Introduction
Epithelial ovarian cancer (OC) is the 2nd most
prevalent malignancy of the gynecological tract and the 5th leading
cause of death in women (1). A
significant proportion (70%) of the patients with epithelial OC,
are diagnosed with advanced stage disease mainly due to the lack of
specific symptomatology keeping the cure rates, even with advances
in modern treatment, at a low stage (2). A proportion of as high as 70% of
patients will be presented with disease relapse within the first 2
years of diagnosis even after optimal treatment (3). Recurrence rates also remain elevated
for early stages accounting for 20-25% (3). The optimal management of patients
with advanced stage OC combines cytoreductive surgery supplemented
with platinum-based chemotherapy. The platinum component is usually
cisplatin or carboplatin combined with a taxane including
paclitaxel or docetaxel and are administered through intravenous
route at 3-4 weeks' intervals. A survival benefit on either overall
survival or progression free survival has been shown for the
patients who are left with no macroscopic residual disease after
complete cytoreduction (4,5). The management of recurrences is
challenging while second-line chemotherapy has been associated with
poor outcomes based on the high chemo-resistance rates (3). For patients with platinum sensitive
recurrent disease 6 cycles of platinum-based chemotherapy are
recommended (6). For relapses with
platinum free interval less than 6 months non platinum based agents
have been used including pegylated liposomal doxorubicin, taxol
once a week, topotecan or doxorubicin (7). Recently, the development of targeted
therapies is now considered of critical importance (3).
Upper abdominal disease is a frequent finding in
numerous cases of advanced OC and has been attributed to diffuse
peritoneal dissemination (8).
Diaphragmatic involvement, especially of the right hemidiaphragm,
has been reported at a range of 42 to 71% of the cases (9). Complete resection of the disease
recognized in the diaphragm at the time of cytoreduction has been
associated with favorable survival outcomes, as it has been shown
that recurrent disease often appears as diaphragmatic residual
lesions. This fact pinpoints the paramount importance of complete
resection of the diaphragmatic disease (8,10).
Diaphragmatic stripping is the most prevalent procedure for the
resection of diaphragmatic disease whereas full thickness excision
is required in cases of additional involvement of the underlying
muscle, despite its association with increased perioperative
complications (11). A plethora of
less invasive ablative methods such as argon and helium beam plasma
have been utilized for the resection of disseminated peritoneal
implants aiming to minimize the risk of postoperative complications
related to extensive surgical procedures to affected structures
with simultaneous achievement of complete resection of the
malignant lesions. We hypothesize that helium gas plasma (J-plasma)
could be beneficial for diaphragmatic stripping, tumor plaque
removal or for diffuse tumor deposits vaporization, combining the
unique properties of cold helium plasma with radiofrequency energy.
This device can adjust radiofrequency energy for greater control of
tissue effect, enabling thus a higher level of precision and
virtually eliminating unintended tissue trauma (12).
The objective of the present study was to present
preliminary data concerning the safety and treatment efficacy of
J-Plasma in cases of advanced OC with upper abdominal involvement
undergoing peritoneal and primarily diaphragmatic resection.
Materials and methods
A retrospective review of a prospectively maintained
database of patients who had diaphragmatic stripping with the use
of a novel device (J-Plasma® Precise open 150 mm) from
January 2016 to September 2020, due to peritoneal dissemination for
advanced stage OC (FIGO stage ≥III) was performed. The records of
patients with diaphragmatic disease who underwent surgery either
primary or interval debulking for the management of their disease,
were retrospectively retrieved and analyzed. We excluded from the
study patients who did not give informed consent at the time of
surgery, those considered unfit for extensive cytoreduction or did
not respond to neoadjuvant chemotherapy as well as those diagnosed
with ovarian metastasis from another primary tumor. The
Institutional Review Board (IRB) of General Hospital ‘Alexandra’
approved the conduction of the study and access to patient database
(no. 800/14-10-2019).
