Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second
most common primary hepatic malignancy, accounting for >5% of
primary liver cancers, and the number of cases is increasing
worldwide (1). The only
potentially curative treatment of ICC is surgical resection,
although some patients subsequently develop recurrence (2). Several combinations of systemic
chemotherapy have shown to improve patients' survival; however, the
etiology and pathogenesis of ICC remain poorly understood (3-5).
Jumonji domain-containing 6 (JMJD6), a member of the
Jumonji C domain-containing family of proteins, was originally
identified as a phosphatidylserine receptor (PSR) on cell surface
(6). Subsequent studies have
demonstrated that JMJD6 is located in the nucleus and has
demethylase and hydroxylase activities toward histone and
non-histone proteins (7,8). There is growing evidence to indicate
that JMJD6 overexpression is associated with advanced
clinicopathological stage, increased aggressiveness, and poor
survival in various types of cancer; however, the impact of JMJD6
on ICC has not been reported yet (9).
Tumor immunology is a hot topic describing the
interaction between the immune system and tumor cells.
Understanding these interactions is important for the development
of novel therapies for cancer. Immune checkpoint inhibitors
function by reducing the suppression of T cells, especially
CD8+ T cells, to improve tumor-specific responses
(10,11). An increasing number of reports
describe the relationship between CD8+ T cells and ICC
prognosis. Our institution has shown that decreased microvessel
density is related to worse prognosis, and Asahi et al also
reported that high CD8 count could be an improved prognostic factor
of ICC (12,13).
In this study, we assessed the clinical relevance
and prognostic significance of JMJD6 expression in ICC. We also
aimed to reveal the possible mechanism and the relationship between
JMJD6 and the tumor immunological environment via in vitro
JMJD6 knockdown studies.
Materials and methods
Patients and tumor samples
We retrospectively examined patients with primary
ICC who underwent surgical resection at the Department of Surgery
and Science, Graduate School of Medical Sciences, Kyushu
University. Fifty-three patients with ICC who were diagnosed
between May 1998 and August 2017 were eligible for inclusion in the
study. The patients provided the written consent for the use of
their tissues for future scientific research at the time of
collection. Paraffin-embedded specimens were retrieved from the
Department of Anatomic Pathology, Graduate School of Medical
Sciences, Kyushu University. The analyzed clinicopathological
features included the age at surgery, sex, pathological stage (8th
edition AJCC/UICC staging manual), microvascular invasion,
laboratory data, and the clinical course of each patient (14). This study was approved by the
Clinical Research Ethics Committee of Kyushu University Hospital
according to the Ethical Guidelines of the Japanese Government
