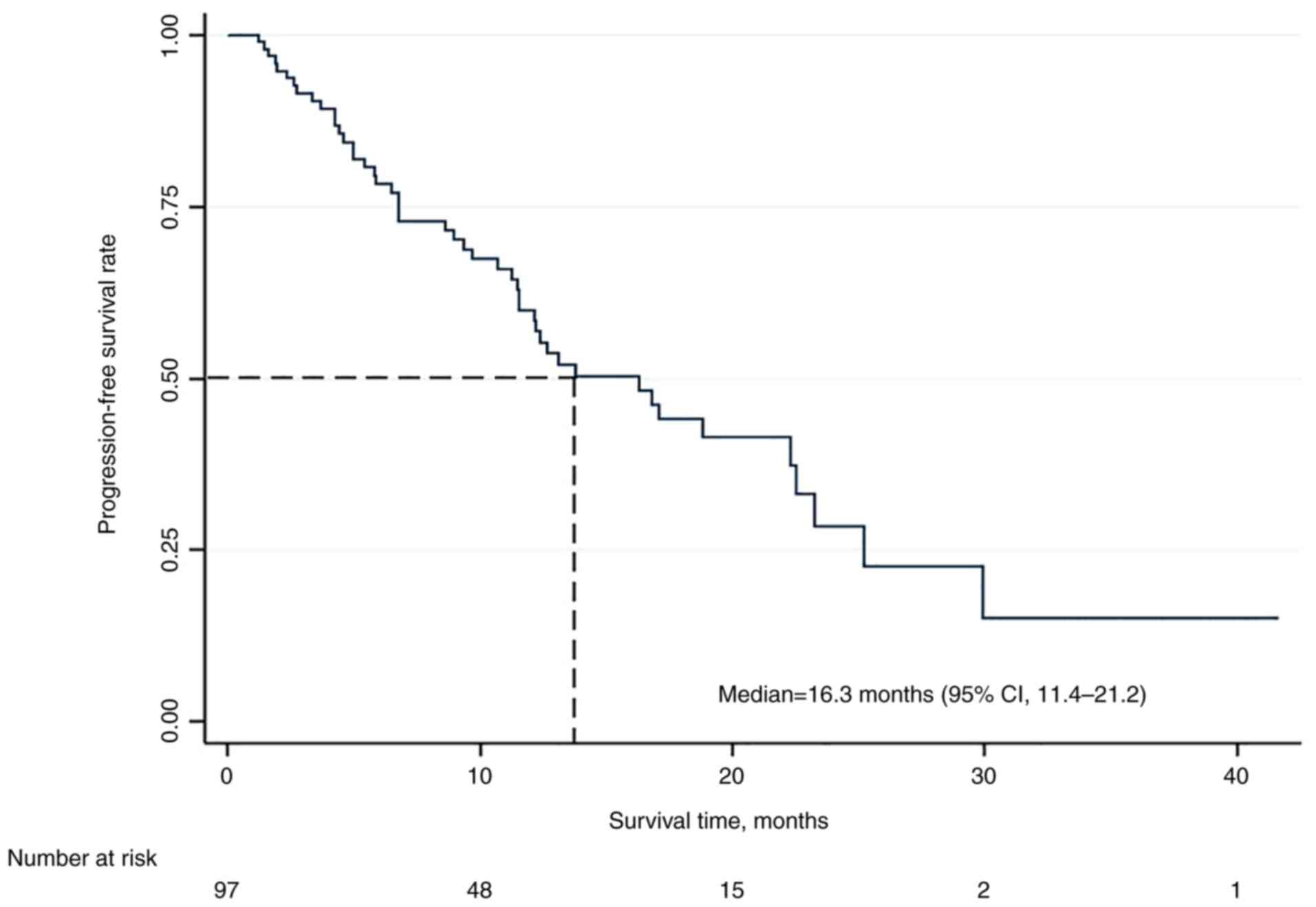
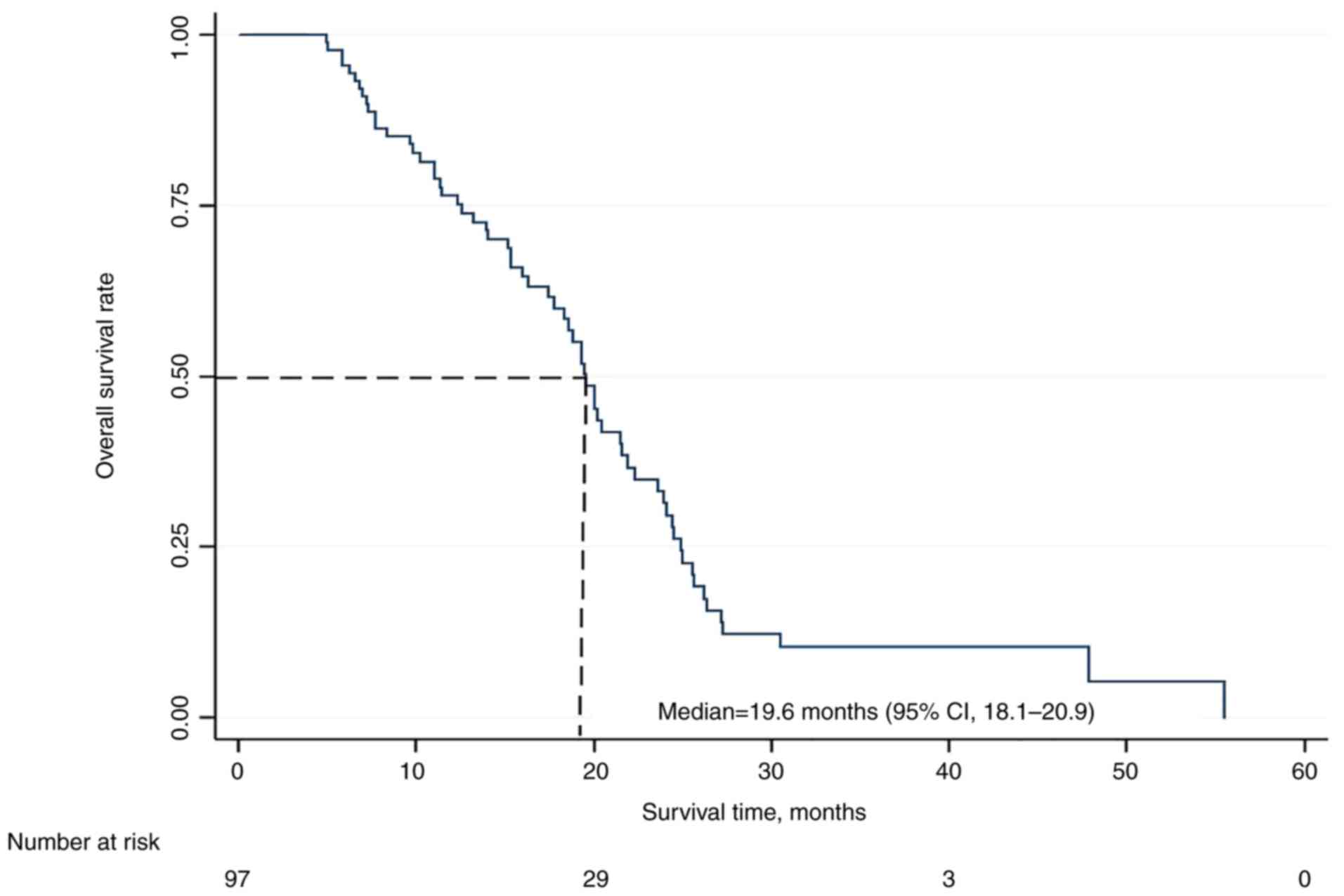
|
1
|
Althubiti MA and Nour Eldein MM: Trends in
the incidence and mortality of cancer in Saudi Arabia. Saudi Med J.
