and (breast cancer, n=14), respectively. The plotted
points of IgG levels (FIU) were pre-vaccination, the end of the 1st
cycle (2-3 months later), the end of the 2nd cycle (7-9 months
later), the end of the 3rd cycle (15-17 months later), and
thereafter (every 3-34 months) during the follow-up. IgG boosting
was observed in 36 of the 43 patients tested at the end of the 1st
cycle, followed by an increase in IgG thereafter. In all 12
patients tested after the vaccine's termination, the IgG boosting
levels were maintained at levels ranging from 23,165 FIU at 34
months to 1,601,706 FIU at 63 months post-termination.
Collectively, among the 44 patients without an
active tumor, IgG boosting against the vaccinated peptides at the
end of the 1st and 2nd cycles of PPV was observed in 36 of 43
patients and all 43 patients whose samples were available,
respectively. Moreover, the PPV induced robust (>50,000 FIU)
humoral immunity for 41 of the 43 tested cancer patients without an
active tumor even after the follow-up period (Fig. 2A-C). IgG boosting at the end of the
1st cycle and the 2nd cycle was observed in three of the seven
recurrent patients and in three of the four recurrent patients
tested, respectively. These results suggest that post-vaccination
IgG boosting was a prognostic marker in PPV patients, which is
consistent with the reported observations (13,14,17).
CTL responses
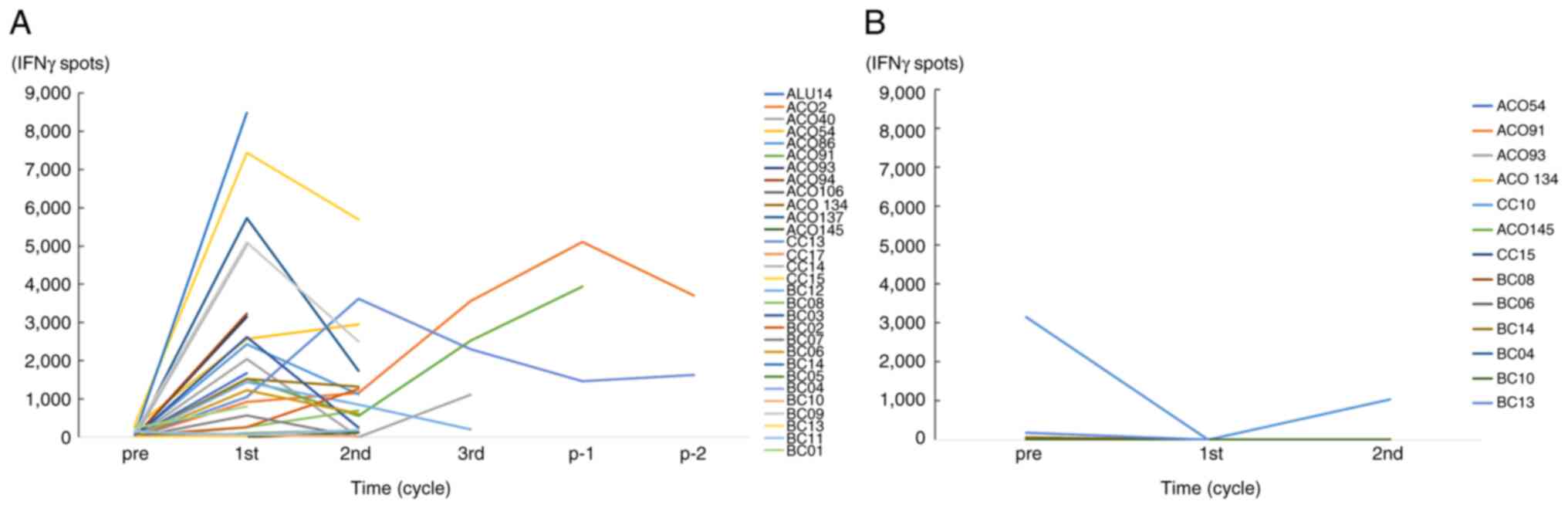
The CTL activity against the vaccinated peptides at
the end of the 1st cycle was boosted in 23 of the 30 tested
patients. However, these elevated CTL activities declined at the
end of the 2nd cycle in most of the tested cases (Fig. 3A). The CTL activity against
non-vaccinated peptides in the 13 patients tested was no boosted at
the 2nd cycle (Fig. 3B).
