Introduction
Neuroblastoma is the most common extracranial solid
tumor in children; approximately half of patients with
neuroblastoma present with metastatic disease at the time of
diagnosis (1,2). Metastasis of neuroblastoma can occur
by the hematogenous route, seeding bone marrow, liver, and bone.
The treatment of patients with disseminated neuroblastoma is one of
the greatest challenges for pediatric oncologists, given that the
five-year survival rate remains as low as 40-45%, despite advanced
treatment options (3). Therefore,
it is essential to develop effective strategies to inhibit tumor
metastasis.
Cancer metastasis begins with the detachment of
metastatic cells from the primary tumor, migration of the cells to
distal sites through blood vessels, settlement, and growth at the
distal site (4). During this
process, metastatic cells go through detachment, migration,
invasion, and adhesion (4). Each
of these processes involves rate-limiting steps influenced by
non-malignant cells of the tumor microenvironment (5). Tumor vascularization is a
rate-limiting step for metastasis. Vascular endothelial growth
factor (VEGF) is a highly potent molecule that increases vessel
permeability, endothelial cell growth, proliferation, migration,
and differentiation (6).
Nafamostat mesylate is a synthetic serine protease
inhibitor approved for pancreatitis and disseminated intravascular
coagulation (DIC) (7). This agent
inhibits thrombin, plasmin, kallikrein, trypsin, and C1 esterase in
the complement system and factors VIIa, Xa, and XIIa in the
coagulation cascade. Moreover, nafamostat mesylate inhibits the
metastasis of colon cancer, pancreatic cancer, breast cancer, and
squamous cell carcinoma (8-14).
However, the effects of nafamostat mesylate on other types of
cancer have been less extensively studied.
In the present study, the effects of nafamostat
mesylate on neuroblastoma regarding cell proliferation, motility,
cell-invasive potential, and growth factor production were
investigated. It is suggested that the results of the present study
will contribute to developing new aspects of treatment for
neuroblastoma.
Materials and methods
Reagents
Nafamostat mesylate was obtained from Nichi-Iko
Pharmaceutical Co., Ltd. (product no. 873999), dissolved in
distilled water, and stored at -80˚C until use.
Cell culture
The murine neuroblastoma cell line, Neuro-2a (cat.
no. ATCC-CCL-131; American Type Culture Collection), was maintained
in RPMI-1640 medium (product no. R8758; Sigma-Aldrich: Merck KGaA)
and 10% fetal bovine serum (FBS; cat. no. CCP-FBS-BR-500; Cosmo Bio
Co., Ltd.). The cells were cultured in a standard T75 cell culture
flask (cat. no. 156499; Thermo Fisher Scientific, Inc.) at 37˚C
with 5% CO2 to a confluency of 80%. The Neuro-2a cells
were then subcultured to 80% confluency, and cells passages three
to eight were used for the experiments. The doubling time was 28.9
h and the doubling rate of the cells remained relatively constant
through passages three to eight.
Cell proliferation assay
The effects of nafamostat mesylate on cell growth
was evaluated using the WST-8 Cell Counting reagent (cat. no.
341-07761; Dojindo Molecular Technologies). A total of
2x103 Neuro-2a cells were seeded in 100 µl RPMI-1640+10%
FBS on 96-well plates which were pre-incubated for 24 h before the
addition of a 50-µM concentration of nafamostat mesylate. WST-8 (10
µl) was added to each well at a 1:10 ratio in a cell culture
medium. After 2.5 h of incubation in a humidified atmosphere at
37˚C with 5% CO2, the absorbance at 450 nm was measured
using a spectrophotometer (cat. no. 51119000; Thermo Fisher
Scientific, Inc.). The results are expressed as the means ± SD from
three independent experiments.
Wound healing assay
Neuro-2a cells (5x106 cells) were seeded
onto a 6-well plate. A 90% confluent monolayer of cells was then
scratched with a pipette tip and susbsequently washed with media to
remove the floating cells. In order to examine whether nafamostat
inhibited the migration of Neuro-2a cells induced by serum, the
cells were then incubated with two concentrations (10 and 50 µM) of
nafamostat mesylate in RPMI-1640+10% FBS as previously described
(15), and images of the cell
migration into the wound at 24 h were captured by light microscope
(15).
Quantification of cytokine secretion
by enzyme-linked immunosorbent assay (ELISA)
A total of 1.5x105 Neuro-2a cells were
seeded onto a 6-well plate. After 24 h of incubation, the cells
were treated with 50 µM nafamostat mesylate for 24 h. Following
harvesting of the culture supernatants, the VEGF levels were
analyzed by ELISA using the Quantikine kit (cat. no. MMV00; R&D
Systems). All experiments were performed at least three times, and
the mean was calculated.
