Introduction
Recurrent intracranial malignant tumours with
complex pathological types may be difficult to treat surgically,
due to their proximity to critical neurovascular structures of the
skull base or previous administration of multi-modality treatment,
such as surgery or radiotherapy (RT). The treatment options for
recurrent intracranial malignant tumours are very limited. Although
reoperation and standard-dose salvage chemotherapy are used in
selected patients, they only provide palliative effects (1). In this context, reirradiation of the
intracranial recurrent lesion may improve local control and prolong
survival; however, caution is required due to cumulative late
central nervous system toxicity and lack of a likely chance of a
cure (2,3).
Photon-based RT remains the standard of care for the
treatment of brain tumours. Notably, carbon ion therapy (CIT) is
becoming more widely available for cranial irradiation (4). Radiation-induced brain necrosis is
one of the most serious adverse events that can lead to
neurological decline and even death (5). Its diagnosis usually requires
advanced imaging techniques to quantify signal changes and
differentiation from tumour recurrence (6). Bevacizumab is an anti-vascular
endothelial growth factor (VEGF) antibody, which has been used to
treat symptomatic cerebral radiation necrosis (RN) (7). However, to the best of our knowledge,
there are no studies on the use of bevacizumab to treat CIT-induced
RN.
A clinical trial (no. KJTJ2018013BOJI) to verify the
safety and effectiveness of CIT was performed in Wuwei Heavy Ion
Hospital (Wuwei, China), between November 2018 and February 2019.
Prior to patient enrolment, the clinical trial was approved by the
ethics committee of the Gansu Provincial Cancer Hospital (approval
no. A201809200024; Lanzhou, China). Written informed consent for
publication of clinical data, including treatment, follow-up and
any subsequent case reports, was obtained from all participants at
the point of recruitment to the trial. A total of 47 subjects were
recruited, including eight cases of recurrent intracranial
malignant tumours. During follow-up 1 year after treatment, two
patients were diagnosed with RN by magnetic resonance imaging
(MRI). The present study reported on these two cases of symptomatic
RN to verify the efficacy and optimal dose pattern of bevacizumab
in the treatment of CIT-induced RN.
Case report
Case 1
A 28-year-old man was admitted to Wuwei Heavy Ion
Hospital in November 2018, who presented with persistent headache,
decreased visual acuity and visual field defect that had persisted
for 1 year. A head gadolinium (Gd)-enhanced MRI brain scan revealed
a mass that showed a hypointense signal in T1-weighted imaging
(T1WI) and a diverse signal in T2WI. The mass measured 3.0x2.2x3.4