IgG response to a lymphocyte-specific
protein tyrosine kinase at positions 486-494 (Lck-486 peptide)
We investigated the IgG response to the Lck-486
peptide, which is capable of inducing HLA-A24-restricted CTL
activity; we did so because this peptide was vaccinated for the
majority of the present HLA-A24 patients (23 of 30). We speculated
that Lck-486 peptide could thus be a suiTable representative for
the evaluation of the kinetics of each peptide. This investigation
was also conducted based on our previous study of a monoclonal
anti-Lck-486 IgG (IgG 2b) peptide capable of inhibiting tumor
growth in vivo with a suppression of tumor-infiltrating T
regulatory cells in a murine model (18).
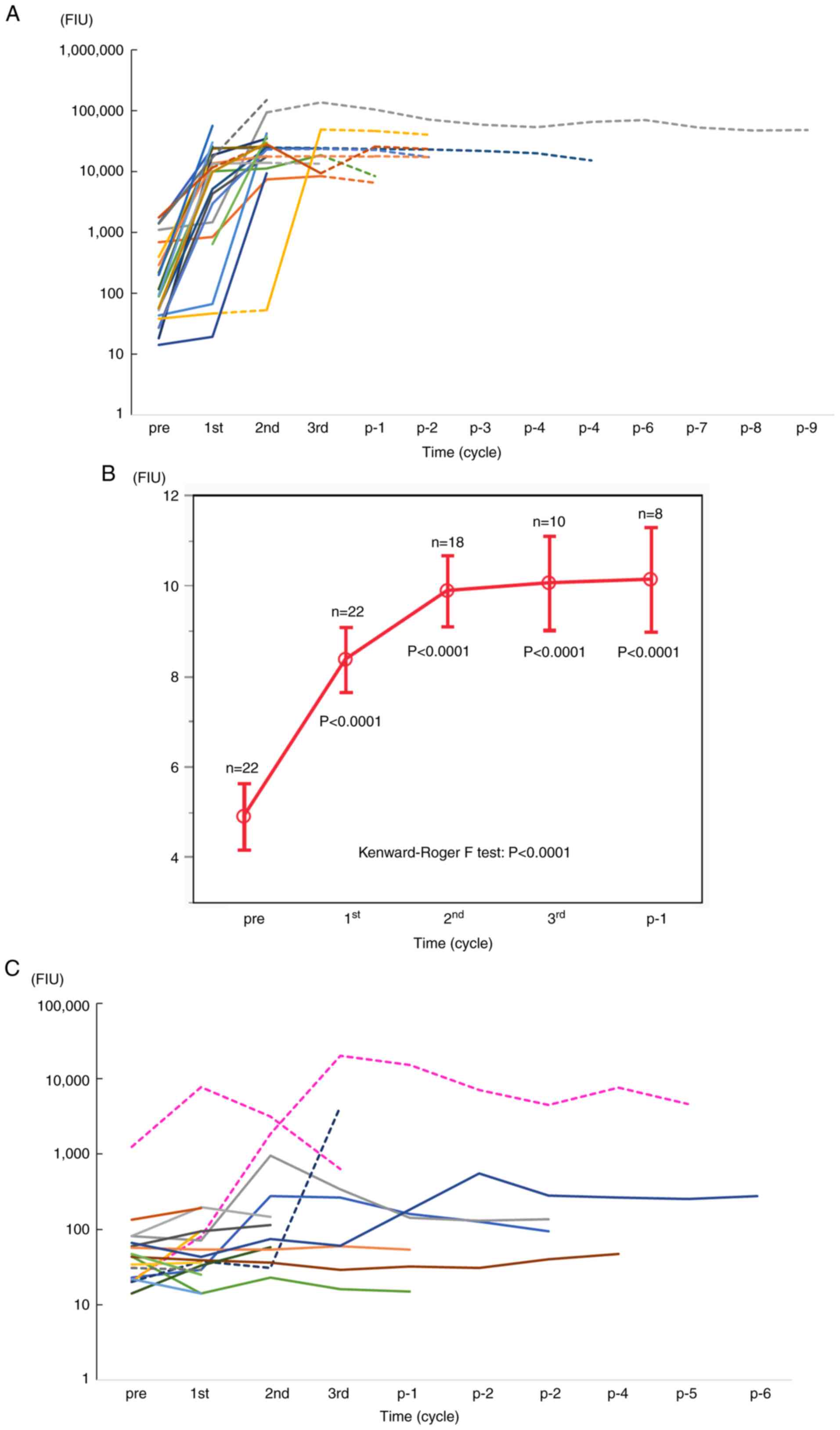
Among the 44 patients, 30 were HLA-A24+ and the
remaining 14 were HLA-A24- (Table
I). Twenty-three of the 30 HLA-A24+ patients received a
vaccination with the Lck-486 peptide; the remaining seven did not.