The use of the J-Plasma device was on a pilot trial
in the department of surgical oncology of our institution with the
intension to evaluate its safety and feasibility. The open surgical
approach was followed for all patients according to the strategies
adopted by the Gynecologic Oncology Unit of the 1st Department of
Obstetrics and Gynecology of General Hospital ‘Alexandra’. A full
pre-operative work up was performed including CT and/or MRI scans
and tumor markers. The procedures performed were individualized
according to each case and aimed for complete cytoreduction. In
selected cases where peritoneal implants of the bowel and the
mesentery were recognized, plasma was used as a means of
vaporization. The J-Plasma device was utilized with a pencil grip
of 15 cm, set at 75% power at 24 watts and a gas flow of 4 L/ml.
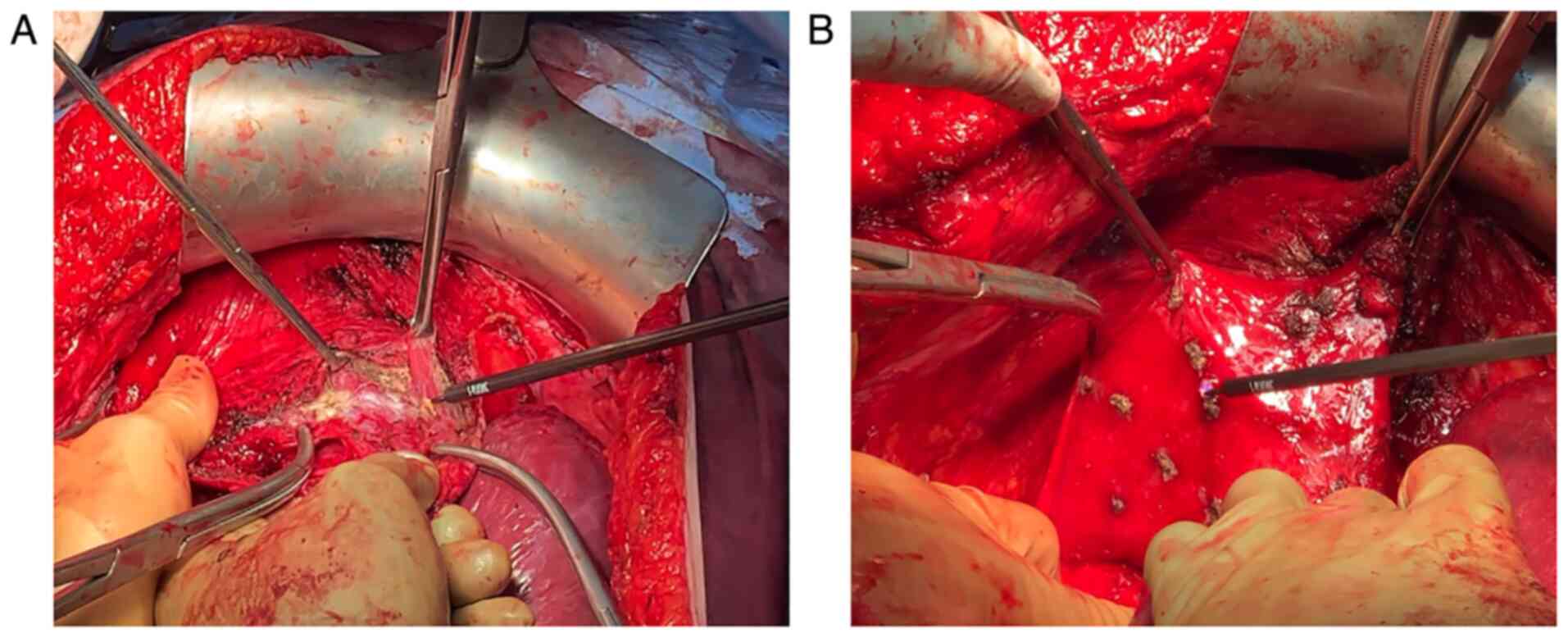
The application of J-plasma in the diaphragmatic lesions consisted
of full-thickness diaphragmatic resection, diaphragmatic peritoneal
excision (Fig. 1A) or ablation of
diaphragmatic implants (Fig. 1B).