Western blotting
Cytoplasmic extracts were obtained as previously
described (16). Briefly, Neuro-2a
cells (1x107 cells) were resuspended in 1 ml of RIPA
buffer (cat. no. 08714-04; Nakalai Tesque, Inc.) at 4˚C for 30 min,
and then the cells were centrifuged (8,000 x g) at 4˚C for 15 min.
The proteins (40 µg/lane), quantified using bicinchoninic acid
assay (BCA), were electrophoresed on a 10% sodium dodecyl sulfate
polyacrylamide gel followed by semi-dry transfer to a
polyvinylidence fluoride (PVDF) membrane (cat. no. 88518;
Invitrogen; Thermo Fisher Scientific, Inc.). The transferred PVDF
blots were pretreated with 5% nonfat dry milk in Tris-buffered
saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature
and incubated with primary antibodies for nuclear factor-κB (NF-κB;
product no. 8242; 1:3,000; Cell Signaling Technology, Inc.) and
actin (cat. no. A2066; 1:3,000; Sigma-Aldrich; Merck KGaA) at 4˚C
overnight. The membranes were then washed three times with TBST and
incubated with horseradish peroxidase-conjugated secondary antibody
(product code ab99697; 1:3,000; Abcam) for 1 h at room temperature.
Following washing three times again, the antibodies bound to the
protein blots were detected using Western Lightning Plus
Chemiluminescence Reagent (cat. no. NEL103E001EA; PerkinElmer Life
Sciences), visualized on a LAS-3000 Mini Digital Imaging System
(FUJIFILM Corporation).
Animals
Female, eight-week-old A/J mice (n=18, weight 17-21
g), which express the MHC haplotype H-2a, were purchased
from Japan SLC, Inc. The mice were maintained in specific
pathogen-free conditions. All animal procedures and experiments
were performed according to a protocol approved by the Animal
Ethics Committee (Permission number 27-34), Mie University Graduate
School of Medicine, Tsu, Mie, Japan.
In vivo experimental animal
models
The mouse neuroblastoma dissemination model was
established by injecting Neuro-2a cells (1x106 cells)
into the lateral tail vein of A/J mice (17). Two groups, a nafamostat-treated
group (n=12) and a control group (n=6), were prepared as follows:
The nafamostat-treated group was intravenously injected with
Neuro-2a cells incubated with nafamostat mesylate (50 µg/ml) for 24
h. The control group was injected with Neuro-2a cells incubated
with a vehicle for 24 h. The mice were intravenously injected with
Neuro-2a cells on day 0. The progression of internal tumors was
monitored daily by assessing survival, clinical conditions and body
weight. Animals were euthanized by cervical dislocation if they
lost >20% of their original body weight, if they lost weight
rapidly (>1 g per day for two consecutive days), or if they
became morbid or exhibited the first sign of any distress.
Statistical analysis
The normality of the distribution was assessed using