Robust IgG boosting (>50,000 FIU) against the Lck-486 peptide
was observed in all 22 HLA-A24+ patients tested, and the boosting
levels were maintained even after vaccine termination (Fig. 4A). The patients' median IgG levels
were significantly higher than their pre-vaccination levels
(P<0.0001) (Fig. 4B). In
contrast, IgG boosting against the Lck-486 peptide was not observed
in any of the seven HLA-A24+ patients without Lck-486 vaccination,
whereas it occurred in two of the 14 HLA-A24- patients without
Lck-486 vaccination (Fig. 4C).
We also assessed the clinical benefits of the
vaccinations, as our earlier study revealed that the patients who
received the Lck-486 vaccination had significantly longer survival
compared to patients with advanced cancer (19). Three of the 23 HLA-A24+ patients
(13%) developed cancer recurrence after receiving the Lck-486
peptide vaccination (among the 23 patients, the recurrence rate was
13%), whereas two of the HLA-A24+ patients (29%) developed cancer
recurrence after Lck-486 peptide vaccination. The RFS values were
11.3, 14.1 and 20.5 months respectively in the former three
patients, and 6.7 and 11.0 months in the latter two patients. The
OS values were 66.7, 43.4 and 46.7 months in the former three
patients, and 24.8 and 20.6 in the latter two, respectively. All
measurements of clinical events (recurrence rate, RFS, and OS) were
favorable in the patients who received the Lck-486 peptide.
Pre-vaccination inflammatory
signatures
We reported that pre-vaccination inflammatory
signatures hampered the clinical benefits of PPV for patients with
advanced cancer (13-17).
Herein, we investigated the following pre-vaccination inflammatory
signatures: 59.9% as the median percentage of neutrophils; 1.9 as
the neutrophil-lymphocyte ratio; 4,800 as the white blood cell
number; 31.2% as the percentage of lymphocytes; and
429x104 as the red blood cell number. The median CRP
level in the 17 tested patients without an active tumor was
0.09.
Discussion
The results of this study demonstrated that the PPV
induced robust (>50,000 FIU) humoral immunity for 41 of the 43
tested cancer patients without an active tumor even after the
follow-up period. In contrast, CTL activity was observed in 23 of
30 non-recurrent patients at the end of the 1st PPV cycle and in
four of six recurrent patients at the end of the 2nd cycle,
followed by a rapid decline. These results suggest that the CTL
induction rate in the non-recurrent cases was not very different
from that of the recurrent cases. The mechanisms involved in this
decline of CTL activity are presently unclear. A kinetic study of
T-cell subsets including regulatory T cells could be critical to
solve this issue. The involvement of myeloid-derived suppressor
cells in PPV-induced CTL suppression should also be fully examined,
since our earlier investigation demonstrated that myeloid-derived
suppressor cells were closely involved in the decline of CTL
activity (13,14,17).
Another of our studies showed that the
administration of monoclonal anti-Lck-486 IgG inhibited tumor
growth and suppressed tumor-infiltrating T regulatory cells in a
murine model, i.e., female BALB/c mice in which can bind to Lck-486
peptide (18). Lck antigen was
reported as a key molecule for T-regulatory cell activity (20,21).
In the present series of cancer patients, potent IgG boosting to
Lck-486 peptide occurred in all 22 of the HLA-A24+ patients who
received this peptide, and the boosting levels were maintained even
after vaccine termination. These results indicate that a robust IgG
response against the Lck-486 peptide may play a role in the
prevention of recurrence among HLA-A24+ patients. However, this
possibility must be confirmed by investigations of the
administration of either PPV-induced anti-Lck-486 IgG or a
monoclonal Lck-486 antibody to cancer patients.
The pre-vaccination inflammatory signatures in the
present patients without active tumors were almost within the
normal ranges. In contrast, we have repeatedly observed that these
signatures were higher than the normal ranges in patients with
advanced-stage cancer and were thus prognostic biomarkers for the
cancer vaccine (13,14). Together our past and present
findings demonstrate that the higher immune induction provided by a
cancer vaccine is a crucial issue for recurrence prevention.