cm (antero-posterior x transverse x craniocaudal) and was located
in the right parasellar region. Physical examination showed equally
large and round bilateral pupils, light reflection, decreased
binocular vision, visual field defect, restricted abduction in the
right eye and right eyelid droop. The patient underwent a tumour
biopsy in the right cavernous sinus area via the sphenoidal sinus
under general anaesthesia by neuroendoscopic navigation in November
2018. The baseline neuro-ophthalmic assessment at this time showed
no deterioration.
Tumor biopsy revealed a highly differentiated
chondrosarcoma in the right cavernous sinus. The
immunohistochemistry results were as follows: CK (-), CK-19 (-),
epithelial membrane antigen (EMA) (-), Ki-67 (8%+), S-100 (+),
IDH-1 (-), Vimentin (+), neuron-specific enolase (-) and Brachyury
(-), which supported the pathological diagnosis. The post-biopsy
MRI showed local bone defects in the sellar base, sphenoid sinus
and occipital slope, as well as structural disorder in the sellar
region and patchy mixed T1WI and T2WI signal shadows with patchy
rings under enhancement. Haemorrhage and partial fillings were
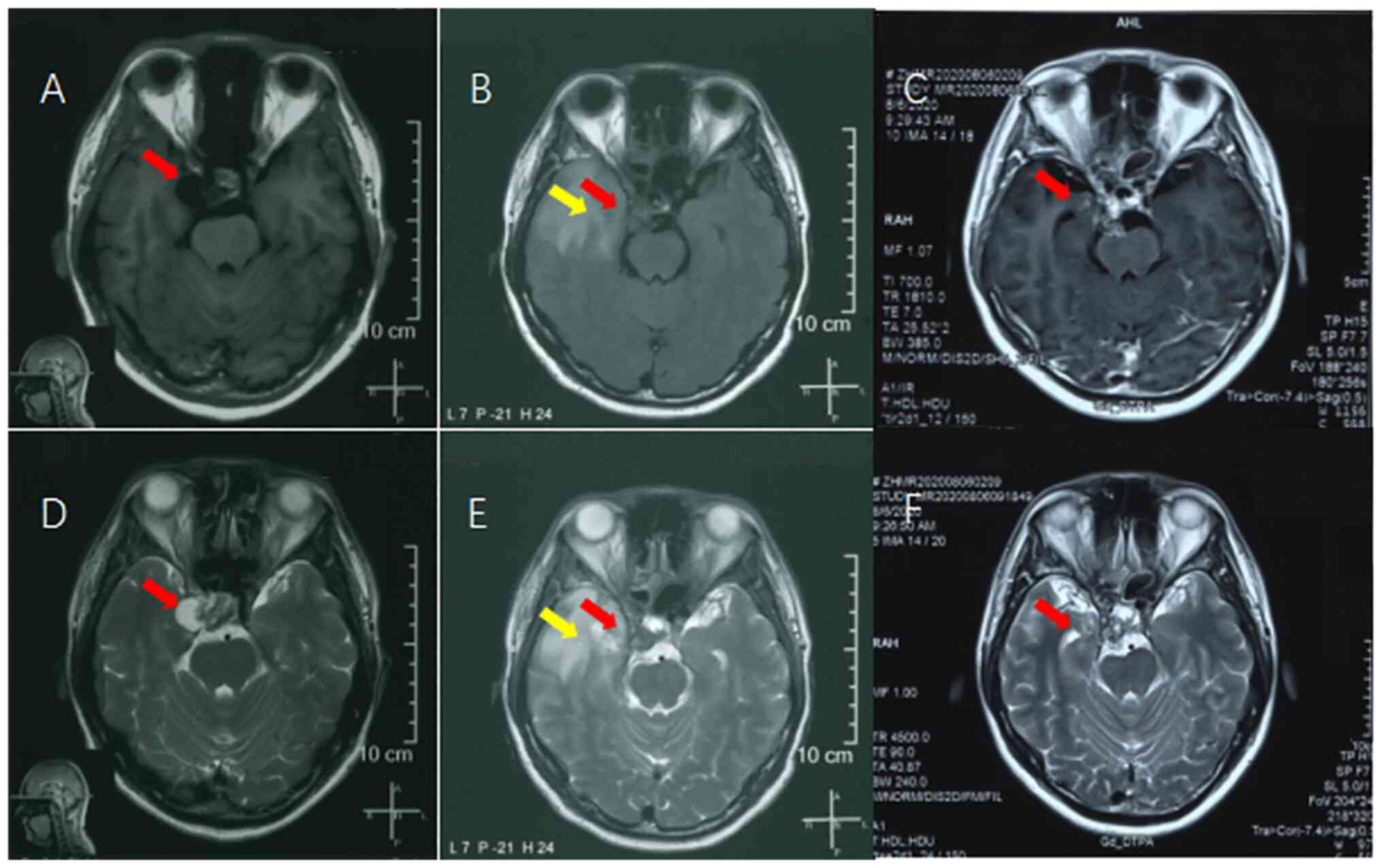
observed in the operative area (Fig.
1A and D).
A total of 1 month after the biopsy, the patient
applied to be enrolled in the clinical trial. After discussion by
the expert group, the patient was considered to meet the inclusion
criteria. In full communication with the patient and after signing
the informed consent, the patient was officially enrolled in the
clinical trial in December 2018. The patient had a definite
diagnosis of chondrosarcoma in the right cavernous sinus, stage Ia
with cT1N0M0, according to the American Joint Council on Cancer 8th
edition staging (8). CIT was
administered to the patient according to the protocol for
chondrosarcoma of the skull base. The target volume was delineated
based on the preoperative MRI and computed tomography (CT) imaging.
The gross tumour volume (GTV) was defined as the primary tumour in
MRI. The clinical target volume (CTV) included the GTV, parietal
wall of the nasopharynx, cranial base slope, the small and great
wings of the sphenoid bone, sella turcica, cavernous sinus,
sphenoid sinus and the posterior ethmoid sinus. The planning target
volume (PTV) was determined by adding 3-mm margins to the CTV. The
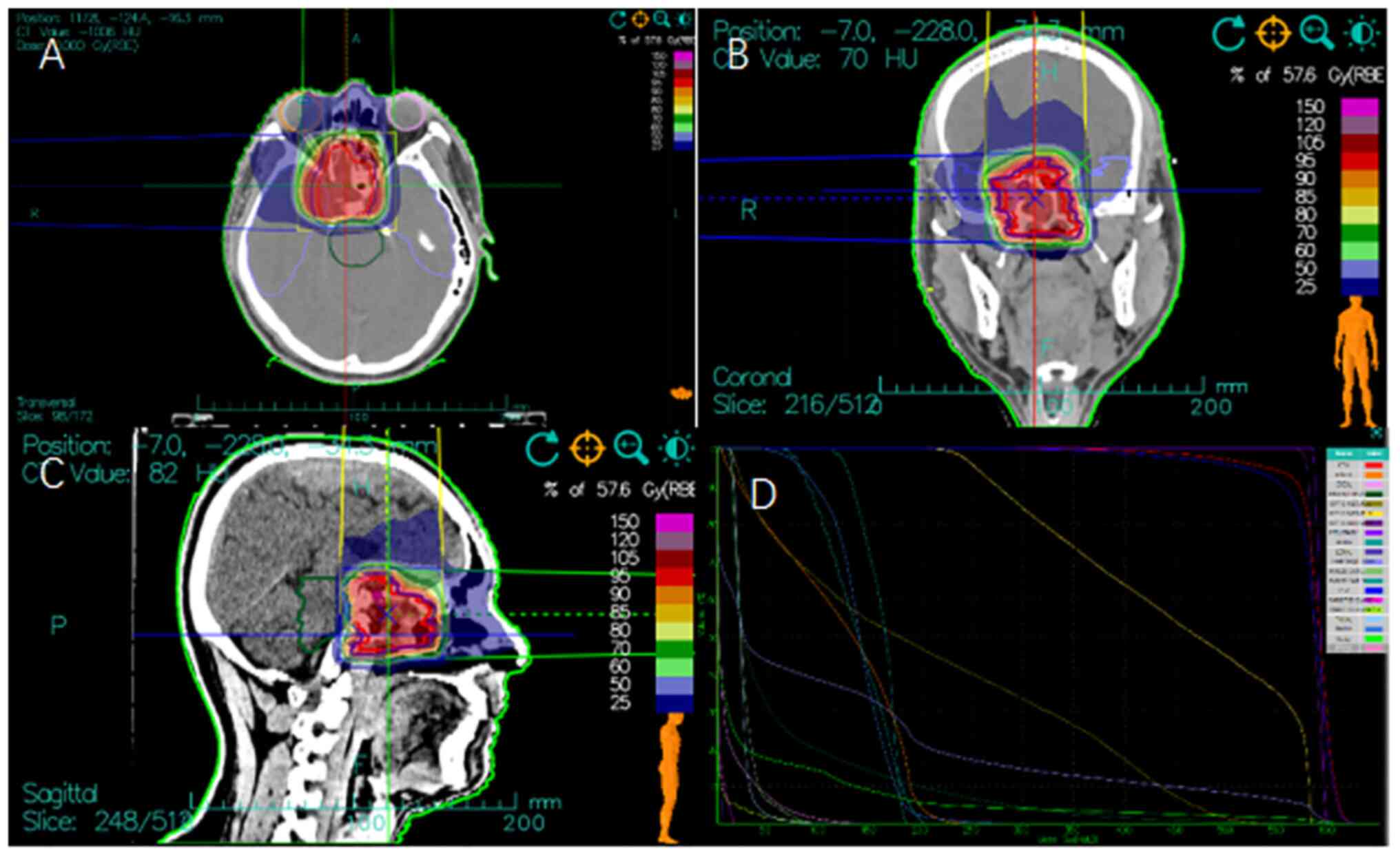
patient was administered a PTV total prescription dose of 57.6 Gy
[relative biological effectiveness (RBE)] in 16 fractions, 3.6 Gy
(RBE) per fraction over 3.2 weeks (Fig. 2). The treatment process was
uneventful, with no acute adverse events base on Radiation Therapy
Oncology Group criteria (9). The
patient reported relief of the symptoms of headache, diplopia and
blurred vision after CIT completion.
