Introduction
Histiocytic sarcoma (HS) is a rare hematological
malignancy with an unknown prevalence (1). The etiology of HS is being
researched; however, HS cells exhibit an immunophenotypically
mature histiocyte phenotype. In the WHO classification (2017)
(1), HS is classified as
histiocytic and dendritic cell neoplasms (2,3). HS
typically involves extranodal sites. The intestine, skin, and soft
tissue are the most common extranodal sites for HS (4). Clinical presentations of HS are
variable, ranging from localized disease with solitary mass to
metastatic diseases with dissemination. Patients with localized HS
is substantially treated with surgical resection, which exerts
relatively better survival. Metastatic or disseminated HS should be
managed with multimodal combination therapy consisting of surgery,
chemotherapy and radiotherapy, because no standard therapy had been
established. Even by intensive treatment optimal outcome have not
been warranted in advanced disease cases.
HS may be caused by pluripotent germ cells (5). Specific markers of HS are CD68 and
CD163 as diagnostic criteria. These surface antigens are originally
expressed in histiocytes (4). In
addition of histiocytic markers, CD31, CD4 and CD45RO are expressed
in HS. Besides identical molecular features appear clonally in HS.
BRAF mutation V600E occasionally identified in HS cells. In
such cases, BRAF inhibitor could be provide as a target
therapy, although BRAF mutation is not detected as universal
alteration in HS. Other target option is immune checkpoint protein
which is recently developing in the field of oncology (5). PDL1/L2 can be expressed
physiologically by histiocytes (6,7). As
a result, HS is produced from histiocytes expressing PD-L1/L2
(6-8).
Although the cell of origin of HS is unknown, it reserves the