(approval no. 30-578).
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded tissue sections
from patients with ICC were immunostained for JMJD6 (PSR H-7,
sc-28348, Santa Cruz Biotechnology, Inc.; 1:100) and PD-L1 (clone
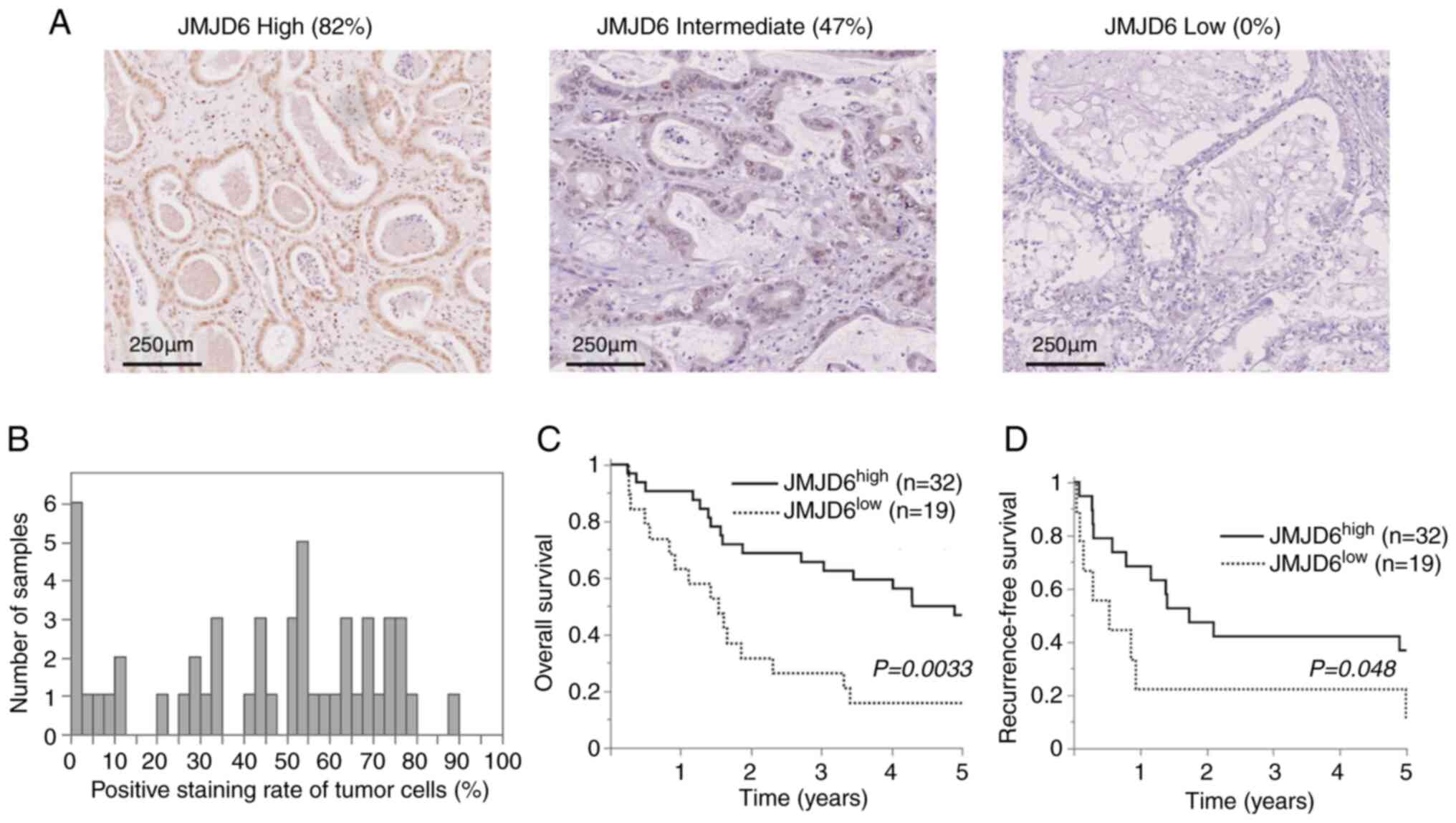
SP142, Spring Bioscience; 1:100). Positive JMJD6 nuclear expression
was defined as nuclear staining of ≥40% (Fig. 1). The programmed death-ligand 1
(PD-L1) expression on the membrane and cytoplasm was defined as
positive when the percentage of positive cells was ≥5% of ICC cells
(15). Immunohistochemical
evaluations were independently performed by two observers (Y.K. and
K.Y.) who were blinded to the clinical backgrounds of the patients.
If the difference between evaluations was >10%, the evaluations
were repeated. The findings of the two observers were averaged and
considered final. The capture of microscopic images and
quantitative analyses were undertaken on the NanoZoomer platform
(Hamamatsu Photonics), and we ensured that the results matched with
the observers' results.
Cell lines
The cholangiocarcinoma cell lines, SSP-25 and
HuH-28, were obtained from Riken Bioresource Center, Tsukuba,
Japan. The cell lines were originally isolated from intrahepatic
cholangiocarcinoma specimens obtained from surgical resection of
Japanese adult patients. The SSP-25 cell line was authenticated by
STR profiling (supplemental document). The cells were incubated at
37˚C and 5% CO2 in RPMI media (Thermo Fisher Scientific,
Inc.) supplemented with heat-inactivated 10% fetal bovine serum and
penicillin-streptomycin.
JMJD6 siRNA transfection
The siRNA transfection was performed as previously
described (16). Lipofectamine
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
transfect the cells with siRNA (17). The siRNAs were obtained from
Dharmacon, Inc. (siJMJD6-1 CAT# J-010363-10-0002,
sequence=GGAGAGCACUCGAGAUGAU, siJMJD6-2 CAT# J-010363-12-0002,
sequence=GGUAUAGGAUUUUGAAGCA, Control (non-targeting pool) CAT#
D-001810-10-05). To facilitate transfection, the cells per well
were incubated in 10% FBS containing RPMI; subsequently, they were
plated to 40% confluence on a 6-well plate during transfection. We
mixed 150 µl of Opti-MEM and 9 µl of RNAiMAX and subjected the
mixture to incubation for 5 min at room temperature. In another
tube, 10 pM siRNA in 3 ml of Opti-MEM and 150 µl of Opti-MEM were
combined. Subsequently, we added siRNA solution to the diluted
RNAiMAX reagent, and 250 µl of the prepared siRNA/RNAiMAX mixtures
per well was used for incubation at room temperature for 25 min.
Afterward, the 2.5x105 cells per well and the solution
were combined. They were incubated for 6 h at 37˚C, and the mixture
was replaced with RPMI with 10% FBS. The cells were incubated for
72 h in maximum, and the transfection efficiency was monitored
every 24 h using western blotting and real-time polymerase chain
reaction (PCR) in order to optimize the appropriate incubation
time, and was decided to be 48 h.