BellCurve (Excel software; Social Survey Research Information Co.,
Ltd.). Unpaired Student's t-tests were used to analyze significant
differences between two groups. Data are expressed as the mean ±
standard deviation. The survival analysis was performed using
Kaplan-Meier curves along with log-rank testing. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of nafamostat mesylate on
neuroblastoma cell migration and proliferation
The majority of patients with neuroblastoma develop
metastatic disease at diagnosis and their prognosis is poor with
current therapeutic approaches (18). To investigate the effects of
nafamostat mesylate on cell motility, a 90% confluent cell layer
was scratched with a pipette tip and treated with nafamostat
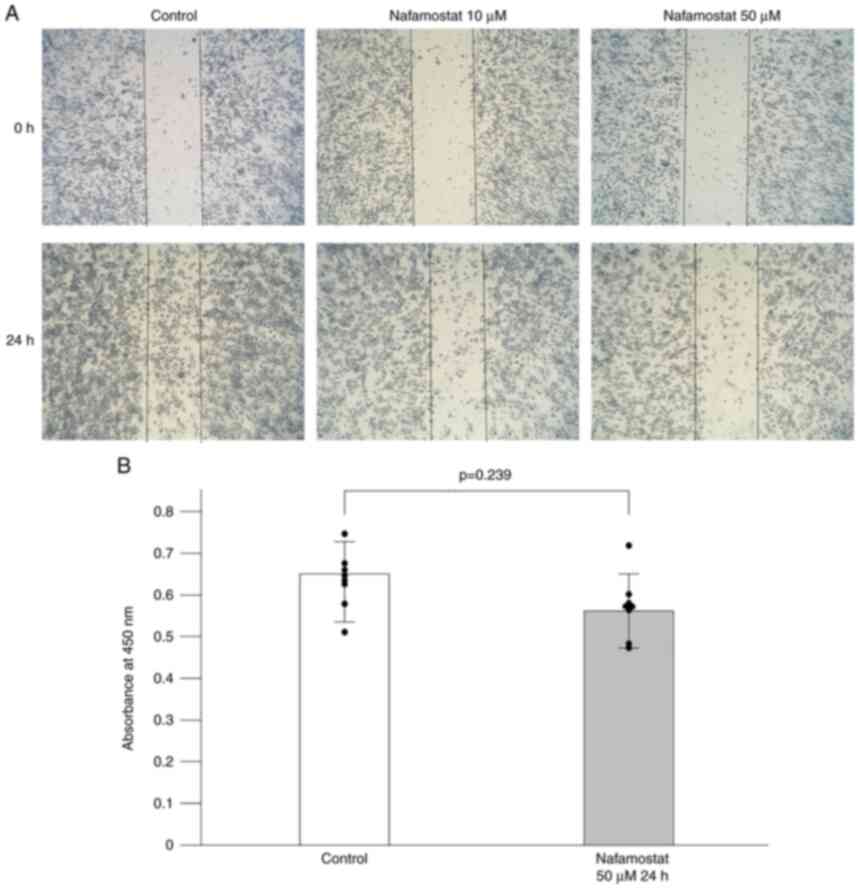
mesylate in RPMI-1640+10% FBS for 24 h. As revealed in Fig. 1A, nafamostat mesylate markedly
reduced the migration of Neuro-2a cells into the scratched area
over 24 h compared with the untreated group in a dose-dependent
manner. Previous studies have demonstrated the inhibitory effect of
nafamostat mesylate on cell proliferation in pancreatic, colon, and
breast cancers (9-11).
To determine whether nafamostat mesylate affected neuroblastoma
cell proliferation, a WST-8 assay was performed. Neuro-2a cells
were exposed to a 50-µM concentration of nafamostat mesylate for 24
h and were then assessed using the WST-8 assay. The cell
proliferation assay revealed that nafamostat mesylate may not
affect cell proliferation because nearly the same absorbance was
observed between the nafamostat-treated and the vehicle-treated
groups at the 24-h interval (Fig.
1B).
Inhibition of neuroblastoma
dissemination by nafamostat mesylate in vivo
In view of the ability of nafamostat mesylate to
potently inhibit Neuro-2a cell migration, the effects of nafamostat
mesylate in vivo were investigated. Neuroblastoma
hematogenously metastasizes to bone marrow, liver, skin, and bone.
To model hematogenous metastasis, murine neuroblastoma Neuro-2a
cells were intravenously injected into the lateral tail vein of A/J
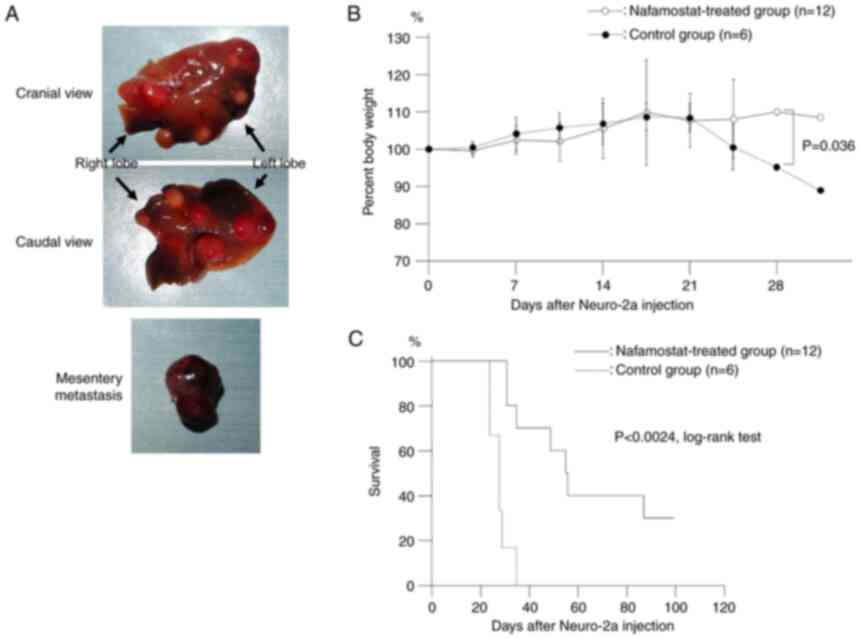
mice as previously described (17). Consequently, multiple liver nodules
were observed (17). Nafamostat
mesylate-treated Neuro-2a cells (nafamostat-treated group) or
vehicle-treated Neuro-2a cells (control group) were intravenously
injected into the tail veins of A/J mice. Representative images of
the liver nodules and mesentery matastasis in the control group at
euthanasia or death after injection of Neuro-2a cells are presented
in Fig. 2A. Liver metastasis was
present in all animals in both the control and nafamostat-treated
groups at euthanasia or death. Approximately 80% of the animals had
at least a 10% weight loss (Fig.