surface antigens of histiocytes such as B7-H1 (PD-L1) (6,7).
Thus, an anti-PD-L1 immune checkpoint inhibitor, representative
nivolumab is expected to be a promising treatment agent (5). Successful treatment of cancers with
PD-1/PD-L1 blockade shows promising clinical outcomes, initially in
melanoma and then in a variety of cancers such as lung cancer,
renal cell carcinoma, and Hodgkin lymphoma. We experienced the case
with metastatic HS treated with nivolumab and revealed a refractory
site among the patient's diseases was proven in pathological
evidence as one of resistant mechanisms.
Case report
We treated a 43-year-old woman who had been
diagnosed with HS; she was admitted to Kagawa University hospital
(Miki, Japan) in May 2018. Her disease had been present for 3
months before her diagnosis. Her initial symptom was left femoral
pain, so she visited to the primary orthopedic clinic near her
hometown. She was referred to our center, and an orthopedic surgeon
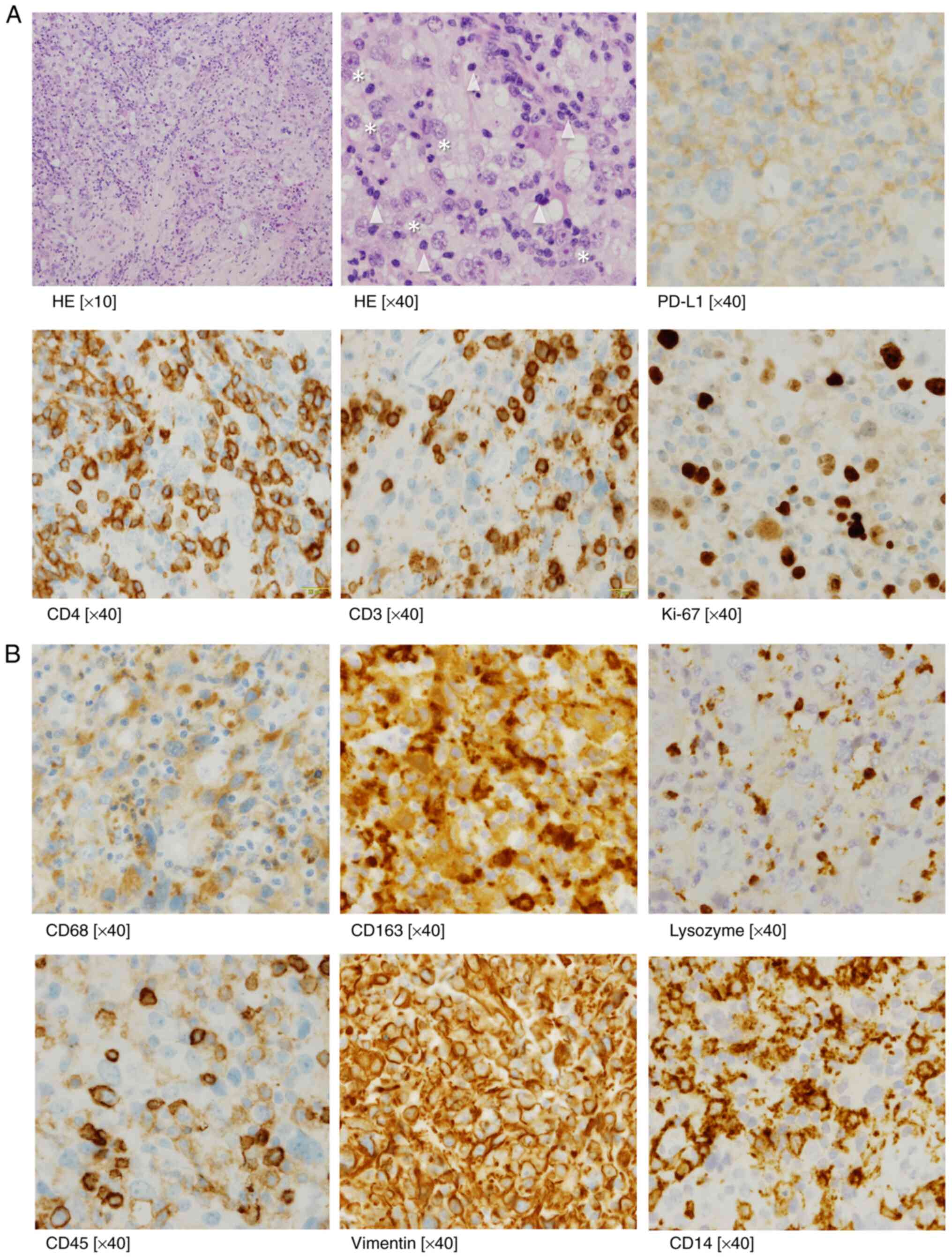
performed a biopsy on her. A biopsy of the right iliac mass
revealed atypical cell nodules with histiocytic immunophenotypes
such as CD68+, CD163+, lysozyme+,
CD45+ (moderate), CD45RO+ (weak),
vimentin+ (Fig. 1A and
B), except for CD30. CD14 staining
were weak in some of the large atypical cells (Fig. 1B), but not in CD34. In tumor cells,
myeloid (myeloperoxidase), T cell (CD3, CD4, and CD8) and B cell
(CD20) lineage markers were all negative. Follicular dendritic cell
markers (CD1a and CD21) were negative. Other specific markers
including SMA, desmin, S-100, AE1/AE3 were all negative. In the
atypical cells, the Ki-67 proliferation index was 70%. These
findings led to a diagnosis of metastatic HS. We also showed the
infiltrating CD3+ CD4+ T cells as evidence of
immune reaction (Fig. 1B).