At the completion of partial resection of the diaphragm for
full-thickness diaphragmatic disease, a ‘bubble test’ was performed
to evaluate the integrity of the diaphragm that was repaired after
the application of the aforementioned technique (13).
Statistical analysis was performed with SPSS v.25.0
statistical software (version 23.0; IBM Corp.). Continuous
variables were interpreted as median and range while categorical
variables were assessed in percentages and total number.
Investigated outcomes
Our primary outcomes included overall survival (OS),
disease free survival (DFS), recurrence rates, postoperative 30-day
mortality and postoperative complications. Additionally, type of
surgery, operative time, blood loss and length of hospital stay
were also recorded. Data on patients' and disease characteristics
included patients' age, BMI, performance status, CA-125,
histological type and disease stage as well as presence of ascites
and pleural effusion in the patients under evaluation.
Disease staging was made according to International
Federation of Gynecology and Obstetrics (FIGO) staging
classification for epithelial OC (14). The patients' performance status was
evaluated according to the Eastern Cooperative Oncology Group
(ECOG) performance status tool (15) while surgical complications were
classified according to the 5 grading system in Dindo-Clavien
classification (16).
Results
Patient and disease
characteristics
A total of 12 patients who underwent diaphragmatic
stripping with the use of J-plasma for cytoreduction due to
advanced OC were included. Median patients age was 65 years (range:
45-75 years) and median BMI was 27 kg/m2 (range: 23-32
kg/m2). Median preoperative CA-125 levels were 403 U/ml
(range: 112-2,115 U/ml). The median performance status of patients
(assessed using the ECOG performance status) was 1 (range: 1-2).
High grade serous ovarian carcinoma was the most common
histological type and was expressed in 11 out of 12 patients
(91.7%). Synchronous presence of high grade serous ovarian and
endometrioid endometrial carcinoma was detected in one patient
whereas another one was diagnosed with ovarian carcinosarcoma as
well. At primary diagnosis, 11 (91.7%) patients were classified as
FIGO stage IIIC (in double primary cancer patient ovarian was stage
IIIC and endometrial IA), whereas the remaining one with
carcinosarcoma had FIGO stage IV disease. Ascites was
preoperatively detected in 11 (91.7%) cases while 1 patient had
also unilateral pleural effusion. Table I depicts baseline patient
characteristics.
 | Table IBaseline patient characteristics. |
Table I
Baseline patient characteristics.
| Characteristic | Value |
|---|
| Median age, years
(range) | 65 (45-75) |
| Median BMI,
kg/m2 (range) | 27 (23-32) |
| ECOG performance
status | 1 (1-2) |
| Median CA-125
(U/ml) | 403 (112-2115) |
| Histological type, n
(%) | |
|
High grade
serous | 10 (83.4%) |
|
Serous high
grade + endometroid endometrial | 1 (8.3%) |
|
Carcinosarcoma | 1 (8.3%) |
| FIGO stage, n
(%) | |
|
IIIC | 11
(91.7%)a |
|
IV | 1 (8.3%) |
| Ascites, n (%) | 11 (91.7%) |
| Pleural effusion, n
(%) | 1 (8.3%) |
Short-term outcomes
Six patients (50%) had primary debulking surgery
whereas the remaining 6 (50%) underwent interval debulking surgery
after 3 cycles of neoadjuvant chemotherapy (NACT) with
carboplatin-paclitaxel. All patients were treated with
hysterectomy, bilateral salpingo-oophorectomy and omentectomy. In 5
patients (41.6%) peritonectomy was performed in addition to
diaphragmatic stripping. Three (25%) patients had splenectomy and
one had additional appendicectomy (8.3%). All patients underwent
resection of the peritoneum of the right diaphragm while 2 of them
(16.6%) had also stripping of the left diaphragmatic peritoneum.