Transwell migration assay and
viability assay
Transwell migration assay: A total of
4x104 SSP-25 cells resuspended in 250 µl RPMI were
placed on an 8.0-µm Transparent PET Membrane (Corning Inc.). The
chamber was placed in a 24-well plate containing 750 µl RPMI and
10% FBS. After incubation at 37˚C overnight, migrating cells were
stained with Diff-Quik (KACLaS) and were counted manually in five
random microscopic fields at x200 magnification and quantified
using ImageJ software (https://imagej.net/). The viability of the cells was
examined by the CellTiter-Glo (CTG) assay (Promega) according to
the manufacturer's instructions. Briefly, the cells were plated in
96-well plates with enough number of cells to 100% confluent in 24
h. The luminescence was read and quantified in 24 h with Multiskan
GO spectrophotometric microplate reader (Thermo Fisher Scientific,
Inc.).
RNA extraction and sequencing
RNA was extracted using the Maxwell(R) RSC simplyRNA
tissue kit (Promega). Whole transcriptome sequencing was applied to
RNA samples using the Illumina HiSeq 3000 platform in a 100-bp
single-end mode. Sequenced reads were mapped to the human reference
genome sequence (hg19) using TopHat version 2.0.13 in combination
with Bowtie 2 version 2.2.3 and SAMTools version 1.0. The number of
fragments per kilobase of exon per million mapped fragments was
calculated using Cuffnorm version 2.2.1. RNA-Seq data were
calculated as the fold change between samples with two-tailed
Student's t-test (P<0.1) using the Subio Platform and Subio
Basic Plug-in (v1.20; Subio, Inc.). Thresholds were set at a fold
change of 2.0 and P-values of <0.05. Raw data of this study were
submitted to Gene Expression Omnibus (accession no. GSE171974).
Reverse transcription-quantitative PCR
(RT-qPCR)
One microgram of total RNA was converted to cDNA
using the SuperScript III First-Strand Synthesis Supermix (Thermo
Fisher Scientific, Inc.) with oligo-dT primers as per
manufacturer's instructions. Quantification was determined using
the ΔΔCt method relative to a β-actin control. The qRT-PCR primers
used were as follows: JMJD6 Hs00397095_m1 and β-actin
Hs01060665_g1. Assays were performed using the TaqMan Fast Advanced
Master Mix (Thermo Fisher Scientific, Inc.) (18,19).
Western blot assay
Western blotting was performed as previously
described (19). Briefly,
whole-cell lysis was performed in RIPA buffer containing protease
inhibitors (Nacalai Tesque). Proteins were separated by
polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes. After blocking with blocking
buffer supplied in the iBind Western System (Thermo Fisher
Scientific, Inc.), membranes were incubated with the primary
antibody. Monoclonal antibodies for PD-L1 (1:1,000, E1L3N; Cell
Signaling Technology), JMJD6 (1:200; Santa Cruz Biotechnology,
Inc.), and β-actin (1:1,000; Cell Signaling Technology) were used.
The JMJD6 antibody was derived from mouse, and the other antibodies
were derived from rabbit. Horseradish peroxidase-conjugated
secondary antibodies for mouse (1:1,000; Abcam) and rabbit
(1:2,000; Abcam) were used. Primary and secondary antibodies were
simultaneously applied on the rockers of the iBind Western System
after setting membranes on a paper filter. Antibody binding was
detected by enhanced chemiluminescence assays, and each band was
detected using an Amersham Imager 600 (GE Healthcare Life
Sciences).
Statistical analysis
Statistical analysis was performed with JMP Pro
16.1.0 statistical software. Comparisons of categorical and
continuous variables were performed using the Chi-square test and
Student's t-test or the Mann-Whitney U test, respectively. A
P-value of <0.05 was considered significant. Cumulative overall
survival (OS) and recurrence-free survival (RFS) rates were
calculated using the Kaplan-Meier method, and differences between
curves were evaluated using the log-rank test.
Results
JMJD6 expression in ICC specimens
Patients with ICC comprised 34 males and 17 females
at an age range of 33-82 years (median=60). JMJD6 expression was
analyzed in ICC specimens. Immunohistochemical analysis revealed
that JMJD6 expression was predominantly noted in the nuclei
(Fig. 2A and B). The percentage of stained tumor cells
was analyzed, and the mean value was selected as the cut-off point
to obtain comparable subgroup sizes.
Association of JMJD6 expression with
clinicopathological features
The relationship between JMJD6 expression and
clinicopathological factors in patients with ICC was evaluated
(Table I). High JMJD6 expression
was observed in 32 of 51 patients (62.7%). JMJD6 expression was not
significantly associated with clinicopathological factors, except
for older age in low-expression samples (65.6 vs. 58.7 years, P=
0.043).
 | Table IClinicopathological factors and JMJD6
expression in IHC. |
Table I
Clinicopathological factors and JMJD6
expression in IHC.