The effects of the PPV on the patients' clinical
outcomes (RFS and OS) were examined. The reported OS rates of lung
adenocarcinoma patients are ~80% for the stage I patients, 50% for
stage II, 25% for stage III, and 8% for stage IV. The respective OS
rates of the colon cancer patients are 90, 80, 77 and 22%, and
those of the breast cancer patients were 92, 93, 77 and 39%; these
data were obtained from a Pfizer Japan source, i.e., https://ganclass.jp/qa/link/qa_family_link02.php.
However, it could be difficult to expect the same post-vaccination
recurrence rates for the patients in the present small-scale study
since the origin, histology, and cancer stages differed among the
44 enrolled patients (Table
II).
An exact molecular basis of anti-tumor humoral
immunity is not yet fully understood although the possible positive
roles are partly reported as cited in the original manuscript
(8-10).
Several review articles might facilitate its deeper understanding.
Dunn et al, well summarized the history of cancer
immunosurveillance controversy from a view of elimination,
equilibrium, and escape of tumor cells (22). Thomas et al, reviewed the
perspectives of humoral and cellular immune responses based on
NY-ESO-1 vaccines (23). Zitvogel
et al, put forward a hypothesis that gut microbial proteins
might be sufficiently similar to human tumor antigens and are thus
capable of eliciting tumor-specific T lymphocytes and antibodies
that can recognize future tumor cells via ‘antigenic mimicry’
(24). Discovery by Sivan et
al reported that commensal Bifidobacterium promoted anti-tumor
immunity and facilitate anti- PD-L1 efficacy, which in turn might
provide us future direction of cancer immunity (25).
In conclusion, our analyses revealed that the PPV
induced robust humoral immunity in the majority of cancer patients
without active tumors.
Supplementary Material
Information of peptide candidates used
for personalized peptide vaccination.
Adverse events during the personalized
peptide vaccination in 44 patients without active tumor.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS, KI and SY conceived and designed the study. SS
and KI collected and assembled data. SS, SY, UT, KY and KI were
involved in the data analysis and interpretation. All authors wrote
the manuscript. All authors have read and approved the final
manuscript. SS and KI confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All protocols were first approved by the ethical
committee of Kurume University and by the regional ethical
committee (Fukuoka Clinical Research Board; approval no. 718004)
and then registered in the UMIN Clinical Trials Registry of the
Japanese government. All of the study protocols were in accordance
with the Declaration of Helsinki and the International Conference
on the Harmonization of Good Clinical Practice guidelines and were
conducted in an outpatient setting. Before their inclusion in the
study, all participants gave written informed consent to
participate in the clinical trial and to have their data used for
research and publication purposes.
Patient consent for publication
Not applicable.
Competing interests
KI has received research funding from Taiho
Pharmaceutical Company. The other authors declare that they have no
competing interests.
References
|
1
|
Vansteenkiste JF, Cho BC, Vanakesa T, De
Pas T, Zielinski M, Kim MS, Jassem J, Yoshimura M, Dahabreh J,
Nakayama H, et al: Efficacy of the MAGE-A3 cancer immunotherapeutic
as adjuvant therapy in patients with resected MAGE-A3-positive
non-small-cell lung cancer (MAGRIT): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 17:822–835.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xiang B, Snook AE, Magee MS and Waldman
SA: Colorectal cancer immunotherapy. Discov Med. 15:301–308.
2013.PubMed/NCBI
|
|
3
|
Mittendorf EA, Lu B, Melisko M, Price
Hiller J, Bondarenko I, Brunt AM, Sergii G, Petrakova K and Peoples
GE: Efficacy and safety analysis of Nelipepimut-S vaccine to
prevent breast cancer recurrence: A randomized, multicenter, phase
III clinical trial. Clin Cancer Res. 25:4248–4254. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Clifton GT, Hale D, Vreeland TJ, Hickerson
AT, Litton JK, Alatrash G, Murthy RK, Qiao N, Philips AV, Lukas JJ,
et al: Results of a randomized phase IIb trial of Nelipepimut-S +
Trastuzumab versus Trastuzumab to prevent recurrences in patients
with high-risk HER2 low-expressing breast cancer. Clin Cancer Res.