Diaphragmatic lesions were the main ablated areas while J-plasma
was also applied for the resection of peritoneal mesenteric and
liver implants in 5/12 patients (41.6%). Diaphragmatic stripping
was applied in 8/12 patients (66.6%), full-thickness resection in
3/12 patients (25%) while in one patient (8.33%) ablation of the
diaphragmatic lesions was performed. Five (41.6%) patients had
concomitant bowel resections due to colorectal involvement.
Complete cytoreduction (RD: 0) was achieved in all 12 patients.
Median operative time was 240 min (range: 200-320
min) while the median estimated blood loss was 400 ml (range:
320-1,500 ml). Median operative time for the diaphragmatic
resection was 25 min (range: 20-40 min). No major intraoperative
complications were recorded except a case of intraoperative
hemorrhage which was successfully managed with intraoperative fluid
replacement, colloids and blood transfusion. No J-plasma related
complications and/or respiratory complications were noted. No
defect in the diaphragmatic integrity and connection with the
pleural cavity following resection were detected as evaluated by
the ‘bubble test’. Grade I postoperative complications were
detected in 8 patients (66.6%), grade II in 2 (16.7%) patients and
grade III complications were recorded in 2 patients (16.7%)
(Table II). Median length of
hospital stay was 9.5 days (range: 7-20 days). No postoperative
mortality was recorded. Table II
depicts the short term surgical outcomes.
 | Table IIShort- and long-term perioperative
outcomes. |
Table II
Short- and long-term perioperative
outcomes.
| Outcome | No. of patients
(%) |
|---|
| Type of debulking
surgery | |
|
PDS, n
(%) | 6(50) |
|
IDS, n
(%) | 6(50) |
| Type of surgical
procedure, n (%) | |
|
TAH/BSO | 12(100) |
|
Omentectomy | 12(100) |
|
Peritonectomy | 5 (41.6) |
|
Splenectomy/Appendicectomy | 3(25)/1 (8.33) |
|
Bowel
resection | 5 (41.6) |
|
Right
diaphragmatectomy | 12(100) |
|
Left
diaphragm stripping | 2 (16.6) |
| Median operative
time, min (range) | 240 (200-320) |
| Median estimated
blood loss, ml (range) | 600 (320-1500) |
| Median length of
hospital stay, days (range) | 9.5 (7-20) |
| Complications, n
(%) | |
|
Nausea and
vomiting | 3(25) |
|
Fever | 1 (8.3) |
|
Surgical
site infection | 2 (16.7) |
|
Anemia | 2 (16.7) |
|
Ileus | 1 (8.3) |
|
Intra-abdominal
abscess | 2 (16.7) |
|
Cardiac
ischemia | 1 (8.3) |
| Complications
(Dindo-Clavien), n (%) | |
|
I | 8 (66.6) |
|
II | 2 (16.7) |
|
III | 2 (16.7) |
| 30 day mortality,
n | 0 |
| Recurrences, n
(%) | 2(17) |
| Median OS, months
(range) | 12 (6-26) |
| Median DFS
(months) | 12 (10-13) |
Survival outcomes
Postoperatively, 6 patients that received NACT, were
treated with 3 cycles of adjuvant chemotherapy using
carboplatin-paclitaxel whilst the remaining 6 patients received 6
cycles of carboplatin-paclitaxel. During a median follow-up period
of 12 months (range:6-26 months), 2 patients (17%) presented with a
disease recurrence (one local pelvic wall recurrence and one
distant peritoneal) while none of the patients died of the disease
during the follow-up period. Median disease free survival was 12
months (range:10-13 months) (Table
II).
Discussion
In the present study we sought to evaluate the
safety and efficacy of a novel device in terms of resection of
diaphragmatic lesions in patients with disseminated advanced OC
with diaphragmatic involvement. Based on the currently available
literature, this is to the best of our knowledge, the first study
which evaluated the potential effect of helium gas cold atmospheric
plasma with the use of J-plasma electrosurgical device for the
management of peritoneal carcinomatosis due to epithelial OC. In
our study the device was primarily used during diaphragmatic
stripping of peritoneal metastases. We observed that J-plasma
effectively allowed complete resection of peritoneal lesions with
low impact on postoperative morbidity. Moreover, none of the
patients experienced recurrence in the site of lesions treated with
J-plasma; thus, depicting its potential treatment efficacy in
controlling the disease, at least during the first year following
primary debulking surgery.
