Introduction
Ovarian cancer has a high mortality rate compared to
other cancer types of the female reproductive organs (1). In 2015, ~1.2 million females
developed ovarian cancer, resulting in 160,000 deaths worldwide
(2-4).
It is called a ‘silent killer’, as the disease usually does not
produce any obvious symptoms in early stages and there is no
effective screening program to date. Therefore, the majority of
patients are diagnosed only at advanced stages and have a poor
survival rate (5). The diagnosis
of ovarian cancer includes careful review of patients' medical
history, physical examination, serum cancer antigen 125 (CA-125)
levels, radiologic findings and histopathologic confirmation
(6). This also helps with the
study of the behaviour of ovarian masses (OMs), whether they are
benign or malignant. Therefore, OM is an important radiological
finding that may indicate ovarian cancer if it is associated with
specific criteria such as fixation, irregularity and nodularity
(7). Approximately 12-20% of OMs
are malignant; however, OMs may also be benign, such as leiomyomas,
ovarian follicular cysts and endometriosis (7). OMs are the main reason for referral
and hospitalization of patients to assess the risk of malignancy.
Accurate initial diagnosis in females with ovarian cancer is
important to obtain an early and correct diagnosis of ovarian
cancer and to avoid the risk of overtreatment. In the clinical
context, there are several methods for assessing the risk of
ovarian malignancy, such as the Risk Malignancy Index (RMI) and the
Risk Algorithm for Ovarian Cancer (6,7). The
RMI is a widely known method for malignancy risk assessment.
However, the primary evaluation of OMs is mainly based on the
initial diagnostic workup, which includes ultrasound findings,
menopausal status and serum CA-125 levels (7,8). An
RMI score of >200 is associated with a high risk of ovarian
cancer in females with OMs (6,7). The
aim of the present study was to investigate the diagnostic value of
the RMI in Libyan females with OMs by using the indices of RMI in
combination with ultrasound (US) findings, menopausal status and
CA-125 levels to distinguish between benign and malignant
tumors.
Patients and methods
With the approval of the Institutional Review Board
of the National Cancer Institute (Misurata, Libya), the present
retrospective study was performed on 51 patients with OMs who were
admitted and underwent surgery at the Gynaecology Department of the
National Cancer Institute (Misurata, Libya) between January 2019
and December 2020. Demographic characteristics, US findings,
menopausal status, serum CA-125 levels and histopathology reports
were collected. OMs were evaluated based on the US findings by
determining the following items: Solid area, irregularity,
nodularity, bilaterality, multilocularity, ascites and
intra-abdominal metastases. The US score was assigned as follows:
1, no abnormality or one abnormality was detected; or 3, two or
more abnormalities were detected.
Serum CA-125 levels were also determined for all
patients and CA-125 >35 U/ml was defined as abnormal (9).
The menopausal status was determined for all
patients and the status was defined as postmenopausal when the
patient presented with amenorrhea for one year or more, or
underwent surgical ablation. The menopausal score was assigned as
follows: 1 patient was premenopausal; or 3, the patient was
postmenopausal.
The RMI was calculated as follows: RMI=US score x
menopausal score x CA-125 level in U/ml (7). The cut-off point for the RMI at 200
was used to distinguish between benign and malignant tumors, as it
provided the best diagnostic accuracy value results in the present
study and others (10-12).
Furthermore, the histopathology reports were
collected and analyzed for the correlation with the RMI.
Statistical analysis
The variables of the collected data were arranged
into logical classes and descriptive statistics were used for the
continuous variables using SPSS 19.0 for Windows (IBM Corporation).
Frequency tables were analyzed using the χ2 test, with
the likelihood ratio (LR) regarding the probability of malignant
disease vs. benign, or Fisher's exact test to assess the
significance of the association between the categorical variables
and to compare demographic, radiological and biological variables
between patients with benign or malignant OMs. The sensitivity,
specificity and positive/negative predictive values of the RMI
based on benign or malignant OMs as a reference test that is able
to indicate the malignancy of a tumor were estimated for all
patients. Different cut-offs points (range from 25 to 1,000) in the
receiver operating characteristic (ROC) curve were used to estimate
the predictive significance of the RMI. P<0.05 was considered to
indicate statistical significance.