After CIT, the patient entered regular follow-up and
did not report any adverse events during the first year. However,
14 months after CIT, a follow-up MRI showed a mass with abnormal
signal in the right temporal lobe, measuring ~18x28 mm in size.
Gd-enhanced scans showed significant enhancement with a wreath
shape in the lesion area, surrounded by a large area of high-signal
oedema. The primary tumour in the right cavernous sinus had
decreased in size (Fig. 1B and
E). The patient experienced
symptoms of olfactory hallucination, dizziness, nausea and petit
mal epilepsy. Glucocorticoids and magnesium valproate were
administered to reduce brain oedema and control the epilepsy.
Fluorodeoxyglucose (FDG)-PET showed decreased tracer uptake in the
lesions in the right temporal lobe, which indicated no progression
in the tumor area. Thus, the aggravation of the clinical symptoms,
combined with the FDG-PET and MRI findings, indicated RN rather
than tumour progression.
Bevacizumab was proposed as the main treatment to
control RN. Thereafter, the patient was administered 5 mg/kg
bevacizumab biweekly for six cycles. MRI after four cycles showed
marked improvement in both T1WI and T2WI. The symptoms of olfactory
hallucination, dizziness and petit mal epilepsy were markedly
improved with this treatment. MRI after six cycles showed that the
abnormal signal mass and surrounding brain oedema had nearly
disappeared and showed a further decrease in primary tumour size
(Fig. 1C and F). Follow-up MRI performed 24 months
after CIT showed no tumour progression with the patient in a stable
state (data not shown).
Case 2
A 50-year-old man was admitted to Wuwei Heavy Ion
Hospital in December 2018, who presented with a recurrent
anaplastic meningioma for 9 years, for which he had undergone three
surgeries and stereotactic RT. The patient first underwent
intracranial tumour resection for an MRI diagnosis of meningioma in
2009, with the postoperative pathology indicating meningioma. No
further adjuvant treatment was administered after the operation. In
June 2015, follow-up MRI showed recurrence of left frontal
meningioma, and a second surgical treatment was performed. The
pathological findings indicated a grade 3 anaplastic meningioma
based on the World Health Organisation (WHO) classification
(10). Due to the presence of
gross residual lesions, adjuvant stereotactic RT was performed in
July 2015, with the 50% isodose line wrapped around the target
volume, and prescribed doses of 30 Gy (5 fractions) in the centre
and 15 Gy (5 fractions) at the edge. In April 2017, the patient
developed frontal redness and swelling, headache and fever. MRI
indicated a recurrent lesion. Surgery was performed for frontal
sinus abscess removal and recurrent meningioma resection. The
pathological diagnosis was meningioma in the right frontal area
(WHO grade 2-3), with local bone invasion. The immunohistochemistry
results were as follows: Vimentin (+), EMA (-), S-100 (-), CD34
(+), STAT6 (+), Ki-67 (20%+), and P53 (+), which support the