The application of J-Plasma is based on the
combination of radiofrequency power with the cold flow of helium
gas. The most important feature of the device is the minimal depth
of diffusion of energy into the adjacent tissues, compared to other
known energy technologies (15).
Consequently, the respective damage to the remaining healthy tissue
is limited. This important feature of J-Plasma technology makes the
device suitable for a plethora of applications and more
specifically, for the destruction or resection of various malignant
lesions in areas requiring subtle manipulations. Moreover, the
effect of the CAP component (Cold Atmospheric Plasma), provides the
unique ability of selective destruction of various types of cancer
and bacterial cells (16). The
energy that is released by the CAP component of J-Plasma, has
proved to interact individually or in combination with tissues
biological cells. Research on this novel observation has
highlighted the benefits of CAP in a number of therapeutic and
cosmetic applications (17). To
that end, ongoing research in this field seems promising in
establishing new medical applications for cold plasma. J-Plasma is
the energy source that harnesses the benefits of CAP in surgery for
open, laparoscopic and robotic surgical procedures.
In clinical practice, the pilot application of
J-Plasma during surgery for gynecological diseases has also been
described for the treatment of endometriosis with encouraging
outcomes in terms of damage to the underlying tissues (17). However, those are still preliminary
outcomes and further studies are needed so as to designate the
exact depth of spread to the surrounding tissues as well as the
impact of this energy source in ovarian reserve.
Given the fact that OC disseminates through the
peritoneum surface, electrosurgical ablative methods and techniques
with minimal penetration to the underlying tissue could be
beneficial ensuring a lower morbidity as well as eradication of
lesions identified in surgically demanding locations (9). Various types of energy sources such
as monopolar ablation, CO2 laser, argon beam plasma and
plasma energy, have been proposed aiming at the complete resection
of the malignant lesions while simultaneously reducing the lateral
spread and penetration to the affected organs (18-20).
Despite the high prevalence of diaphragmatic
involvement in cases of advanced OC with involvement of the upper
abdomen, diaphragmatic resection is technically challenging and is
performed by less than 30% of the gynecologic oncologists in the
UK, precluding thus complete cytoreduction in these patients
(12). Moreover, Eskander et al
have showed that ~39% of the patients with advanced OC reported to
have undergone optimal cytoreduction, still have disease on the
right and left diaphragmatic area (21). A plethora of less invasive
techniques have reported favorable outcomes in the treatment of
diaphragmatic implants including peritoneal stripping,
radiofrequency ablation and argon beam plasma ablation (8,22).
In the present study, we sought to apply J-Plasma as a minimal
invasive electrosurgical method for resection of diaphragmatic
implants and peritoneal lesions. The median operative time for the
diaphragmatic resection of 20 min, including the application of the
technique was comparable to those reported by other studies
reporting outcomes of various diaphragmatic interventions (22,23).
Among them, Muallem et al reported a significantly prolonged
operative time in patients who had diaphragmatic surgery compared
to those who had cytoreductive procedures without diaphragmatic
resection, which could probably be attributed to the surgically
demanding diaphragmatic areas as well as to the operative technique
(22). In the same study, the mean
difference in operative time among procedures, which include
diaphragmatic resection and those, which did not, was 38.5 min
which was approximately double as much as that of diaphragmatic
resection found in our study (21 min). This could indicate the
potential beneficial effect of J-Plasma in minimizing the time of
diaphragmatic resection based on its ability to simultaneous
coagulate the small vessels that require coagulation. Overall, ~2/3
of our patients required diaphragmatic stripping for the management
of the disease while full-thickness resection was performed in one
fourth of them. Those rates are in accordance to those reported in
the literature for diaphragmatic resection techniques (22). Compared to full-thickness
diaphragmatic excision it has been proved that performing only
peritoneal diaphragmatic stripping has been associated with
significantly decreased pulmonary complication rates (33-72.7 vs.