2B). Survival analysis was performed using Kaplan-Meier with
log-rank testing, for animals with experimental liver dissemination
by Neuro-2a, and the median survival times in the
nafamostat-treated and the control groups were 56 and 26 days,
respectively. The survival time of the nafamostat-treated group was
statistically longer than that of the control group (P<0.05,
log-rank test).
Effects of nafamostat mesylate on
Neuro-2a cells
Angiogenesis, the formation of new blood vessels,
plays a central role in the process of tumor growth and metastasis.
VEGF has been confirmed as the most potent inducer of angiogenesis
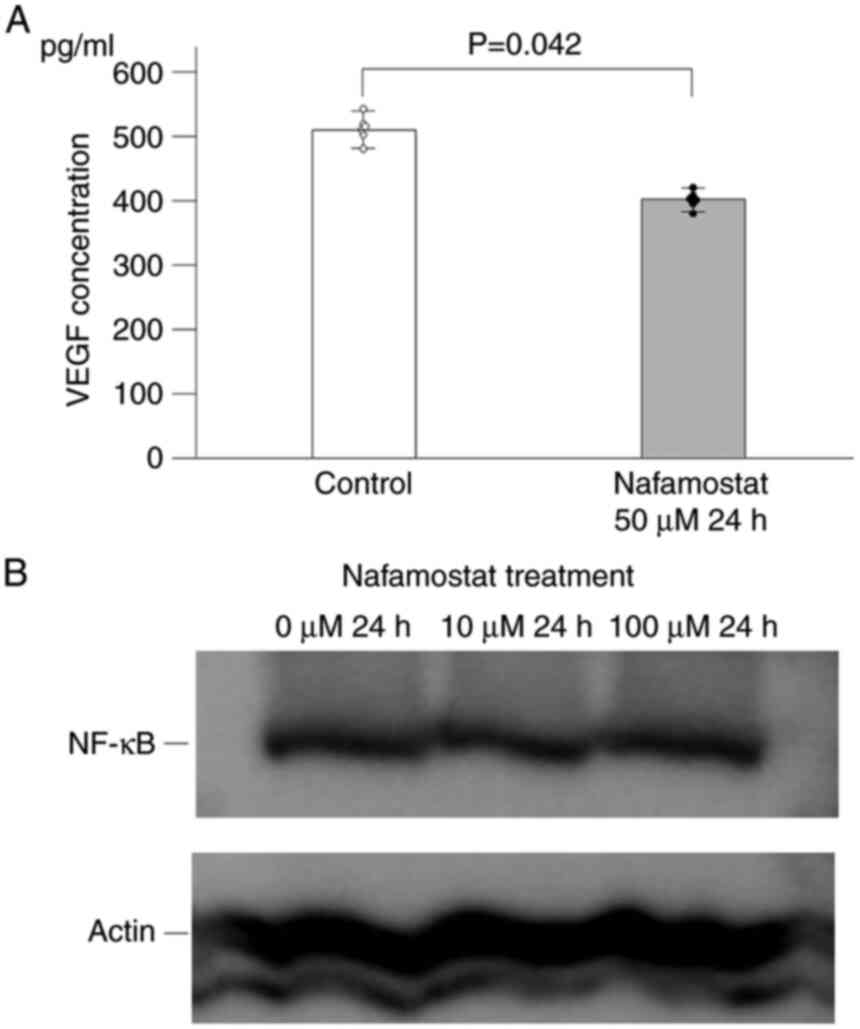
(6). To investigate the effects of
nafamostat mesylate on angiogenesis, the concentration levels of
VEGF in the cell culture supernatants were evaluated by ELISA. In
the presence of nafamostat mesylate, the concentration of VEGF in
the culture supernatant was significantly decreased for Neuro-2a
cells (Fig. 3A). Inhibition of
NF-κB by nafamostat mesylate has been reported to suppress VEGF
expression (8,12). To determine whether nafamostat
mesylate inhibits the activity of the NF-κB pathway, western blot
analysis was performed to assess the expression levels of NF-κB in
Neuro-2a cells after nafamostat mesylate treatment. The results
indicated that nafamostat mesylate did not inhibit the expression
levels NF-κB (Fig. 3B).