pathological diagnosis. In September 2018, the patient developed
noticeable swelling in the left frontal area. Brain Gd-enhanced and
FLAIR MRI confirmed a crescent-shaped T1-hyperintense and
T2-hypointense signal mass measuring 5.7x1.8x4.0 cm (transverse x
antero-posterior x craniocaudal) under the left frontal cranial
plate. The anterior horn of the adjacent left ventricle was
broadened and obtuse. The proposed diagnosis was a recurrence of
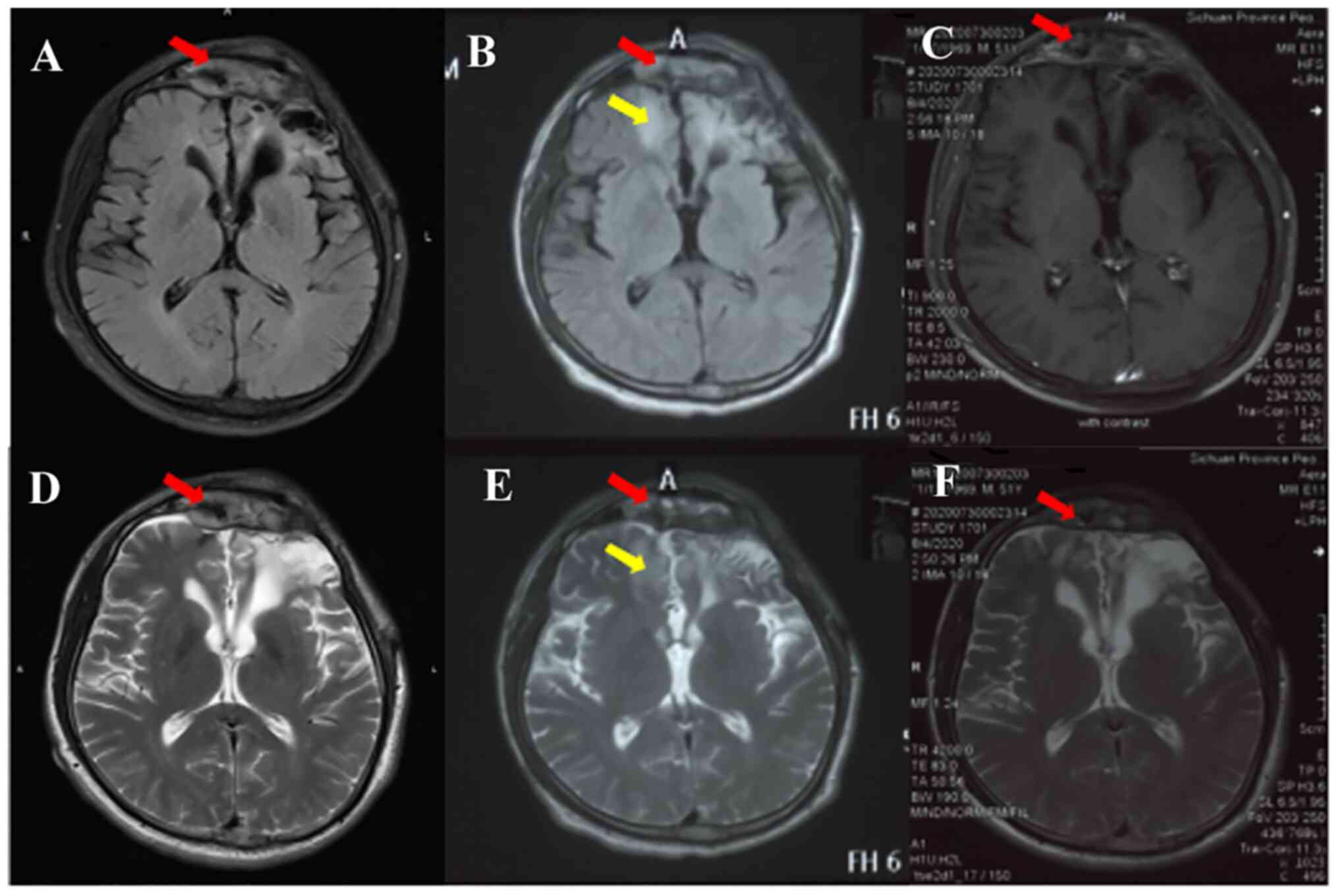
the meningioma (Fig. 3A and
D). On physical examination, the
left eyebrow arch was slightly raised with local swelling and the
bilateral pupils were equally large and round. Light reflection and
normal binocular vision were also observed.
The patient applied to be enrolled in the clinical
trial and was administered CIT according to the approved protocol.
The target volume was delineated based on MRI and CT imaging. The
GTV included the enhanced lesions observed in MRI. The planning GTV
(PGTV) was generated by adding a 3-mm margin to the GTV. The CTV
included the GTV and tumour bed area. The PTV was generated by
applying 10-mm margins to the CTV. The PTV or PGTV were shrunk in
the presence of bony structures or parts beyond the body. The
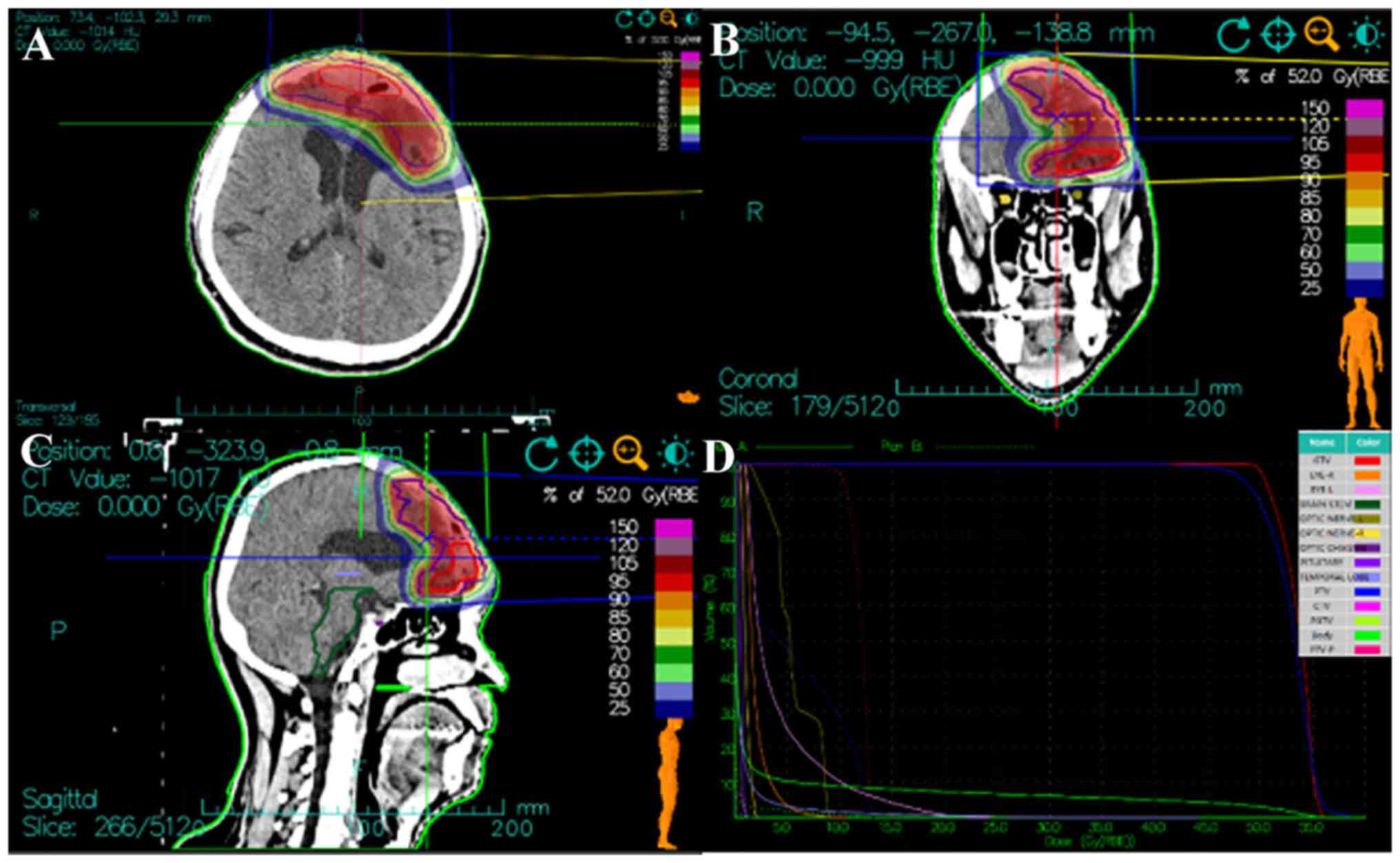
patient received the total prescription dose of 52 Gy (RBE) in 13
fractions to the PTV and 64 Gy (RBE) in 16 fractions to the PGTV,
at 4 Gy (RBE) per fraction over 3.2 weeks (Fig. 4). The treatment course was smooth
and the patient reported relief of the symptoms of headache with
grade 1 adverse events, including alopecia and cutaneous
pigmentation, when finishing CIT.
After CIT, the patient entered regular follow-up and
did not report any severe adverse events during the first year. The
follow-up MRI performed at 13 months showed large areas with long
T1 and T2 signals in the bilateral frontal lobes; Gd-enhanced
imaging showed significant enhancement with multiple nodules. The
anterior horn of the left lateral ventricle was also enlarged
(Fig. 3B and E). The primary meningioma in the frontal
lobe was stable. The patient experienced mild dizziness, nausea and
headache. Combined with their medical history and MRI
characteristics, the patient was considered to have developed
RN.
Bevacizumab was proposed as the main treatment for
RN control. Thereafter, the patient was administered 5 mg/kg
bevacizumab biweekly for four cycles. Subsequent MRI showed
reduction of the Gd-enhanced areas in the bilateral frontal lobes
(Fig. 3C and F). In addition, the symptoms of mild
dizziness, nausea and headache improved markedly with the
treatment. Follow-up MRI performed 24 months after CIT showed no RN
and no tumour progression, and the patient was in a stable state
(data not shown).
Discussion
Limited evidence shows no obvious histological
difference between RN and pseudoprogression (psPD). Notably, psPD
often occurs within the first 2 months of treatment completion,
whereas RN may show a latency of >3 months to years after RT
(11). Thus, during the follow-up