26:2515–2523. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Harper DM, Franco EL, Wheeler CM, Moscicki
AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa
Clemens SA and Dubin G: HPV Vaccine Study group. Sustained efficacy
up to 4.5 years of a bivalent L1 virus-like particle vaccine
against human papillomavirus types 16 and 18: Follow-up from a
randomised control trial. Lancet. 367:1247–1255. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rosenbaum P, Artaud C, Bay S, Ganneau C,
Campone M, Delaloge S, Gourmelon C, Loirat D, Medioni J, Pein F, et
al: The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine
induces tumor-specific cytotoxic antibodies in breast cancer
patients. Cancer Immunol Immunother. 69:703–716. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gupta S, McDonald JD, Ayabe RI, Khan TM,
Gamble LA, Sinha S, Hannah C, Blakely AM, Davis JL and Hernandez
JM: Targeting CA 19-9 with a humanized monoclonal antibody at the
time of surgery may decrease recurrence rates for patients
undergoing resections for pancreatic cancer, cholangiocarcinoma and
metastatic colorectal cancer. J Gastrointest Oncol. 11:231–235.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van der Meijden PM, van Klingeren B,
Steerenberg PA, de Boer LC, de Jong WH and Debruyne FM: The
possible influence of antibiotics on results of bacillus
Calmette-Guérin intravesical therapy for superficial bladder
cancer. J Urol. 146:444–446. 1991.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schwartzentruber DJ, Lawson DH, Richards
JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K,
Pockaj B, et al: gp100 peptide vaccine and interleukin-2 in
patients with advanced melanoma. N Engl J Med. 364:2119–2127.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bezu L, Kepp O, Cerrato G, Pol J, Fucikova
J, Spisek R, Zitvogel L, Kroemer G and Galluzzi L: Trial watch:
Peptide-based vaccines in anticancer therapy. Oncoimmunology.
7(e1511506)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sasada T, Yamada A, Noguchi M and Itoh K:
Personalized peptide vaccine for treatment of advanced cancer. Curr
Med Chem. 21:2332–2345. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: A new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Narita Y, Arakawa Y, Yamasaki F, Nishikawa
R, Aoki T, Kanamori M, Nagane M, Kumabe T, Hirose Y, Ichikawa T, et
al: A randomized, double-blind, phase III trial of personalized
peptide vaccination for recurrent glioblastoma. Neuro Oncol.
21:348–359. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Noguchi M, Fujimoto K, Arai G, Uemura H,
Hashine K, Matsumoto H, Fukasawa S, Kohjimoto Y, Nakatsu H,
Takenaka A, et al: A randomized phase III trial of personalized
peptide vaccination for castration-resistant prostate cancer
progressing after docetaxel. Oncol Rep. 45:159–168. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Suekane S, Yutani S, Yamada A, Sasada T,
Matsueda S, Takamori S, Toh U, Kawano K, Yoshiyama K, Sakamoto S,
et al: Identification of biomarkers for personalized peptide
vaccination in 2,588 cancer patients. Int J Oncol. 56:1479–1489.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsueda S, Itoh K and Shichijo S:
Antitumor activity of antibody against cytotoxic T lymphocyte
epitope peptide of lymphocyte-specific protein tyrosine kinase.
Cancer Sci. 109:611–617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Noguchi M, Koga N, Moriya F, Suekane S,
Yutani S, Yamada A, Shichijo S, Kakuma T and Itoh K: Survival
analysis of multiple peptide vaccination for the selection of
correlated peptides in urological cancers. Cancer Sci.
109:2660–2669. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Taskén K: Waking up regulatory T cells.
Blood. 114:1136–1137. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bardhan K, Patsoukis N, Weaver J, Freeman
G, Li L and Boussiotis VA: PD-1 inhibits the TCR signaling cascade
by sequestering SHP-2 phosphatase, preventing its translocation to
lipid rafts and facilitating Csk-mediated inhibitory
phosphorylation of Lck. J Immunol 196 (Suppl 1). 128(15)2016.
|
|
22
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thomas R, Al-Khadairi G, Roelands J,
Hendrickx W, Dermime S, Bedognetti D and Decock J: NY-ESO-1 based
immunotherapy of cancer: Current perspectives. Front Immunol.
9(947)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zitvogel L, Ayyoub M, Routy B and Kroemer
G: Microbiome and anticancer immunosurveillance. Cell. 165:276–287.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015.PubMed/NCBI View Article : Google Scholar
|