Results
Patient characteristics
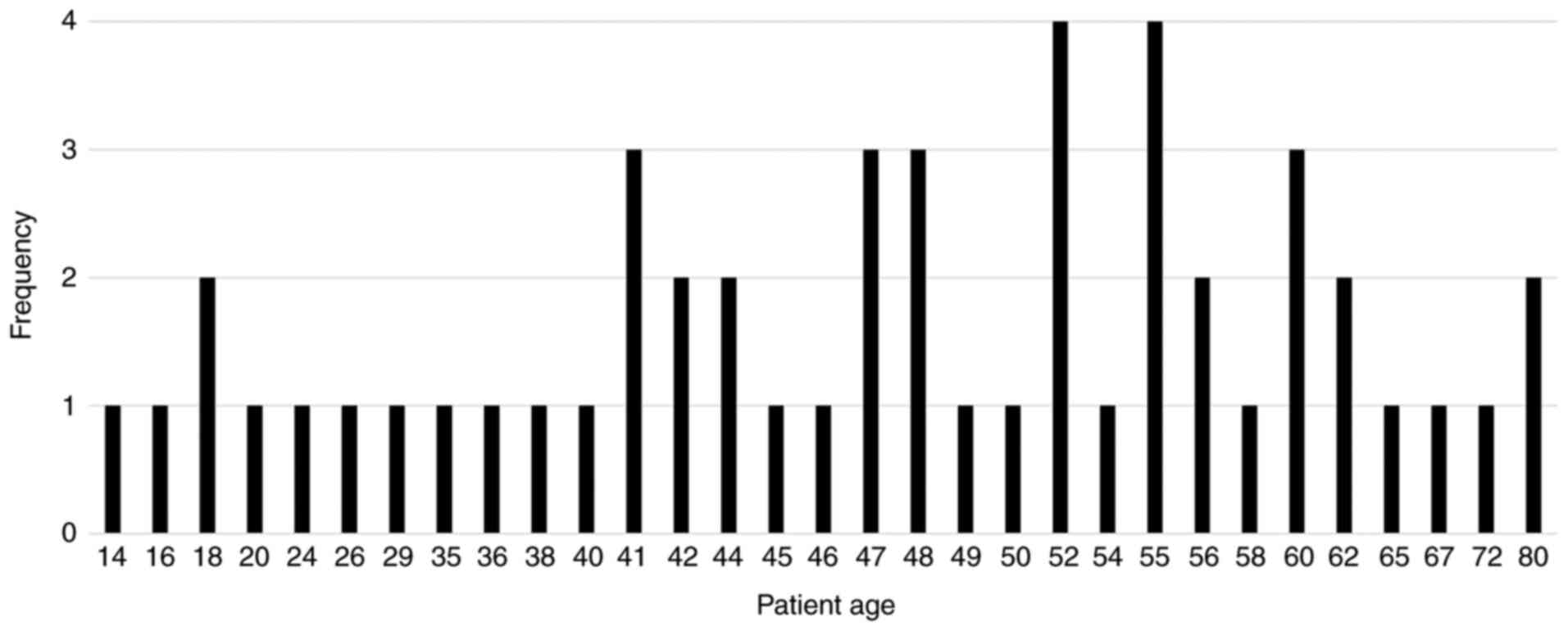
The mean age of the patients was 47 years (range,
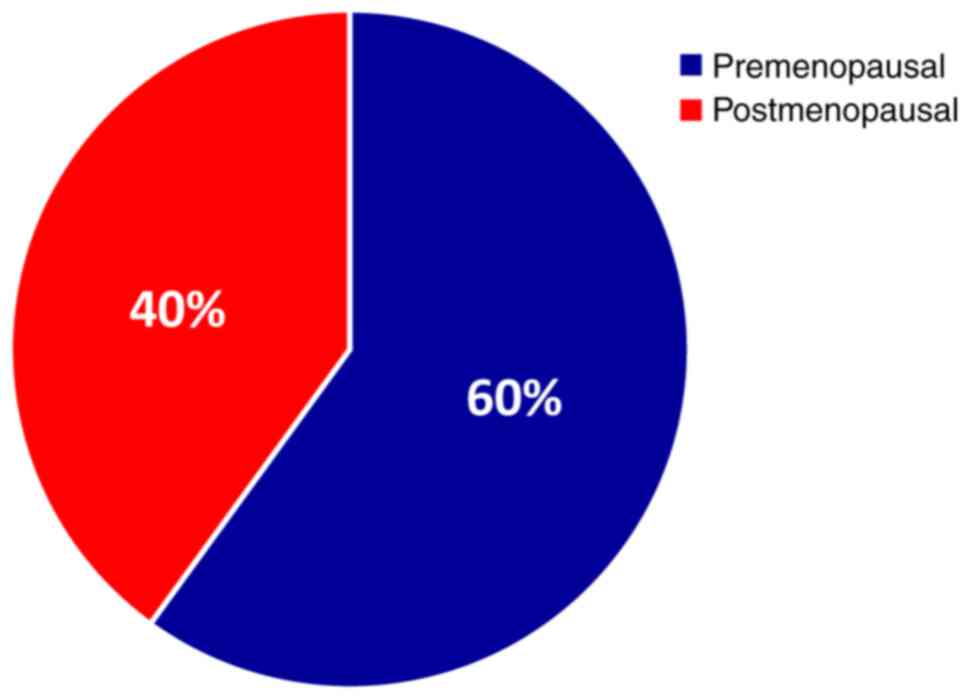
14-90 years) (Fig. 1) and 60% of
the patients were premenopausal (Fig.
2). A total of 51 patients with OMs were enrolled in the
present study; malignant tumors were confirmed in 8 (15.6%)
patients and 43 (84.4%) patients had benign tumors.
Patient characteristics and disease
status
The patient characteristics according to disease
status, including age, menopausal status, ultrasound findings and
serum CA-125 levels are presented in Table I. A significant association was
noted between US score and disease status (P<0.0001). The mean
CA-125 expression was 41 U/ml in females with benign tumors and 635
U/ml in females with malignant tumors (P=0.017). The age at
diagnosis and menopausal status were not significantly associated
with disease status (P=0.095 and 0.237, respectively). In the
present study, it was also observed that 90.7% of females with
benign disease had an RMI score <200, while an RMI score ≥200
was observed in 87.5% of females with malignant tumors.
 | Table IDistribution of subjects by age,
menopausal status, ultrasound features, serum CA-125 levels and RMI
risk. |
Table I
Distribution of subjects by age,
menopausal status, ultrasound features, serum CA-125 levels and RMI
risk.