of patients with a history of brain malignancy and RT,
inexperienced physicians may conclude that the disease has actually
progressed if abnormal lesions are observed on radiography. Both of
the present cases were diagnosed with disease progression by a
radiologist based on abnormal signals in the tumour bed and oedema
in the surrounding area using conventional MRI protocols, as these
non-specific findings may also be observed in tumour progression.
For patients with malignant brain tumours after RT, distinguishing
between progressive disease and RN is key for the timely
administration of the correct treatment regimen.
The incidence of RN is influenced by numerous
factors, including RT modality, total dose, dose fractionation,
intracranial pathology and diagnostic imaging modality. A previous
study reported an RN incidence of 14-15% based on conventional RT
modalities (12). Precision RT
techniques, such as intensity-modulated RT, image-guided RT,
stereotactic radiosurgery (SRS) and particle beam RT have minimized
the risk of RN by decreasing the radiation injury to normal tissue
(13). As a type of high linear
energy transfer (LET) ray, CIT is considered more suitable for the
treatment of radiation-resistant and recurrent tumours, due to its
physical and biological advantages. CIT can be used to produce
highly compact dose distributions that significantly reduce
exposure to normal tissues compared with traditional photon RT.
Carbon ion beam dose depositions follow the so-called Bragg curve
as a function of tissue depth (14,15);
therefore, because of the higher density of ionization events along
the direction of carbon ions entering into tissue, CIT is a
fundamentally different form of radiation with regard to its
biological effects (16). Because
of this, numerous uncertainties exist regarding the clinical and
physical properties of carbon ions (17-19).
Previous studies have investigated the robustness of scanned ion
therapy, as well as uncertainties in treatment planning, treatment
delivery and patient alignment (20,21).
For example, if a patient is offset or the tumour volume changes
during the treatment course, ion Bragg peaks may miss the planned
target location, resulting in a potential underdose or overdose to
critical structures outside the target. These uncertainties may
lead to more severe damage to organs at risk (OARs) or tumour
recurrence. Another phenomenon that cannot be ignored is the
trailing effect of the carbon ion dose deposition curve.
Particularly for large-volume tumours, the widening of the
spread-out Bragg peak (SOBP) leads to an increased dose at the tail
of the dose curve, which may also lead to increased toxicity to the
OARs behind the tumour (22). Due
to limited clinical experiences with CIT, more attention should be
paid to its toxicities in clinical practice. In the present two
cases, RN occurred 1 year after CIT, despite the very low radiation
dose in the necrotic area according to the dose distribution and
dose-volume histogram in the treatment plan.