19.5-46.6%, respectively) (13).
In the present study, we did not record any pulmonary complications
not even in the patients who underwent full-thickness diaphragmatic
excisions.
Despite J-Plasma's promising effect, our results
should be interpreted as preliminary as our study primarily aimed
at evaluating short term complications of J-plasma and not its
treatment efficacy. The small-sample size, the short follow-up
period and the retrospective nature of the present study are
considered the main limitations of our study. However, future
randomized trials should be organized with an adequate follow-up
time which will permit extraction of safe conclusions concerning
the impact of J-plasma on long-term control of treated metastases
and evaluation of patterns of relapse in these patients.
Concluding, the findings of our study support that
J-plasma can be used during diaphragmatic stripping as it is
associated with low rates of short-term morbidity and less
operative time needed for stripping which are lower compared to the
one that follows traditional peritoneal technic. Furthermore, in
terms of disease control it seems to be particularly effective as
none of our patients experienced disease relapse in abdominal
regions that were treated with J-plasma. However, given the absence
of a control group and the lack of an adequate follow-up time its
effect on the long term survival of OC patients undergoing
debulking operations remains elusive.
Acknowledgements
The abstract was presented in the 27th European
Congress of Obstetrics and Gynaecology Sep 2-4 2021 in Athens,
Greece and published as abstract no. 410 in Eur J Obstet Gynecol
Reprod Biol 270 (e109-e110): Mar 2022.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH conceived, supervised and validated the study and
methodology, performed investigation, supervised and wrote,
reviewed and edited the original draft. VT analyzed and curated the
data, obtained resources and wrote the original draft. AP analyzed
and curated the data, performed investigation, designed
methodology, obtained resources and wrote the original draft. ES
performed investigation, visualized the data and wrote and reviewed
the original draft. NA interpreted the data, wrote and reviewed the
original draft. AR conceived, designed, supervised the study and
reviewed and edited the original draft. All authors have read and
approved the final manuscript. VT and AP confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
This retrospective chart review study involving
human participants was in accordance with the ethical standards of
the institutional and national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. The Human Investigation Committee (IRB) of General
Hospital ‘Alexandra’ approved this study (approval no.
800/14-10-2019).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baek MH, Lee SW, Park JY, Rhim CC, Kim DY,
Suh DS, Kim JH, Kim YM, Kim YT and Nam JH: Preoperative predictive
factors for complete cytoreduction and survival outcome in
epithelial ovarian, tubal, and peritoneal cancer after neoadjuvant
chemotherapy. Int J Gynecol Cancer. 27:420–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rehmani H, Li Y, Li T, Padia R, Calbay O,
Jin L, Chen H and Huang S: Addiction to protein kinase Cɩ due to
PRKCI gene amplification can be exploited for an aptamer-based
targeted therapy in ovarian cancer. Signal Transduct Target Ther.
5(140)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liang MI, Prendergast EN, Staples JN,
Holschneider CH, Cohen JG and Cass I: Prognostic role of pathologic
response and cytoreductive status at interval debulking surgery
after neoadjuvant chemotherapy for advanced epithelial ovarian
cancer. J Surg Oncol. 120:779–785. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eggink FA, Koopmans CM and Nijman HW:
Surgery for patients with newly diagnosed advanced ovarian cancer:
Which patient, when and extent? Curr Opin Oncol. 29:351–358.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim SI, Hwang WY, Lee M, Kim HS, Kim K,
Chung HH, No JH, Kim JW, Kim YB, Park NH, et al: Survival impact of
extended cycles of second-line chemotherapy in platinum-sensitive
relapsed ovarian cancer patients with residual tumor after six
cycles. BMC Cancer. 20(1199)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lindemann K, Gao B, Mapagu C, Fereday S,
Emmanuel C, Alsop K and Traficante N: Australian Ovarian Cancer
Study Group. Harnett PR, Bowtell DDL and deFazio A: Response rates
to second-line platinum-based therapy in ovarian cancer patients
challenge the clinical definition of platinum resistance. Gynecol
Oncol. 150:239–246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bacalbasa N, Balescu I, Balalau C, Ionescu
O and Stoica C: Association of diaphragmatic surgery as part of
cytoreductive effort in advanced stage ovarian cancer. In Vivo.