| Factors
(Category) | JMJD6 High expression
(n=32) | JMJD6 Low expression
(n=19) | P-value |
|---|
| Median age, years
(IQR) | 58.7 (55-63) | 65.6 (60-71) | 0.043a |
| Sex | | | 0.68 |
|
Male
(%) | 22(69) | 12(63) | |
|
Female
(%) | 10(31) | 7(37) | |
| Albumin (g/dl) | 4.11 (3.97-4.27) | 4.03 (3.84-4.23) | 0.49 |
| Total bilirubin
(mg/dl) | 0.72 (0.31-1.13) | 1.31 (0.78-1.84) | 0.081 |
| Tumor size (mm) | 44.9 (37.3-52.4) | 46.6 (37.3-55.9) | 0.77 |
|
Solitary/Multiple | 23/9 | 10/9 | 0.16 |
| Microvascular
invasion | 16/14 | 13/5 | 0.20 |
|
Perihilar/Peripheral | 12/20 | 10/9 | 0.29 |
| Differentiation
(well/mod/por) | 10/20/2 | 9/8/1 | 0.42 |
| CEA (ng/ml) | 3.37
(1.88-4.86) | 3.12
(1.18-5.06) | 0.84 |
| CA19-9 (U/ml) | 1349 (0-6,946) | 7,778
(863-14,693) | 0.15 |
JMJD6 expression and survival
analysis
According to univariate analysis, low JMJD6
expression was significantly associated with poor overall survival
(OS, P=0.0033) (Fig. 1C) and
recurrence-free survival (RFS, P=0.048) (Fig. 1D).
Univariate analysis revealed that JMJD6 expression,
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9) were unfavorable predictors for OS. In the multivariate
analysis, low JMJD6 expression and high CA19-9 were revealed to be
the independent worse prognostic factors for OS (Table II). In the analyses regarding RFS,
univariate analysis showed that low JMJD6 expression, low serum
albumin level, high carcinoembryogenic antigen (CEA), and
microvascular invasion were unfavorable predictors for RFS. In the
multivariate analysis, low JMJD6 expression, low serum albumin
level and high carcinoembryogenic antigen (CEA) were independent
worse prognostic factors of RFS (Table III). The analyses were also
performed in the subgroups of peripheral and perihilar ICC and did
not reveal any specific differences (data not shown).
 | Table IIUnivariate and multivariate Cox
proportional hazard analyses of overall survival in patients with
ICC. Bold numbers indicate statistically significant correlations
(P<0.05). |
Table II
Univariate and multivariate Cox
proportional hazard analyses of overall survival in patients with
ICC. Bold numbers indicate statistically significant correlations
(P<0.05).
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factors | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| JMJD6, low
expression | 2.73
(1.36-5.48) | 0.0047a | 3.47
(1.62-7.45) | 0.0014a |
| Age ≥60 (year) | 1.20
(0.60-2.37) | 0.6070 | | |
| Sex, male | 1.39