Animal models of proton- or carbon-induced RN are
notably sparse. In one study, researchers irradiated the right
hemisphere of rat brains with large single-fraction doses of proton
or helium ion beams. The animals were then subjected to continuous
MRI (23). T2WI showed abnormal
lesions consistent with the histological analysis findings of
necrotic changes. Similar studies have been conducted with carbon
ions (24,25), in which physical doses of 30 and 50
Gy with carbon particles (290 MeV/nucleon; 5 mm SOBP) in a single
fraction were delivered to the left cerebral hemispheres of adult
Sprague-Dawley rat brains. Histological examination revealed
necrotic tissue damage, haemorrhage in the thalamus and
vasodilatations around the necrotic region a total of 8 weeks after
50 Gy irradiation. The damaged tissue regions correlated well with
those expected from the radiation-dose distribution, thus
suggesting an advantage of charged carbon particles for irradiating
restricted brain regions. While such experimental setups are
complex, the use of fractionated radiation, and spatial correlation
of imaging and histological changes with dose and LET may improve
knowledge on carbon-induced neurotoxicity (26).
The utility of CIT is majorly limited by RN, since
administering large doses in hypo-fractions or reirradiation of
recurrent tumours are expected to result in significant RN. Mayer
and Sminia (27) reported a
cumulative dose of >100 Gy for reirradiation to be the threshold
beyond which RN occurred. To reduce the incidence of RN, the
real-time dose distribution should be evaluated, in addition to
paying close attention to the RT history of the patient and
estimated cumulative doses of the tumour target volume and
OARs.
Among theories on the development of RN in the
brain, the role of VEGF and hypoxia-inducible factor-1α (HIF-1α) in
the pathogenesis of RN has become increasingly obvious. Radiation
exposure damages vascular tissue around the tumour, subsequently
leading to impaired oxygen diffusion between the tissue and blood
vessels, and tissue hypoxia, which can initiate increased HIF-1α
expression. Secondly, hypoxia and increased HIF-1α expression in
tumour tissues stimulate reactive astrocytes to secrete VEGF, which
is an angiogenic factor. High VEGF expression can lead to abnormal
neovascularization, in which the vessels lack normal vascular
structure, and exhibit structural disorder, fragility and high
permeability. Abnormal neovascularization also promotes blood
plasma exudation to the surrounding tissue and brain oedema. In
turn, local high intracranial pressure can be caused by cerebral
oedema, which can induce ischemia and hypoxia, forming a vicious
cycle of local hypoxia, eventually progressing to RN (5,28,29).
Steroids have been effectively applied to treat RN
and have been used to provide prompt relief of symptoms. Notably,
steroids can reduce cytokine levels and inflammatory responses, not
only improving brain oedema, but also reducing the risk of
subsequent blood vessel changes and inflammation (30). Thus, for decades, steroids have
been recommended as the front-line therapeutic strategy, including
pulse-dose intravenous steroids, which are more effective than oral
steroids (31). While the
conventional treatment with steroids is dehydration combined with
immunosuppressants (such as glucocorticoids), the reported response
rate is only 20-30% and the long-term use of glucocorticoids can
cause a series of adverse reactions, including metabolic disorders,
gastrointestinal bleeding and immunosuppression-associated
infection (31,32). Bevacizumab, as an antagonist of
VEGF binding to its receptor, serves a role in vascular pruning,
regulating vascular permeability, reducing brain oedema caused by
brain necrosis and treating brain necrosis; however, its effect on
RN has been reported only in small-sample clinical studies
(33). Levin et al
(34) conducted a randomized
double-blind placebo-controlled trial of bevacizumab for the
treatment of symptomatic radiation necrosis of the brain in 14
patients, reporting that all of the bevacizumab-treated patients,
but none of the placebo-treated patients, exhibited an improvement
in neurological symptoms. In addition to several case reports,
studies have further established the clinical efficacy of
bevacizumab for the treatment of brain RN, concluding that
bevacizumab exhibits good short-term efficacy for RN, no matter
whether SRS was applied to brain metastases or if conventional
fraction RT was applied to high-grade glioma (33,35).
However, the optimization of bevacizumab administration is a
complex issue, involving dose, treatment course and discontinuation
criteria. Researchers have administered various doses of
bevacizumab (2.5-10 mg/kg) but the field has not yet produced a
consensus on doses. Due to the vascular mechanisms of brain
necrosis and the features of anti-angiogenic therapy, most experts
recommend low-dose bevacizumab (2.5-5 mg/kg) in clinical practice,
because of the associated treatment costs and treatment goals
(36-38).
Regarding treatment course, patients in previous studies typically
received at least two doses of bevacizumab every 2-4 weeks (no
maximum) (39,40). As the goal of bevacizumab treatment
is symptom relief, not prolonging survival, treatment should be
provided until symptoms are relieved and imaging improves, rather
than being given as a long-term treatment (25).
The use of bevacizumab in the treatment of
CIT-induced RN has rarely been reported (33). In the present two cases,
bevacizumab doses of 5 mg/kg were administered every 2 weeks for
four cycles. Both patients achieved good symptom remission and
imaging improvement, with no recurrence of brain necrosis observed
after 2 years of follow-up. Since bevacizumab was effective in the
treatment of CIT-induced RN, it remains to be determined if it
should be administered as early as possible to prevent the
occurrence of RN. This administration remains controversial based
on the results of published studies on the use of bevacizumab to
prevent photon radiation-induced RN. Two studies have reported
anti-angiogenic drug resistance (41,42),
and premature or intermittent administration has been shown to
increase bevacizumab resistance in patients with radiation brain
necrosis. Moreover, Jeyaretna et al (43) reported that excess bevacizumab
treatment may cause excessive vessel pruning, thereby aggravating
localized ischemia and hypoxia of the necrotic area, and resulting
in brain necrosis recurrence. Therefore, administrating bevacizumab
to patients that have undergone brain radiation prior to the
progression of brain necrosis may do more harm than good.