| Variable | Benign (n=43) | Malignant (n=8) | P-value |
|---|
| Age/years (mean, 47
years; range, 14-90) | | | 0.095 |
|
>30 | 8 | 0 | |
|
30-44 | 9 | 2 | |
|
45-54 | 14 | 1 | |
|
≥55 | 12 | 5 | |
| Menopausal
status | | | 0.237 |
|
Pre | 26 | 3 | |
|
Post | 17 | 5 | |
| US score | | | <0.0001 |
|
1 | 30 | 0 | |
|
3 | 13 | 8 | |
| CA-125, U/ml | | | 0.017 |
|
Mean | 41 | 635 | |
|
Median | 25 | 662 | |
|
Minimum | 2.5 | 22 | |
|
Maximum | 212 | 1,125 | |
| RMI | | | <0.0001 |
|
<200 | 39 | 1 | |
|
≥200 | 4 | 7 | |
Diagnostic value of the RMI
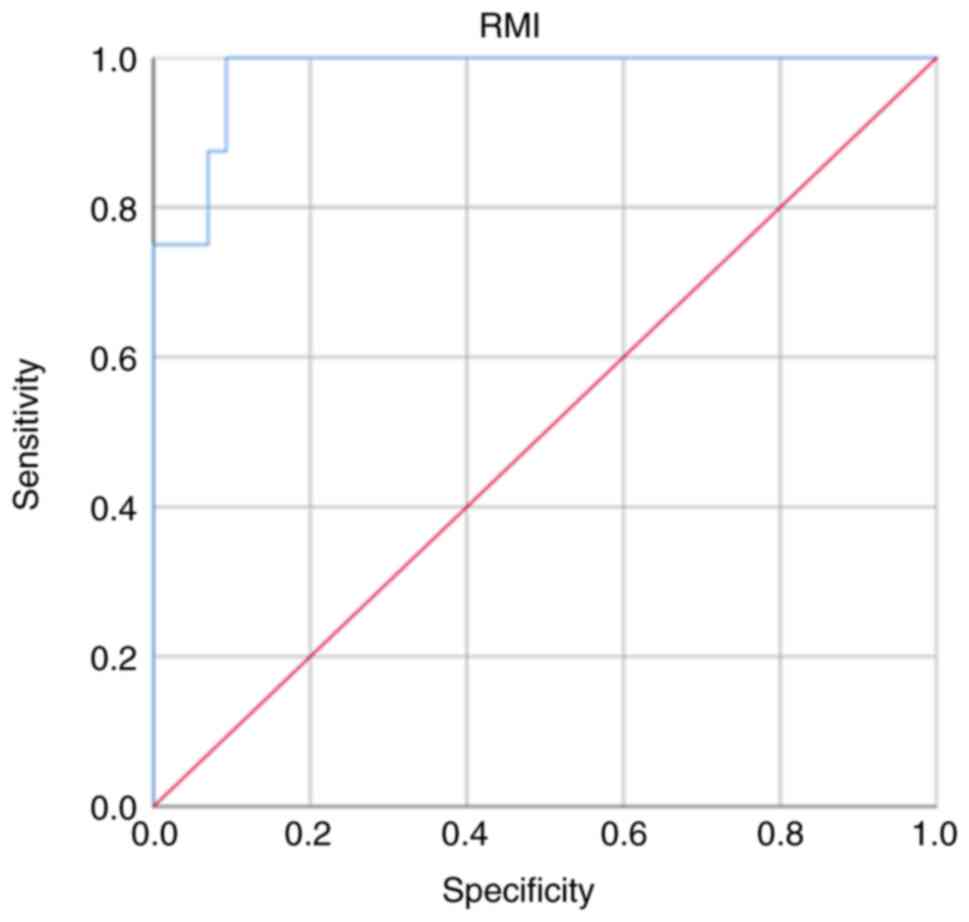
As presented in Fig.
3, an ROC curve was plotted and different cut-off points of RMI
were used. The RMI at a cut-off point of 200 had high sensitivity
and specificity of (87.5 and 90.7%, respectively) with positive and
negative predictive values of 63.6 and 97.5%, respectively, for
distinguishing between benign and malignant OMs (Table II). The results also suggested
that the area under the curve (AUC) was large (0.94, 95% CI,
0.798-1.000) and the RMI at a cut-off point of 200 was the best
criterion to identify ovarian malignant tumor in females with OMs
(Table III). Furthermore, as
presented in Table IV, 11 of 51
patients had an RMI ≥200, of which 7 (87.5%) patients had
histopathological malignancy and 4 (9.3%) patients had benign
tumors. In addition, 40 patients had an RMI <200, of which 39
(90.7%) had a benign tumor and 1 (12.5%) had a malignant tumor
(P<0.0001).
 | Table IIPredictive value of RMI, menopausal
status, serum CA-125 levels and ultrasound score for benign and
malignant ovarian masses. |
Table II
Predictive value of RMI, menopausal
status, serum CA-125 levels and ultrasound score for benign and
malignant ovarian masses.