(0.66-2.91) | 0.3890 | | |
| Albumin ≤3.5
(g/dl) | 1.87
(0.45-7.85) | 0.3917 | | |
| Total bilirubin
≥2.0 (mg/dl) | 2.99
(0.67-13.4) | 0.1522 | | |
| CEA ≥5 (U/ml) | 2.76
(1.18-6.46) | 0.0189a | 1.52
(0.61-3.81) | 0.3671 |
| CA19-9 ≥50
(U/ml) | 2.08
(1.01-4.28) | 0.0461a | 2.72
(1.25-5.89) | 0.0114a |
| Tumor size ≥5-cm
(cm) | 1.98
(0.99-3.97) | 0.0530 | | |
| MVI, positive | 1.94
(0.91-4.12) | 0.0845 | | |
| Location,
perihilar | 1.26
(0.63-2.51) | 0.5108 | | |
| Number of tumors
>1 | 1.95
(0.97-3.91) | 0.0593 | | |
 | Table IIIUnivariate and multivariate Cox
proportional hazard analyses of recurrence-free survival in
patients with ICC. |
Table III
Univariate and multivariate Cox
proportional hazard analyses of recurrence-free survival in
patients with ICC.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factors | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| JMJD6, low
expression | 2.20
(1.13-4.26) | 0.0206a | 2.33
(1.16-4.68) | 0.0179a |
| Age ≥60 (year) | 1.30
(0.67-2.50) | 0.4388 | | |
| Sex, male | 1.73
(0.83-3.60) | 0.1428 | | |
| Albumin ≤3.5
(g/dl) | 3.45
(1.03-11.5) | 0.0448a | 5.18
(1.45-18.5) | 0.0113a |
| Total bilirubin
≥2.0 (mg/dl) | 3.69
(0.84-16.2) | 0.0835 | | |
| CEA ≥5 (U/ml) | 4.10
(1.70-9.88) | 0.0016a | 3.80
(1.53-9.46) | 0.0041a |
| CA19-9 ≥50
(U/ml) | 1.58
(0.79-3.14) | 0.1973 | | |
| Tumor size ≥5
(cm) | 1.98
(0.99-3.97) | 0.0530 | | |
| MVI, positive | 2.32
(1.12-4.78) | 0.0231a | 1.97
(0.92-4.21) | 0.0804 |
| Location,
perihilar | 0.94
(0.55-2.06) | 0.8586 | | |
| Number of tumors
>1 | 1.93
(0.97-3.81) | 0.0592 | | |
In vitro JMJD6 expression, cellular
assays and RNA sequencing
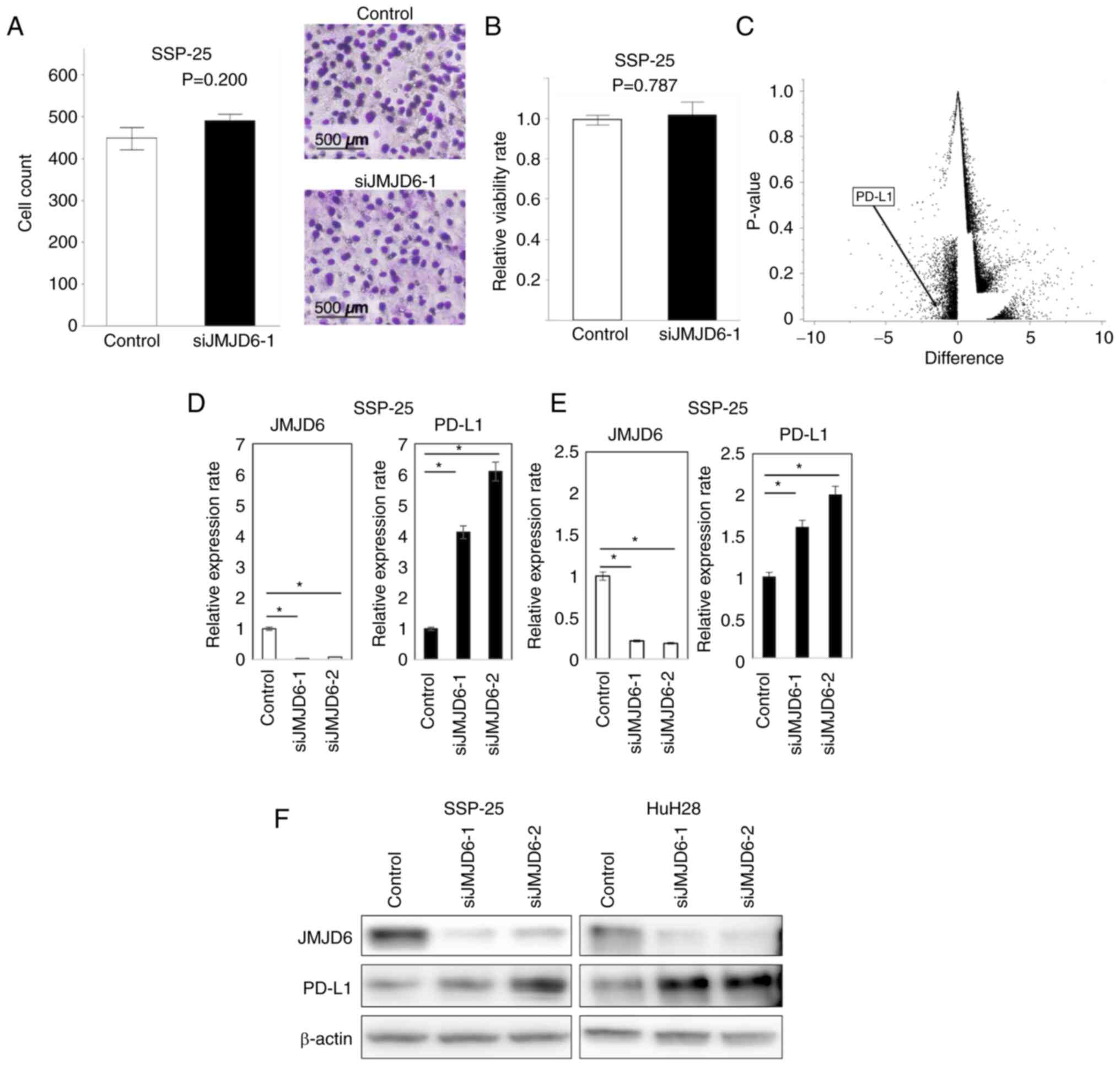
To explore the importance of JMJD6 in ICC, JMJD6 was
knocked down in SSP-25 cells. The migration assay (Fig. 2A) and the viability assay (Fig. 2B) did not show any significant
difference between JMJD6-knockeddown cells and the control. RNA
sequencing revealed 91 genes whose expression were positively
altered and 167 negatively altered genes in JMJD6-depleted cells
(Fig. 2C and D, Table
SI). Among those, we focused on immunology-related genes to
evaluate the impact of tumor immunology on the prognosis of ICC.
Notably, JMJD6 knockdown increased the expression of PD-L1. An
increase in PD-L1 expression under JMJD6 depletion was also
identified using RT-PCR (Fig. 2E).
Western blotting also revealed similar increase of PD-L1 expression
in ICC cells after JMJD6 knockdown (Fig. 2F).