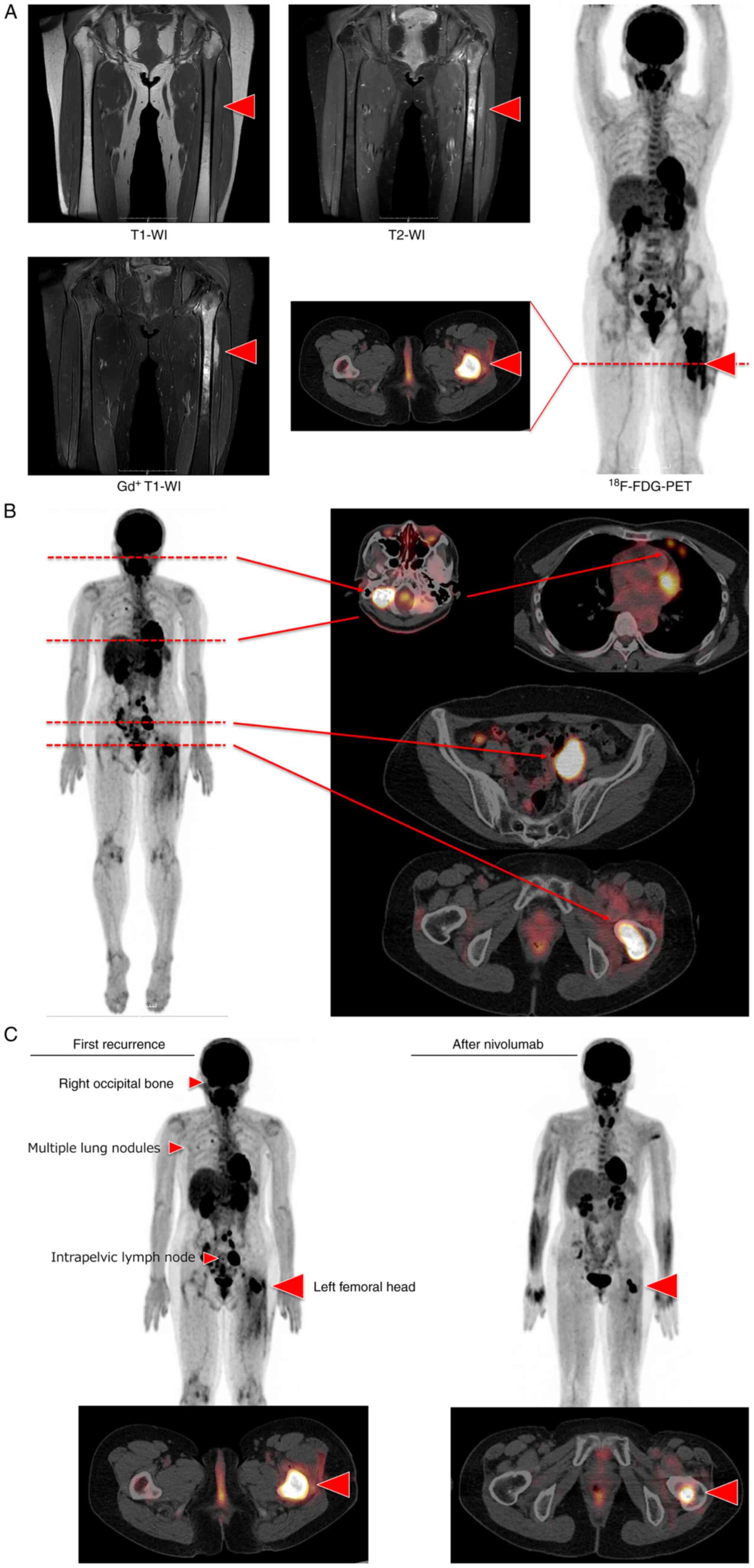
Her disease was restricted to the left femoral bone,
but it spread to the bone cortex and bone marrow (Fig. 2A). Initially, surgical resection
was not recommended. We tried two chemotherapy regimens on her;
CHOP (9) (cyclophosphamide,
hydroxydaunorubicin, oncovin, and prednisolone) and
cladribine-cytarabine (10). In a
stable disease, neither cytotoxic chemotherapy regimen could reduce
her tumor mass. We were looking for a method that would accurately
assess treatment responses. Because chemotherapy was ineffective,
the patient was treated with radiation therapy (RT) at the primary
site on her left femoral bone. Thirty Gy of RT had been completed.