| Variable | Benign, % | Malignant, % | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95%, CI) |
|---|
| RMI | | | 87.5 | 90.7 | 63.6 | 97.5 | 0.94
(0.798-1.000) |
|
<200 | 90.7 | 12.5 | | | | | |
|
≥200 | 9.3 | 87.5 | | | | | |
| Menopausal
status | | | 62.5 | 60.5 | 22.7 | 89.7 | 0.61
(0.401-0.828) |
|
Pre | 60.5 | 37.5 | | | | | |
|
Post | 39.5 | 62.5 | | | | | |
| US score | | | 100 | 69.8 | 38.1 | | 0.849
(0.743-0.954) |
|
1 | 69.8 | 0.00 | | | | | |
|
3 | 30.2 | 100.0 | | | | | |
| CA-125, U/ml | | | 87.5 | 58.1 | 28.0 | 96.2 | 0.728
(0.566-0.900) |
|
<35 | 58.1 | 12.5 | | | | | |
|
≥35 | 41.9 | 87.5 | | | | | |
 | Table IIISensitivity, specificity and LR for
malignant ovarian masses given a positive or negative result for
different cut-off points of the RMIs. |
Table III
Sensitivity, specificity and LR for
malignant ovarian masses given a positive or negative result for
different cut-off points of the RMIs.
| RMI | Sensitivity, % | Specificity, % | Positive LR | Negative LR |
|---|
| 25 | 98.6 | 34.9 | 1.51 | 0.04 |
| 50 | 98.6 | 53.5 | 2.12 | 0.02 |
| 75 | 98.6 | 58.1 | 2.35 | 0.02 |
| 100 | 97.7 | 69.8 | 3.23 | 0.03 |
| 125 | 97.7 | 81.4 | 5.30 | 0.02 |
| 150 | 97.7 | 81.4 | 5.30 | 0.02 |
| 175 | 96.8 | 83.7 | 5.93 | 0.03 |
| 200 | 87.5 | 97.7 | 38.04 | 0.12 |
| 225 | 87.5 | 90.7 | 9.40 | 0.13 |
| 250 | 87.5 | 90.7 | 9.40 | 0.13 |
| 500 | 87.5 | 93.0 | 12.5 | 0.13 |
| 1000 | 75.5 | 93.0 | 10.78 | 0.16 |
 | Table IVDistribution of subjects by the RMI
above/below the cut-off of 200. |
Table IV
Distribution of subjects by the RMI
above/below the cut-off of 200.
| RMI | Benign (n=43) | Malignant
(n=8) | P-value |
|---|
| <200 | 39 (90.7) | 1 (12.5) | <0.0001 |
| ≥200 | 4 (9.3) | 7 (87.5) | |
Discussion
Cancer-related deaths continue to be a major problem
worldwide. Ovarian cancer (OC) in particular is among the deadliest
malignancies in females. The reasons for this high mortality rate
are mainly the advanced stage at diagnosis and the frequent
recurrence after surgical resection and adjuvant therapy (13). However, the major challenges in
treating OC include early diagnosis, prognosis, prediction,
development of resistance to anticancer drugs and recurrence. With
no effective treatment for OC, early diagnosis remains an important
step to support current clinical approaches and improve patient
outcomes (14,15).
The present study was tailored to investigate the
diagnostic value of the RMI in evaluating and differentiating
between benign and malignant OMs in Libyan females for effective
early diagnosis. For this purpose, the RMI was used as an index
calculated from the US features, menopausal status and serum CA-125
levels.
In the present study, numerous important and
valuable observations have been made, all suggesting that the
assessment of the RMI in Libyan subjects provides important useful
information. However, comparisons with other studies are difficult,
as the present study is somewhat limited by the small size of the
cohort. The mean age of all patients was 47 years and that of
patients with malignant and benign disease was 59.5 and 44.8 years,
respectively. A high percentage of patients aged >50 years
presented with OC, as reported in other studies (16,17).