PD-L1 in clinical samples
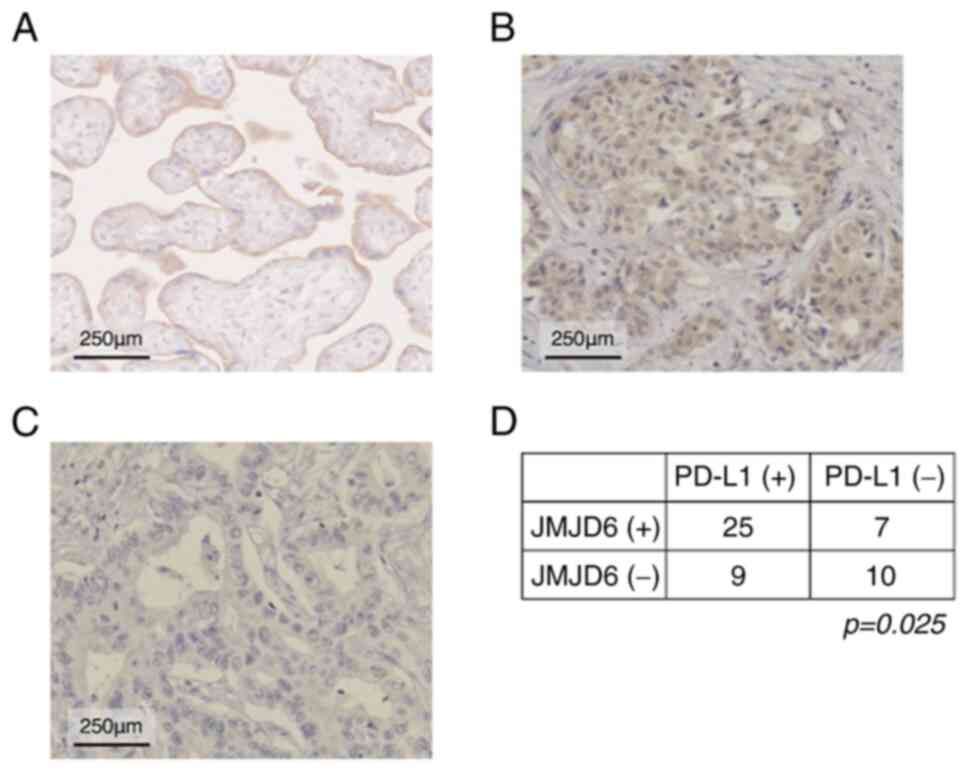
To investigate the PD-L1 expression in clinical
specimens, we performed IHC for PD-L1 in the same samples used for
the IHC for JMJD6 (Fig. 3A). The
result showed that 34 of 51 samples were positive for PD-L1. The
rate of high PD-L1 expression was higher in the low JMJD6 group and
vice versa (Fig. 3B; P=0.025). We
also performed the survival analysis of PD-L1 expression, but it
did not show any significant results (P-value for OS=0.3070,
P-value for PFS=0.0687, data not shown).
Discussion
Patients with ICC have a poor prognosis, and
curative treatments, such as surgical resection, are limited to
early-stage disease. Systemic therapy options and their
effectiveness are also limited. In contrast to the dramatic
decrease of hepatocellular carcinoma owing to the development of
virological treatment and newly emerged systemic therapy options,
ICC treatment is limited and still requires further investigation
(20).
In this study, we showed that the epigenetic
regulator JMJD6 is a favorable prognostic factor for ICC and that
JMJD6 is a possible regulator of PD-L1 expression. A mechanism that
can explain these results is that JMJD6 modifies the promoter
region of PD-L1 and inhibits PD-L1 expression. JMJD6 demethylates
histones and other proteins, and it promotes or inhibits gene
expression depending on which amino acid and on what site they are
located. Also, PD-L1 expression is reported to be upregulated
through epigenetic regulation with histone demethylases (21). Thus, JMJD also has the possibility
to regulate the expression of PD-L1.
The relationship between JMJD6 and tumor has
previously been reported as an unfavorable prognostic factor in
various cancers, including breast cancer, colon cancer, oral
squamous cancer, melanoma, and hepatocellular carcinoma. However,
to the best of our knowledge, the role of JMJD6 in ICC has not been
investigated (22-24).
Our results showed that JMJD6 is a good prognostic
factor, in contrast to past reports. As JMJD6 is an epigenetic
modifier, the influence of the protein varies depending on what
type of protein to regulate, e.g., CDK4 in HCC, p53 in colon
cancer. Therefore, we performed RNA sequencing to elucidate the
genes regulated by JMJD6, which resulted in the identification of
171 candidate genes. Our institution has previously reported the
impact of tumor immunology on cancer prognosis (12,25,26);
thus, we focused on immune-related genes. We found that PD-L1 mRNA
levels increased in response to JMJD6 knockdown, indicating that
JMJD6 regulates PD-L1 expression. This inverse relationship between
JMJD6 and PD-L1 expression was confirmed by PCR, western blotting,
and IHC.