Two months after RT, FDG-PET/CT depicted a clearance of FDG
accumulation in the lesions including the left femoral head, which
showed complete remission.
She succumbed to disease progression five months
after being in remission. Right occipital bone, multiple lung
nodules, intrapelvic right lymph node, and primary site were the
recurrent disease sites (Fig. 2B).
RT was administered to her lung nodules and primary site. Because
pulmonary irradiation is toxic, and the primary site was already
irradiated during the initial treatment. As a result, we
investigated the BRAF V600E mutation, the microsatellite
instability (MSI) test, and the PD-L1 expression pathological
sample at the onset. The first two tests came back negative, but
PD-L1 was strongly positive in her HS tissue sample. As a result,
we began administering nivolumab 200 mg biweekly. All metastatic
lesions, including the lungs, were in remission after 12 cycles
(Fig. 2C). Only a center lesion of
the primary site were remained positive accumulation of FDG uptake
(Fig. 2C). She underwent primary
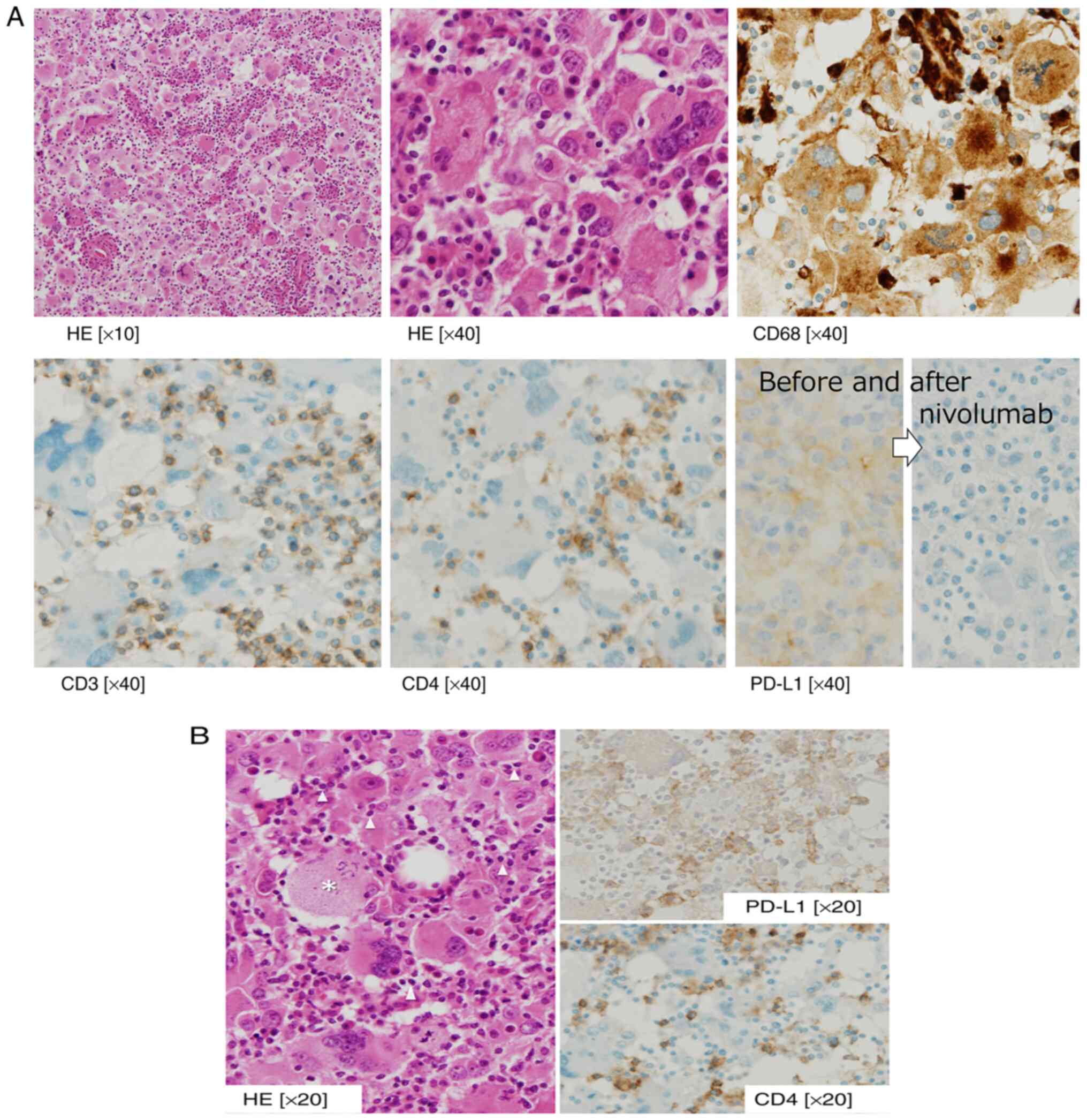
site resection and left femoral head replacement surgery. The
pathological findings draw plenty of T cells within the tumor cells
defect with PD-L1 antigen (Fig.
3A). This implicated HS tumor cells escaping from cell
immunity. And in the area surrounding the residual tumor, HS tumor
cells remain positive for PD-L1, in where HS was attacked and
eliminated by active T cells (Fig.
3B).
Discussion
The clinical outcome of HS is unfavorable (11). So far, no standard chemotherapy has
been reported. Multimodality treatment is important for treatment
outcomes not only in cases of limited disease but also in cases of
metastatic disease (12,13). Anecdotal evidence suggests that
various treatable agents, such as CHOP, etoposide, and alkylating
drugs, are available. Furthermore, novel approaches are being
investigated, including thalidomide, alemtuzumab, vemurafenib,
purine analogs, and vinblastine (12). A Mayo Clinic case report described