32:431–436. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pounds R, Phillips A, Kehoe S, Nevin J,
Sundar S, Elattar A, Teo HG, Singh K and Balega J: Diaphragm
disease in advanced ovarian cancer: Predictability of pre-operative
imaging and safety of surgical intervention. Eur J Obstet Gynecol
Reprod Biol. 226:47–53. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grimm C, Harter P, Heitz F and du Bois A:
The sandwich technique of diaphragmatic stripping or full-thickness
resection for advanced ovarian cancer: How to keep it short and
simple. Int J Gynecol Cancer. 25:131–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Majd HS, Ferrari F, Manek S, Gubbala K,
Campanile RG, Hardern K and Tozzi R: Diaphragmatic peritonectomy
vs. full thickness resection with pleurectomy during
visceral-peritoneal debulking (VPD) in 100 consecutive patients
with stage IIIC-IV ovarian cancer: A surgical-histological
analysis. Gynecol Oncol. 140:430–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Panuccio E, Leunen K, Van Nieuwenhuysen E,
Neven P, Lambrechts S and Vergote I: Use of plasmajet for
peritoneal carcinomatosis in ovarian cancer. Int J Gynecol Cancer.
26:1521–1524. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kehoe SM, Eisenhauer EL and Chi DS: Upper
abdominal surgical procedures: Liver mobilization and diaphragm
peritonectomy/resection, splenectomy, and distal pancreatectomy.
Gynecol Oncol. 111 (Suppl 2):S51–S55. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Prat J: FIGO Committee on Gynecologic
Oncology. Staging classification for cancer of the ovary, fallopian
tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–656. 1982.PubMed/NCBI
|
|
16
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Sur. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Parsa M: Retroperitoneal dissection of
ovarian endometrioma using J-Plasma technology. J Minim Invasive
Gynecol. 22(S140)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Renaud MC and Sebastianelli A: Optimal
cytoreduction with neutral argon plasma energy in selected patients
with ovarian and primitive peritoneal cancer. J Obstet Gynaecol
Can. 35:49–52. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sonoda Y, Olvera N, Chi DS, Brown CL,
Abu-Rustum NR and Levine DA: Pathologic analysis of ex vivo plasma
energy tumor destruction in patients with ovarian or peritoneal
cancer. Int J Gynecol Cancer. 20:1326–1330. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Prodromidou A, Pandraklakis A and Iavazzo
C: The emerging role of neutral argon plasma (PlasmaJet) in the
treatment of advanced stage ovarian cancer: A systematic review.
Surg Innov. 27:299–306. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eskander RN, Kauderer J, Tewari KS, Mannel
RS, Bristow RE, O'Malley DM, Rubin SC, Glaser GE, Hamilton CA,
Fujiwara K, et al: Correlation between Surgeon's assessment and
radiographic evaluation of residual disease in women with advanced
stage ovarian cancer reported to have undergone optimal surgical
cytoreduction: An NRG oncology/gynecologic oncology group study.
Gynecol Oncol. 149:525–530. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Muallem MZ, Almuheimid J, Richter R,
Braicu EI, Osman S and Sehouli J: Diaphragmatic surgery in advanced
ovarian, tubal and peritoneal cancer. A 7-year retrospective
analysis of the tumor bank ovarian cancer network. Anticancer Res.
36:4707–4713. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsolakidis D, Amant F, Van Gorp T, Leunen
K, Neven P and Vergote I: Diaphragmatic surgery during primary
debulking in 89 patients with stage IIIB-IV epithelial ovarian
cancer. Gynecol Oncol. 116:489–496. 2010.PubMed/NCBI View Article : Google Scholar
|