Although in vitro experiments have revealed
the connection between JMJD6 and PD-L1, this was not sufficient to
explain that PD-L1 expression is controlled by JMJD6, and the key
to connect them is the epigenetic modification. CD274, the gene
encoding PD-L1, is located on chromosome 9p24.1. In this region,
genomic regulation has been proven to upregulate PD-L1 expression,
resulting in immune escape (27).
Among these types of regulation, H3K4me3 is upregulated by MLL1, an
H3K4 methylation-specific histone methyltransferase in pancreatic
cancer (28). The present data
suggest that a similar mechanism is possibly activated to mediate
PD-L1 expression.
PD-L1 induces cancer cell immune evasion by binding
to the PD-1 receptor on activated T cells, which results in
tolerance of tumor-reactive T cells, rendering tumor cells
resistant to CD8+ T cells (29).
In the current study, although the positivity of PD-L1 was not
sufficient to demonstrate any correlation with the prognosis of
ICC, the high JMJD6 expression, which inversely reflected PD-L1
expression, impacted the prognosis of ICC. Also, the use of immune
checkpoint inhibitor combined with the conventional systemic
therapy has emerged to be effective in prolonging the survival of
biliary tract cancer patients. Thus, JMJD6 is a potential biomarker
to prove the susceptibility of ICI in each individual.
This study has several limitations. First, the
current study was derived from only one institution and the number
of samples was small. Second, we did not perform any epigenetic
experiments to directly prove the mechanism, by analysis with
chromatin immunoprecipitation (ChIP) for example. Also, our
cellular experiments only focused on viability and migration, and
we did not perform the ones regarding apoptosis or cell cycle,
which were reported in previous JMJD6 reports. Therefore, there is
a room for future research about these factors. Additionally, the
current research only presented the in vitro experiments and
clinical sample study. Further studies using in vivo tumor
mouse models and anti-PD-L1 agents are required to obtain more
persuasive evidence.
In conclusion, JMJD6 is an independent favorable
prognostic factor for ICC and is a candidate target protein for the
treatment of ICC, focusing on the tumor microenvironment.
Supplementary Material
RNA sequenc ing results of altered
gene expression in JMJD6 depleted cells
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the following grants:
Grants-in-Aid (KAKENHI) from the Ministry of Health, Labour and
Welfare, Japan (grant no. JP-19K09198).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YKF and SI carried out the molecular studies,
participated in the sequence alignment and drafted the manuscript.
KY carried out the evaluation of IHC. TF and DO performed the RNA
sequencing. SI, TT and NH participated in the design of the study.
YO, TY and MM conceived of the study and participated in its design
and coordination and helped to draft the manuscript. YKF and SI
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Clinical Research
Ethics Committee of Kyushu University Hospital according to the
Ethical Guidelines of the Japanese Government (approval no.
30-578). The patients provided the written consent for the use of
their tissues for future scientific research at the time of
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang H, Yang T, Wu M and Shen F:
Intrahepatic cholangiocarcinoma: Epidemiology, risk factors,
diagnosis and surgical management. Cancer Lett. 379:198–205.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Doherty B, Nambudiri VE and Palmer WC:
Update on the diagnosis and treatment of cholangiocarcinoma. Curr
Gastroenterol Rep. 19(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Blechacz B and Gores GJ:
Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and
treatment. Hepatology. 48:308–321. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yugawa K, Itoh S, Kurihara T, Yoshiya S,
Mano Y, Takeishi K, Harada N, Ikegami T, Soejima Y, Mori M and
Yoshizumi T: Skeletal muscle mass predicts the prognosis of
patients with intrahepatic cholangiocarcinoma. Am J Surg.
218:952–958. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sakamoto Y, Kokudo N, Matsuyama Y,
Sakamoto M, Izumi N, Kadoya M, Kaneko S, Ku Y, Kudo M, Takayama T,
et al: Proposal of a new staging system for intrahepatic
cholangiocarcinoma: Analysis of surgical patients from a nationwide
survey of the liver cancer study group of Japan. Cancer. 122:61–70.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fadok VA, Bratton DL, Rose DM, Pearson A,
Ezekewitz RA and Henson PM: . A receptor for
phosphatidylserine-specific clearance of apoptotic cells. Nature.
405:85–90. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Chang B, Chen Y, Zhao Y and Bruick RK:
JMJD6 is a histone arginine demethylase. Science. 318:444–447.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Unoki M, Masuda A, Dohmae N, Arita K,
Yoshimatsu M, Iwai Y, Fukui Y, Ueda K, Hamamoto R, Shirakawa M, et
al: Lysyl 5-hydroxylation, a novel histone modification, by Jumonji
domain containing 6 (JMJD6). J Biol Chem. 288:6053–6062.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang J, Chen S, Yang Y, Ma X, Shao B, Yang
S, Wei Y and Wei X: Jumonji domain-containing protein 6 protein and
its role in cancer. Cell Prolif. 53(e12747)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nolz JC: Molecular mechanisms of CD8(+) T
cell trafficking and localization. Cell Mol Life Sci. 72:2461–2473.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Farhood B, Najafi M and Mortezaee K:
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A
review. J Cell Physiol. 234:8509–8521. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yugawa K, Itoh S, Yoshizumi T, Iseda N,
Tomiyama T, Toshima T, Harada N, Kohashi K, Oda Y and Mori M:
Prognostic impact of tumor microvessels in intrahepatic
cholangiocarcinoma: Association with tumor-infiltrating
lymphocytes. Mod Pathol. 34:798–807. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asahi Y, Hatanaka KC, Hatanaka Y, Kamiyama
T, Orimo T, Shimada S, Nagatsu A, Sakamoto Y, Kamachi H, Kobayashi
N, et al: Prognostic impact of CD8+ T cell distribution
and its association with the HLA class I expression in intrahepatic
cholangiocarcinoma. Surg Today. 50:931–940. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ronnekleiv-Kelly SM and Pawlik TM: Staging
of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr.