a potential effect of RT in HS (8). After excluding patients with
involvement of the bone marrow, spleen, or reticuloendothelial
system, patients who were managed surgically had higher overall
survival than those who were not (11). Finally, in the era of
immune-oncology, an immune-checkpoint inhibitor brings a favorable
response to this rare soft tissue sarcoma. Bose et al
reported the first nivolumab is effective immunotherapy for HS and
durable response in metastatic HS (14). Because HSs generally express the
surface antigen PD-L1(13), it is
expected to respond well to immunotherapy (13). In our case, the tumor cells with
PD-L1 were diminished with nivolumab, and the tumor cells defect
PD-L1 escaped from the therapy with nivolumab. This resistant
mechanism was proven in our case pathologically. Immunotherapy with
PD-L1 and PD-1 antibodies could then be a novel and promising
treatment option.
In literature reviews, a subset of patients with HS
arises as a secondary neoplasm from hematological malignancies such
as malignant lymphoma (4,12,13).
In such cases, the underlying hematological disease is indolent,
this subset of HS is considered a transformed or
transdifferentiated (12). Lineage
switching is used to explain this disease's trans-malignancy.
Although there are no distinctive molecular markers for HS, some
cases of trans-malignancy exhibit specific molecular genetical
markers such as BRAF V600E (15) If this molecular marker is
identified, it will be a target for BRAF inhibitors such as
vemurafenib and dabrafenib (16).
In our case, MSI was tested, but microsatellite instability-high
status is rarely positive in HS. The hematological disease had not
been mentioned in our case. The patient; however, was primarily
resistant to cytotoxic agents such as the CHOP regimen. This
intrinsic resistance has yet to be identified. A significant
proportion of sporadic HS patients have a B-cell genotype (17). More clinical research for novel
agents to target HS is needed in this regard.
Some prognostic factors are known in this neoplasm
(11,20,21).