39:1259–1262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Howlader N, Altekruse SF, Li CI, Chen VW,
Clarke CA, Ries LA and Cronin KA: US incidence of breast cancer
subtypes defined by joint hormone receptor and HER2 status. J Natl
Cancer Inst. 106(dju055)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Global Burden of Disease Cancer
Collaboration. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–1568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pan H, Gray R, Braybrooke J, Davies C,
Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, et
al: 20-Year risks of breast-cancer recurrence after stopping
endocrine therapy at 5 years. N Engl J Med. 377:1836–1846.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
O'Leary B, Finn RS and Turner NC: Treating
cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol.
13:417–430. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roberto M, Astone A, Botticelli A,
Carbognin L, Cassano A, D'Auria G, Fabbri A, Fabi A, Gamucci T,
Krasniqi E, et al: CDK4/6 inhibitor treatments in patients with
hormone receptor positive, Her2 negative advanced breast cancer:
Potential molecular mechanisms, clinical implications and future
perspectives. Cancers (Basel). 13(332)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rugo HS, Diéras V, Gelmon KA, Finn RS,
Slamon DJ, Martin M, Neven P, Shparyk Y, Mori A, Lu DR, et al:
Impact of palbociclib plus letrozole on patient-reported
health-related quality of life: Results from the PALOMA-2 trial.
Ann Oncol. 29:888–894. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Verma S, O'Shaughnessy J, Burris HA,
Campone M, Alba E, Chandiwana D, Dalal AA, Sutradhar S, Monaco M
and Janni W: Health-related quality of life of postmenopausal women
with hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer treated with ribociclib
+ letrozole: Results from MONALEESA-2. Breast Cancer Res Treat.
170:535–545. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Booth CM, Karim S and Mackillop WJ:
Real-world data: Towards achieving the achievable in cancer care.
Nat Rev Clin Oncol. 16:312–325. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Murthy VH, Krumholz HM and Gross CP:
Participation in cancer clinical trials: Race-, sex-, and age-based
disparities. JAMA. 291:2720–2726. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Turner NC, Slamon DJ, Ro J, Bondarenko I,
Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al:
Overall survival with palbociclib and fulvestrant in advanced
breast cancer. N Engl J Med. 379:1926–1936. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Turner NC, Ro J, André F, Loi S, Verma S,
Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, et al:
Palbociclib in hormone-receptor-positive advanced breast cancer. N
Engl J Med. 373:209–219. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cristofanilli M, Turner NC, Bondarenko I,
Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et
al: Fulvestrant plus palbociclib versus fulvestrant plus placebo
for treatment of hormone-receptor-positive, HER2-negative
metastatic breast cancer that progressed on previous endocrine
therapy (PALOMA-3): Final analysis of the multicentre,
double-blind, phase 3 randomised controlled trial. Lancet Oncol.
17:425–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Odan N, Kikawa Y, Matsumoto H, Minohata J,
Suwa H, Hashimoto T, Okuno T, Miyashita M, Saito M, Yamagami K and
Takao S: Real-world outcomes of treating advanced breast cancer
patients with palbociclib: A multicenter retrospective cohort study
in Japan-the KBCOG-14 study. Breast Cancer (Auckl).
14(1178223420983843)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vuppalanchi R, Saxena R, Storniolo AMV and
Chalasani N: Pseudocirrhosis and liver failure in patients with
metastatic breast cancer after treatment with palbociclib.
Hepatology. 65:1762–1764. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
No authors listed: LiverTox: Clinical and
Research Information on Drug-Induced Liver Injury [Internet].
National Institute of Diabetes and Digestive and Kidney Diseases,
Bethesda, MD, 2012.
|
|
21
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Overall survival with ribociclib plus fulvestrant in
advanced breast cancer. N Engl J Med. 382:514–524. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
He J, Morales DR and Guthrie B: Exclusion
rates in randomized controlled trials of treatments for physical
conditions: A systematic review. Trials. 21(228)2020.PubMed/NCBI View Article : Google Scholar
|