In conclusion, CIT differs fundamentally from photon
radiation in both physical and biological characteristics. Data
comparing the effects of different types of radiation on the
occurrence of RN are surprisingly limited. Notably few, if any,
studies of normal tissue toxicity following CIT have attempted to
link biological effects to physical factors, not just dose. In
addition, considering the trend of hypofractionation with CIT, an
evaluation of various fractionation schemes is required. Notably,
early treatment is necessary once symptomatic brain necrosis
occurs. Bevacizumab is currently recognized as one of the best
medicines for the control of RN based on the principle of
anti-angiogenesis. The available evidence suggests that the number
of administered cycles of bevacizumab should be based on the
purpose of RN treatment, and long-term or prophylactic applications
are not recommended; this is considered to be the best strategy to
reduce the incidence of RN through protecting critical structures
and avoiding severe damage in a clinical setting.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Talent Innovation and
Venture Project of Lanzhou City (grant nos. 2021-RC-125 and
2020-RC-113), the Key R&D Program of Science and Technology
Department of Gansu Province (grant no. 20YF8FA116), the 2021
‘Hundred Cities and Hundred Parks’ Action Project of Lanzhou
National High-tech Zone ((grant no. LZGXQ-21-15), and the
authorized project of Lanzhou KejinTaiji Corporation, Ltd. (grant
no. BMP-B-02-002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RL performed the patient treatment plans and
analyzed the image data, and was a major contributor in writing the
manuscript. QZ designed clinical trial protocol, and analyzed and
interpreted data. HL, YG and XZ were the attending physicians of
these two cases, who formulated the diagnosis and treatment plans,
observed efficacy, followed up and collected medical data. ZL and
SS were responsible for dose calibration, quality control and
implementation of carbon ion radiotherapy. XW contributed to design
and conception. RL and QZ confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient consented to the collection of data and
images for the purpose of research and for their publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Krauze AV, Peters C, Cheng J, Ning H,
Mackey M, Rowe L, Cooley-Zgela T, Smart DD and Camphausen K:
Re-irradiation for recurrent glioma- the NCI experience in tumor
control, OAR toxicity and proposal of a novel prognostic scoring
system. Radiat Oncol. 12(191)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nieder C, Andratschke NH and Grosu AL:
Re-irradiation for recurrent primary brain tumors. Anticancer Res.
36:4985–4995. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kanai T, Endo M, Minohara S, Miyahara N,
Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka
T, et al: Biophysical characteristics of HIMAC clinical irradiation
system for heavy-ion radiation therapy. Int J Radiat Oncol Biol
Phys. 44:201–210. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miyatake S, Nonoguchi N, Furuse M,
Yoritsune E, Miyata T, Kawabata S and Kuroiwa T: Pathophysiology,
diagnosis, and treatment of radiation necrosis in the brain. Neurol
Med Chir (Tokyo). 55:50–59. 2015.PubMed/NCBI
|
|
6
|
Soliman HM, ElBeheiry AA, Abdel-Kerim AA,
Farhoud AH and Reda MI: Recurrent brain tumor versus radiation
necrosis; can dynamic susceptibility contrast (DSC) perfusion
magnetic resonance imaging differentiate? Egyptian J Radiol Nuclear
Med. 49:719–726. 2018.
|
|
7
|
Delishaj D, Ursino S, Pasqualetti F,
Cristaudo A, Cosottini M, Fabrini MG and Paiar F: Bevacizumab for
the treatment of radiation-induced cerebral necrosis: A systematic
review of the literature. J Clin Med Res. 9:273–280.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC eds..et al: AJCC cancer staging manual. 8th ed.
NewYork: Springer; 2017.
|
|
9
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the radiation therapy oncology group (RTOG) and the
European organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gritsch S, Batchelor TT and Gonzalez
Castro LN: Diagnostic, therapeutic, and prognostic implications of
the 2021 World Health Organization classification of tumors of the
central nervous system. Cancer. 128:47–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Parvez K, Parvez A and Zadeh G: The
diagnosis and treatment of pseudoprogression, radiation necrosis
and brain tumor recurrence. Int J Mol Sci. 15:11832–11846.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rahmathulla G, Marko NF and Weil RJ:
Cerebral radiation necrosis: A review of the pathobiology,
diagnosis and management considerations. J Clin Neurosci.
20:485–502. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ali FS, Arevalo O, Zorofchian S, Patrizz
A, Riascos R, Tandon N, Blanco A, Ballester LY and Esquenazi Y:
Cerebral radiation necrosis: incidence, pathogenesis, diagnostic
challenges, and future opportunities. Curr Oncol Rep.
21(66)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tessonnier T, Mairani A, Brons S, Haberer
T, Debus J and Parodi K: Experimental dosimetric comparison of
1H, 4He, 12C and 16O
scanned ion beams. Phys Med Biol. 62:3958–3982. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Suit H, DeLaney T, Goldberg S, Paganetti
H, Clasie B, Gerweck L, Niemierko A, Hall E, Flanz J, Hallman J and
Trofimov A: Proton vs. carbon ion beams in the definitive radiation
treatment of cancer patients. Radiother Oncol. 95:3–22.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tinganelli W and Durante M: Carbon ion
radiobiology. Cancers (Basel). 12(3022)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sakama M and Kanematsu N: An evaluation
method of clinical impact with setup, range, and radiosensitivity
uncertainties in fractionated carbon-ion therapy. Phys Med Biol.