6:35–43. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fontugne J, Augustin J, Pujals A,
Compagnon P, Rousseau B, Luciani A, Tournigand C, Cherqui D,
Azoulay D, Pawlotsky JM and Calderaro J: PD-L1 expression in
perihilar and intrahepatic cholangiocarcinoma. Oncotarget.
8:24644–24651. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yugawa K, Itoh S, Yoshizumi T, Iseda N,
Tomiyama T, Morinaga A, Toshima T, Harada N, Kohashi K, Oda Y and
Mori M: CMTM6 stabilizes PD-L1 expression and is a new prognostic
impact factor in hepatocellular carcinoma. Hepatol Commun.
5:334–348. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Berardo C, Siciliano V, Di Pasqua LG,
Richelmi P, Vairetti M and Ferrigno A: Comparison between
Lipofectamine RNAiMAX and GenMute transfection agents in two
cellular models of human hepatoma. Eur J Histochem.
63(3048)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Izumi T, Sakata K, Okuzaki D, Inokuchi S,
Tamura T, Motooka D, Nakamura S, Ono C, Shimokawa M, Matsuura Y, et
al: Characterization of human pegivirus infection in liver
transplantation recipients. J Med Virol. 91:2093–2100.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shimokawa M, Yoshizumi T, Itoh S, Iseda N,
Sakata K, Yugawa K, Toshima T, Harada N, Ikegami T and Mori M:
Modulation of Nqo1 activity intercepts anoikis resistance and
reduces metastatic potential of hepatocellular carcinoma. Cancer
Sci. 111:1228–1240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Feng M, Pan Y, Kong R and Shu S: Therapy
of primary liver cancer. Innovation (Camb).
1(100032)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sheng W, LaFleur MW, Nguyen TH, Chen S,
Chakravarthy A, Conway JR, Li Y, Chen H, Yang H, Hsu PH, et al:
LSD1 ablation stimulates anti-tumor immunity and enables checkpoint
blockade. Cell. 174:549–563.e19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wan J, Liu H, Yang L, Ma L, Liu J and Ming
L: JMJD6 promotes hepatocellular carcinoma carcinogenesis by
targeting CDK4. Int J Cancer. 144:2489–2500. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wong M, Sun Y, Xi Z, Milazzo G, Poulos RC,
Bartenhagen C, Bell JL, Mayoh C, Ho N, Tee AE, et al: JMJD6 is a
tumorigenic factor and therapeutic target in neuroblastoma. Nat
Commun. 10(3319)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang F, He L, Huangyang P, Liang J, Si W,
Yan R, Han X, Liu S, Gui B, Li W, et al: JMJD6 promotes colon
carcinogenesis through negative regulation of p53 by hydroxylation.
PLoS Biol. 12(e1001819)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Itoh S, Yoshizumi T, Yugawa K, Imai D,
Yoshiya S, Takeishi K, Toshima T, Harada N, Ikegami T, Soejima Y,
et al: Impact of immune response on outcomes in hepatocellular
carcinoma: Association with vascular formation. Hepatology.
72:1987–1999. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yugawa K, Itoh S, Iseda N, Kurihara T,
Kitamura Y, Toshima T, Harada N, Kohashi K, Baba S, Ishigami K, et
al: Obesity is a risk factor for intrahepatic cholangiocarcinoma
progression associated with alterations of metabolic activity and
immune status. Sci Rep. 11(5845)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ikeda S, Okamoto T, Okano S, Umemoto Y,
Tagawa T, Morodomi Y, Kohno M, Shimamatsu S, Kitahara H, Suzuki Y,
et al: PD-L1 is upregulated by simultaneous amplification of the
PD-L1 and JAK2 genes in non-small cell lung cancer. J Thorac Oncol.
11:62–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu C, Paschall AV, Shi H, Savage N, Waller
JL, Sabbatini ME, Oberlies NH, Pearce C and Liu K: The MLL1-H3K4me3
axis-mediated PD-L1 expression and pancreatic cancer immune
evasion. J Natl Cancer Inst. 109(djw283)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6(8)2018.PubMed/NCBI View Article : Google Scholar
|