An epidemiological cohort study showed metastatic cases and
secondary cases were independently poor prognostic factors
(11,18). In this study, elevated LDH, ECOG PS
2-4, and Ann Arbor stage III-IV were independent risk factors
(19). Without surgical treatment,
stage IV disease has a poor prognosis (11). Multimodality combination therapy is
a cornerstone of curative treatment (18). Following this, a
chemotherapy-refractory case, such as ours, would have a poor
prognosis. The pathogenesis or etiology of chemotherapy resistance,
on the other hand, has not been investigated (20). Some molecular markers for treatment
have been identified, primarily in activating driver mutations in
the MAPK/ERK pathway (20-22).
In some case reports, molecular target therapy, such as MEK
inhibitors, trametinib, and vemurafenib, is highly effective in the
treatment of recurrent/refractory HS (20,21).
The efficacy of stem cell transplantation, including cell therapy,
is being studied (23-25),
and clinical results are anecdotal.
In conclusion, primary resistant or advanced HS can
be safely treated with a PD-L1 antibody. Precision medicine policy
plays an important role in this rare tumor. Treatable molecular
targets should be screened for an efficient treatment
procedure.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by JSPS KAKENHI (grant no.
JP19K17927).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
OI and MU confirm the authenticity of all the raw
data. OI managed the patient's case, contributed to the literature
search and wrote the manuscript. HF and NK made substantial
contributions to the conception and design of this report. MU
analyzed the patient's data and suggested important intellectual
content. HF, NK and MU took part in critical discussions. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
We obtained ethics approval from the Kagawa
University Hospital Institutional Review Board (approval no.
H23-023). This research was conducted ethically in accordance with
the World Medical Association Declaration of Helsinki.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO classification of tumours of
haematopoietic and lymphoid tissues. Revised Fourth Edition World
Health Organization classification of tumours. IARC, Lyon,
2017.
|
|
2
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the world health organization
classification of lymphoid neoplasms. Blood. 127:2375–2390.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Emile JF, Abla O, Fraitag S, Horne A,
Haroche J, Donadieu J, Requena-Caballero L, Jordan MB, Abdel-Wahab
O, Allen CE, et al: Revised classification of histiocytoses and
neoplasms of the macrophage-dendritic cell lineages. Blood.
127:2672–2681. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hung YP and Qian X: Histiocytic sarcoma.
Arch Pathol Lab Med. 144:650–654. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gatalica Z, Bilalovic N, Palazzo JP,
Bender RP, Swensen J, Millis SZ, Vranic S, Von Hoff D and Arceci
RJ: Disseminated histiocytoses biomarkers beyond BRAFV600E:
Frequent expression of PD-L1. Oncotarget. 6:19819–19825.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Keir ME, Francisco LM and Sharpe AH: PD-1
and its ligands in T-cell immunity. Curr Opin Immunol. 19:309–314.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
May JM, Waddle MR, Miller DH, Stross WC,
Kaleem TA, May BC, Miller RC, Jiang L, Strong GW, Trifiletti DM, et
al: Primary histiocytic sarcoma of the central nervous system: A
case report with platelet derived growth factor receptor mutation
and PD-L1/PD-L2 expression and literature review. Radiat Oncol.
13(167)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gounder M, Desai V, Kuk D, Agaram N,
Arcila M, Durham B, Keohan ML, Dickson MA, D'Angelo SP, Shukla N,
et al: Impact of surgery, radiation and systemic therapy on the
outcomes of patients with dendritic cell and histiocytic sarcomas.
Eur J Cancer. 51:2413–2422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iwabuchi H, Kawashima H, Umezu H, Takachi
T, Imamura M, Saitoh A, Ogose A and Imai C: Successful treatment of
histiocytic sarcoma with cladribine and high-dose cytosine
arabinoside in a child. Int J Hematol. 106:299–303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kommalapati A, Tella SH, Durkin M, Go RS
and Goyal G: Histiocytic sarcoma: A population-based analysis of
incidence, demographic disparities, and long-term outcomes. Blood.