63(135003)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eley JG, Newhauser WD, Richter D,
Lüchtenborg R, Saito N and Bert C: Robustness of target dose
coverage to motion uncertainties for scanned carbon ion beam
tracking therapy of moving tumors. Phys Med Biol. 60:1717–1740.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kamp F, Brüningk S, Cabal G, Mairani A,
Parodi K and Wilkens JJ: Variance-based sensitivity analysis of
biological uncertainties in carbon ion therapy. Phys Med.
30:583–587. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Meyer J, Bluett J, Amos R, Levy L, Choi S,
Nguyen QN, Zhu XR, Gillin M and Lee A: Spot scanning proton beam
therapy for prostate cancer: Treatment planning technique and
analysis of consequences of rotational and translational alignment
errors. Int J Radiat Oncol Biol Phys. 78:428–434. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lomax AJ: Intensity modulated proton
therapy and its sensitivity to treatment uncertainties 1: The
potential effects of calculational uncertainties. Phys Med Biol.
53:1027–1042. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Malouff TD, Mahajan A, Krishnan S, Beltran
C, Seneviratne DS and Trifiletti DM: Carbon ion therapy: A modern
review of an emerging technology. Front Oncol.
10(82)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kondo N, Sakurai Y, Takata T, Takai N,
Nakagawa Y, Tanaka H, Watanabe T, Kume K, Toho T, Miyatake S, et
al: Localized radiation necrosis model in mouse brain using proton
ion beams. Appl Radiat Isot. 106:242–246. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun XZ, Takahashi S, Kubota Y, Zhang R,
Cui C, Nojima K and Fukui Y: Experimental model for irradiating a
restricted region of the rat brain using heavy-ion beams. J Med
Invest. 51:103–107. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Takahashi S, Sun XZ, Kubota Y, Takai N and
Nojima K: Histological and elemental changes in the rat brain after
local irradiation with carbon ion beams. J Radiat Res. 43:143–152.
2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Grosshans DR, Duman JG, Gaber MW and
Sawakuchi G: Particle radiation induced neurotoxicity in the
central nervous system. Int J Part Ther. 5:74–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mayer R and Sminia P: Reirradiation
tolerance of the human brain. Int J Radiat Oncol Biol Phys.
70:1350–1360. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nonoguchi N, Miyatake S, Fukumoto M,
Furuse M, Hiramatsu R, Kawabata S, Kuroiwa T, Tsuji M, Fukumoto M
and Ono K: The distribution of vascular endothelial growth
factor-producing cells in clinical radiation necrosis of the brain:
Pathological consideration of their potential roles. J Neurooncol.
105:423–431. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhuang H, Shi S, Yuan Z and Chang JY:
Bevacizumab treatment for radiation brain necrosis: Mechanism,
efficacy and issues. Mol Cancer. 18(21)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shaw EG and Robbins ME: The management of
radiation-induced brain injury. Cancer Treat Res. 128:7–22.
2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lam TC, Wong FC, Leung TW, Ng SH and Tung
SY: Clinical outcomes of 174 nasopharyngeal carcinoma patients with
radiation-induced temporal lobe necrosis. Int J Radiat Oncol Biol
Phys. 82:e57–e65. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhuo X, Huang X, Yan M, Li H, Li Y, Rong
X, Lin J, Cai J, Xie F, Xu Y, et al: Comparison between high-dose
and low-dose intravenous methylprednisolone therapy in patients
with brain necrosis after radiotherapy for nasopharyngeal
carcinoma. Radiother Oncol. 137:16–23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Khan M, Zhao Z, Arooj S and Liao G:
Bevacizumab for radiation necrosis following radiotherapy of brain
metastatic disease: A systematic review & meta-analysis. BMC
Cancer. 21(167)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel
JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR and Jackson
EF: Randomized double-blind placebo-controlled trial of bevacizumab
therapy for radiation necrosis of the central nervous system. Int J
Radiat Oncol Biol Phys. 79:1487–1495. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lubelski D, Abdullah KG, Weil RJ and Marko
NF: Bevacizumab for radiation necrosis following treatment of high
grade glioma: A systematic review of the literature. J Neurooncol.
115:317–322. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tye K, Engelhard HH, Slavin KV, Nicholas
MK, Chmura SJ, Kwok Y, Ho DS, Weichselbaum RR and Koshy M: An
analysis of radiation necrosis of the central nervous system
treated with bevacizumab. J Neurooncol. 117:321–327.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bodensohn R, Hadi I, Fleischmann DF,
Corradini S, Thon N, Rauch J, Belka C and Niyazi M: Bevacizumab as
a treatment option for radiation necrosis after cranial radiation
therapy: A retrospective monocentric analysis. Strahlenther Onkol.
196:70–76. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Y, Pan L, Sheng X, Mao Y, Yao Y, Wang
E, Zhang N and Dai J: Reversal of cerebral radiation necrosis with
bevacizumab treatment in 17 Chinese patients. Eur J Med Res.
17(25)2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gonzalez J, Kumar AJ, Conrad CA and Levin
VA: Effect of bevacizumab on radiation necrosis of the brain. Int J
Radiat Oncol Biol Phys. 67:323–326. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Furuse M, Nonoguchi N, Kawabata S,
Yoritsune E, Takahashi M, Inomata T, Kuroiwa T and Miyatake S:
Bevacizumab treatment for symptomatic radiation necrosis diagnosed
by amino acid PET. Jpn J Clin Oncol. 43:337–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tejpar S, Prenen H and Mazzone M:
Overcoming resistance to antiangiogenic therapies. Oncologist.
17:1039–1050. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jeyaretna DS, Curry WT Jr, Batchelor TT,
Stemmer-Rachamimov A and Plotkin SR: Exacerbation of cerebral
radiation necrosis by bevacizumab. J Clin Oncol. 29:e159–e162.
2011.PubMed/NCBI View Article : Google Scholar
|