Females with advanced age had an elevated risk of OC, as more
mutations and accumulations in cells may cause cancer (6).
In the present study, 84% of OMs were observed to be
benign. This result is consistent with those of studies on OMs,
which reported that 70-90% of OMs were benign and 12-20% were
malignant (16-19).
Benign OMs were observed to be more common than malignant OMs. US
has been widely used as the primary imaging modality to define and
characterize OMs (20-22).
Vaginal US examination was frequently the best and first imaging
method when OMs were detected. However, numerous features of OMs
indicated malignant features, such as solid area, multilocularity,
papillary features and irregularity of internal septations.
Extensive experience from numerous centers around the world
suggested that the accuracy of assessment of OMs was 90% based on
US findings (23). The significant
value of US in evaluating OMs to assess the risk of malignancy was
investigated in several studies and the results suggested that
sensitivity, specificity and positive predictive value were high
(24). High sensitivity of the US
method was observed in the early stages of ovarian cancer.
Therefore, the method was encouraged as the first test for
malignancy risk assessment in patients with OMs (24). Of note, in the present study, it
was determined that all malignant cases had a US score of 3
(P<0.0001). Furthermore, US has higher sensitivity (100% vs.
87.5%) than the RMI and a lower specificity (69.8% vs. 90.7%) than
the RMI. These results are consistent with the findings of other
studies (20-22).
Furthermore, CA-125 is useful as a biological marker
for differential diagnosis and follow-up of patients with OMs.
Numerous studies have investigated the value of CA-125 in assessing
malignancy risk in females with OMs. The results suggested that
CA-125 values were inaccurate in early-stage ovarian cancer and
almost 50% of stage I patients had normal CA-125 values (6,19).
The CA-125 level may also be elevated in benign disease (25). Furthermore, due to its low
sensitivity and specificity, CA-125 is ineffective for screening
early ovarian cancer when the test is used alone (7,19).
Be that as it may, to this day, CA-125 is widely used as a
biological tumor marker for the detection of ovarian cancer.
However, while CA-125 used separately may have poor specificity,
when coupled with the RMI, the specificity is markedly enhanced
(18). The present study indicated
that CA-125 was highly expressed (≥35 U/ml) in 87.5% of patients
with malignant ovarian tumors and in 41.9% of patients with benign
ovarian tumors. In comparison, it was noted that CA-125 had the
same sensitivity (87.5 vs. 87.5%) as the RMI, but lower specificity
(58.1 vs. 90.7%) than the RMI. This was consistent with the results
of previous studies (15,18,26).
The RMI and estimation scores based on initial
diagnostic workups, including CA-125 levels, US and patient age,
are widely used for estimating the risk of malignancy in patients
with Oms (7,8). In patients with OMs, an RMI score of
>200 is associated with an increased risk of malignancy
(7).
In the present study, different cut-off points of
the RMI (25-1,000) were assessed to determine the best predictive
value for malignancy risk. The cut-off point of 200 provided the
highest sensitivity, specificity and positive predictive value
(87.5, 97.7 and 38.4% respectively).
In addition, the ROC curve analysis indicated that
at a cut-off value of 200 for the RMI, the likelihood of having
malignant disease was 38.4%, while the likelihood of having benign
disease was only 0.12% in females with OMs. The RMI with a cut-off
value of 200 had the highest significance in discriminating OMs
(<25, 25-250 and >250, respectively) (27). The present observations were in
agreement with numerous studies, which also noted that RMI at a
cut-off point of 200 may serve as a quantitative criterion for
splitting Libyan patients with OM into two groups (benign vs.
malignant) depending on malignancy risk (28,29).
While certain unexpected but minor fluctuations of the negative LR
below the threshold were observed, in general, the RMI of OMs was
strongly discriminated by the 200 cut-off value. However, further
confirmation of the present findings may only be provided by more
intensive studies with a large sample size in Libya. In addition, a
randomized controlled trial (RCT) is the most effective scientific
method to evaluate the effectiveness of such clinical research.