131:265–268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ansari J, Naqash AR, Munker R, El-Osta H,
Master S, Cotelingam JD, Griffiths E, Greer AH, Yin H, Peddi P and
Shackelford RE: Histiocytic sarcoma as a secondary malignancy:
Pathobiology, diagnosis, and treatment. Eur J Haematol. 97:9–16.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Skala SL, Lucas DR and Dewar R:
Histiocytic Sarcoma: Review, discussion of transformation from
B-cell lymphoma, and differential diagnosis. Arch Pathol Lab Med.
142:1322–1329. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bose S, Robles J, McCall CM, Lagoo AS,
Wechsler DS, Schooler GR and Van Mater D: Favorable response to
nivolumab in a young adult patient with metastatic histiocytic
sarcoma. Pediatr Blood Cancer. 66(e27491)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tzankov A, Kremer M, Leguit R, Orazi A,
van der Walt J, Gianelli U and Hebeda KM: Histiocytic cell
neoplasms involving the bone marrow: Summary of the workshop cases
submitted to the 18th meeting of the European association for
haematopathology (EAHP) organized by the European bone marrow
working group, basel 2016. Ann Hematol. 97:2117–2128.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bubolz AM, Weissinger SE, Stenzinger A,
Arndt A, Steinestel K, Brüderlein S, Cario H, Lubatschofski A,
Welke C, Anagnostopoulos I, et al: Potential clinical implications
of BRAF mutations in histiocytic proliferations. Oncotarget.
5:4060–4070. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takahashi E and Nakamura S: Histiocytic
sarcoma: An updated literature review based on the 2008 WHO
classification. J Clin Exp Hematop. 53:1–8. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kommalapati A, Tella SH, Go RS and Goyal
G: Predictors of survival, treatment patterns, and outcomes in
histiocytic sarcoma. Leuk Lymphoma. 60:553–555. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shimono J, Miyoshi H, Arakawa F, Sato K,
Furuta T, Muto R, Yanagida E, Sasaki Y, Kurita D, Kawamoto K, et
al: Prognostic factors for histiocytic and dendritic cell
neoplasms. Oncotarget. 8:98723–98732. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Z, Xiao Y, Liu X, Li Q, Liu T, Zhu F,
Wu G and Zhang L: Case Report: Long-term response to radiotherapy
combined with targeted therapy in histiocytic sarcoma harboring
mutations in MAPK and PI3K/AKT pathways. Front Oncol.
11(755893)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu B, Patel JL, Tao R, Cannon RB, Monroe M
and Goyal G: Near complete response to trametinib treatment in
histiocytic sarcoma harboring a somatic KRAS mutation. J Natl Compr
Canc Netw. 24:1–4. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Branco B, Comont T, Ysebaert L, Picard M,
Laurent C and Oberic L: Targeted therapy of BRAF V600E-mutant
histiocytic sarcoma: A case report and review of the literature.
Eur J Haematol. 103:444–448. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tomlin J, Orosco RK, Boles S, Tipps A,
Wang HY, Husseman J and Wieduwilt M: Successful treatment of
multifocal histiocytic sarcoma occurring after renal
transplantation with cladribine, high-dose cytarabine, G-CSF, and
mitoxantrone (CLAG-M) followed by allogeneic hematopoietic stem
cell transplantation. Case Rep Hematol. 2015(728260)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gergis U, Dax H, Ritchie E, Marcus R,
Wissa U and Orazi A: Autologous hematopoietic stem-cell
transplantation in combination with thalidomide as treatment for
histiocytic sarcoma: A case report and review of the literature. J
Clin Oncol. 29:e251–e253. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Visonneau S, Cesano A, Tran T, Jeglum KA
and Santoli D: Successful treatment of canine malignant
histiocytosis with the human major histocompatibility complex
nonrestricted cytotoxic T-cell line TALL-104. Clin Cancer Res.
3:1789–1797. 1997.PubMed/NCBI
|