RCTs are undoubtedly of high value in Libya and will be considered
and planned for patients with cancer in the future.
In conclusion, calculating the RMI is the best
method and the most reliable tool for defining subsequent
diagnostic, management and therapeutic strategies for benign and
malignant OMs. However, due to the limitation of the small sample
size in the present study, further research is warranted.
Acknowledgements
The authors would like to thank Professor Mohamed
Ahmed Elfagieh, Director of the National Cancer Institute
(Misurata, Libya), for his continuous support of the medical
research.
Funding
Funding: No funding was received.
Availability of data and materials
All relevant raw data from the study are freely
available to any researcher upon request.
Authors' contributions
AH: Study design and demographical and
clinicopathological data collection; AA: Data collection; MA:
Analysis and interpretation of the results, and writing and
proofreading of the manuscript; AB: Statistical analysis of the
data, preparation of figures, and writing and proofreading of the
manuscript. AB: preparation of the figures, review the study
manuscript and proofreading; EE: Statistical analysis, study design
and manuscript drafting. Both AH and EE reviewed and approved the
authenticity of the raw data, and all authors reviewed and approved
the final version of the manuscript.
Ethics approval and consent to
participate
This study is part of the medical research studies
approved by the Institutional Review Board of the National Cancer
Institute (Misurata, Libya; ethical approval no. 7-20121).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Group UCSW: United States cancer
statistics: 1999-2010 incidence and mortality web-based report.
Atlanta: US Department of Health and Human Services, Centers for
Disease Control and Prevention and National Cancer Institute 201,
2013.
|
|
2
|
GBD 2015 Mortality and Causes of Death
Collaborators: Global, regional, and national life expectancy,
all-cause mortality, and cause-specific mortality for 249 causes of
death, 1980-2015: A systematic analysis for the global burden of
disease study 2015. Lancet. 388:1459–1544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wild CP, Stewart BW and Wild C: World
Cancer Report 2014. World Health Organization, Switzerland,
2014.
|
|
4
|
Coburn SB, Bray F, Sherman ME and Trabert
B: International patterns and trends in ovarian cancer incidence,
overall and by histologic subtype. Int J Cancer. 140:2451–2460.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rossing MA, Wicklund KG, Cushing-Haugen KL
and Weiss NS: Predictive value of symptoms for early detection of
ovarian cancer. J Natl Cancer Inst. 102:222–229. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hoffman BL, Schorge JO, Schaffer JI,
Halvorson LM, Bradshaw KD and Cunningham FG: Epithelial ovarian
cancer. In: Williams Gynecology. 2nd edition. McGraw-Hill,
pp853-878, 2012.
|
|
7
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
No authors listed: Surveillance Report
(Exceptional Review) 2017-Ovarian Cancer: Recognition and initial
management (2011) NICE guideline CG122. National Institute for
Health and Care Excellence, London, 2017.
|
|
9
|
Javdekar R and Maitra N: Risk of
malignancy index (RMI) in evaluation of adnexal mass. J Obstet
Gynaecol India. 65:117–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Andersen ES, Knudsen A, Rix P and Johansen
B: Risk of malignancy index in the preoperative evaluation of
patients with adnexal masses. Gynecol Oncol. 90:109–112.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Terzić M, Dotlić J, Ladjević IL,
Atanacković J and Ladjević N: Evaluation of the risk malignancy
index diagnostic value in patients with adnexal masses. Vojnosanit
Pregl. 68:589–593. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moolthiya W and Yuenyao P: The risk of
malignancy index (RMI) in diagnosis of ovarian malignancy. Asian
Pac J Cancer Prev. 10:865–868. 2009.PubMed/NCBI
|
|
13
|
Akdeniz N, Kuyumcuoğlu U, Kale A,
Erdemoğlu M and Caca F: Risk of malignancy index for adnexal
masses. Eur J Gynaecol Oncol. 30:178–180. 2009.PubMed/NCBI
|
|
14
|
Escudero JM, Auge JM, Filella X, Torne A,
Pahisa J and Molina R: Comparison of serum human epididymis protein
4 with cancer antigen 125 as a tumor marker in patients with
malignant and nonmalignant diseases. Clin Chem. 57:1534–1544.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Myers ER, Bastian LA, Havrilesky LJ,
Kulasingam SL, Terplan MS, Cline KE, Gray RN and McCrory DC:
Management of adnexal mass. Evid Rep Technol Assess (Full Rep)
1-145, 2006.
|
|
16
|
Bindal J and Bankey S: Prevalence of
ovarian tumours among ovarian mass lesions in Gajra Raja Medical
College, Gwalior, India. Int J Reprod Contracept Obstet Gynecol.
6:3907–3911. 2017.
|
|
17
|
Jha R and Karki S: Histological pattern of
ovarian tumors and their age distribution. Nepal Med Coll J.
10:81–85. 2008.PubMed/NCBI
|
|
18
|
McGuire V, Hartge P, Liao LM, Sinha R,
Bernstein L, Canchola AJ, Anderson GL, Stefanick ML and Whittemore
AS: Parity and oral contraceptive use in relation to ovarian cancer
risk in older women. Cancer Epidemiol Biomarkers Prev.
25:1059–1063. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Al-Musalhi K, Al-Kindi M, Ramadhan F,
Al-Rawahi T, Al-Hatali K and Mula-Abed WA: Validity of Cancer
Antigen-125 (CA-125) and risk of malignancy index (RMI) in the
diagnosis of ovarian cancer. Oman Med J. 30:428–434.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu J, Xu Y and Wang J: Ultrasonography,
computed tomography and magnetic resonance imaging for diagnosis of
ovarian carcinoma. Eur J Radiol. 62:328–334. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Radiology ACo: ACR Appropriateness
Criteria 2008: Clinically suspected adnexal mass. American College
of Radiology Web site. Available from: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonWomenImaging/SuspectedAdnexalMasses-Doc11.
Accessed November 9, 2009.
|
|
22
|
Valentin L, Ameye L, Jurkovic D, Metzger
U, Lécuru F, Van Huffel S and Timmerman D: Which extrauterine
pelvic masses are difficult to correctly classify as benign or
malignant on the basis of ultrasound findings and is there a way of
making a correct diagnosis? Ultrasound Obstet Gynecol. 27:438–444.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Patel MD: Practical approach to the
adnexal mass. Radiol Clin North Am. 44:879–899. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rein BJ, Gupta S, Dada R, Safi J, Michener
C and Agarwal A: Potential markers for detection and monitoring of
ovarian cancer. J Oncol. 2011(475983)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nazneen T, Begum SA, Mahmud T, Khatoon F,
Islam F and Amatullah M: Preoperative analysis of CA-I25 and its
relation with histopathological study in ovarian tumours.
Mymensingh Med J. 30:402–409. 2021.PubMed/NCBI
|
|
26
|
Liao XY, Huang GJ, Gao C and Wang GH: A
meta-analysis of serum cancer antigen 125 array for diagnosis of
ovarian cancer in Chinese. J Cancer Res Ther. (Suppl 10):C222–C224.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Van Calster B, Timmerman D, Valentin L,
McIndoe A, Ghaem-Maghami S, Testa AC, Vergote I and Bourne T:
Triaging women with ovarian masses for surgery: Observational
diagnostic study to compare RCOG guidelines with an international
ovarian tumour analysis (IOTA) group protocol. BJOG. 119:662–671.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ulusoy S, Akbayir O, Numanoglu C, Ulusoy
N, Odabas E and Gulkilik A: The risk of malignancy index in
discrimination of adnexal masses. Int J Gynaecol Obstet.
96:186–191. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chia YN, Marsden DE, Robertson G and
Hacker NF: Triage of ovarian masses. Aust N Z J Obstet Gynaecol.
48:322–328. 2008.PubMed/NCBI View Article : Google Scholar
|