Discussion
Neuroblastoma is usually highly aggressive in
patients over one year of age and leads to poor outcomes with a
high percentage of metastatic cases (19). Therefore, suppressing tumor cell
migration and invasion could be a unique therapeutic approach to
inhibit metastasis. Nafamostat mesylate is a synthetic serine
protease inhibitor that has been used to treat DIC and acute
pancreatitis. Although the anticancer activity of nafamostat was
demonstrated in several cancers (8,10-12,14),
the effect of nafamostat mesylate on neuroblastoma has not yet been
explored. The present study demonstrated that nafamostat mesylate
suppressed neuroblastoma cell dissemination by inhibiting VEGF
production from neuroblastoma cells. Moreover, nafamostat mesylate
did not affect neuroblastoma cell proliferation at 24 h.
The anticancer activity of nafamostat mesylate is
still controversial. Nafamostat mesylate reportedly inhibited cell
proliferation and cell invasion in colorectal cancer and squamous
cell carcinoma via inhibition of the NF-κB (10,14).
However, Saito et al (12)
and Fujiwara et al (8)
reported that nafamostat mesylate suppressed cell adhesion and
invasion, but not cell viability, in pancreatic cancer by
downregulating NF-κB levels. Although NF-κB upregulation in cancer
cells is the main stimulator of cell proliferation, nafamostat
mesylate did not alter the expression level of NF-κB in Neuro-2a
cells at 24 h (data not shown). This result suggests that
nafamostat mesylate inhibits cell proliferation with cancer-type or
cell-type specificity and does not regulate the signaling pathways
related to cell proliferation in certain types of cancer.
Investigating the mechanisms of cancer invasion and
designing tools targeted at cancer cell metastasis in combination
with current therapies could open up new possibilities to reduce
mortality in highly invasive neuroblastoma cases. A recent
breakthrough study by Kitchen et al highlighted the
importance of re-purposing drugs that target signaling pathways to
treat central nervous system (CNS) edema when there are no
therapeutic options available. They demonstrated that the
translocation of water channel protein aquaporin-4 (AQP4) to the
surface of astrocyte cells was mediated by protein kinase A (PKA)
and calmodulin (CaM), resulting in CNS edema due to increased water
flux (20). Furthermore, the CaM
inhibitor trifluoperazine, which is used for treating
schizophrenia, was reported to effectively reduce CNS edema by
inhibiting AQP4 localization on the cell surface (20,21).
Existing approved drug, nafamostat mesylate, may be re-purposed for
the treatment of disseminated neuroblastoma for which there is no
effective therapeutic option available. Further studies should
evaluate the molecular mechanisms of nafamostat mesylate treatment
for inhibition of neuroblastoma metastasis.
Targeting the molecular and signaling mechanisms
rather than only using traditional approaches is important to meet
the urgent, unmet clinical needs of a number of patients for whom
no pharmacological interventions are available. AQP1 is
predominantly expressed in the brain, and plays a role in the
migration of neural crest cells, with differential AQP1 levels
affecting migration speed, direction, and filopodia length
(22-24).
Neuroblastoma, which originates from neural crest cells, exhibits
enhanced cell migration by AQP1 under hypoxic conditions (24). Therefore, the AQP1 ion channel
blocker AqB011 holds promise as a possible adjunct treatment to
control neuroblastoma metastasis (23). The subcellular localization of AQP1
is controlled by phosphorylation via two different protein kinases
(protein kinase C and PKA) and CaM may be implicated in the
regulation of AQP1(22). With the
development of specific modulators, AQP1 could serve as a promising
therapeutic target for selective intervention in neuroblastoma
dissemination.
Angiogenesis, the formation of new blood vessels,
plays a central role in tumor growth and metastasis. VEGF is the
most potent inducer of angiogenesis (6). Several investigators have reported
that inhibition of NF-κB by nafamostat mesylate suppressed VEGF
expression (8,12). However, the present study
demonstrated that nafamostat mesylate suppressed VEGF production
from Neuro-2a cells without NF-κB inhibition. Transcriptional
factors, such as hypoxia-inducible factor 1, truncated
glioma-associated oncogene homolog 1, and signal transducers and
activators of transcription 3 reportedly regulate VEGF
transcription and enhance expression VEGF (25-28).
Therefore, these transcription factors in addition to NF-κB will be
assessed in future studies by the authors. Angiogenesis inhibition
by nafamostat mesylate must be validated in humans by using
humanized self-organized models, organoids, 3D cultures and human
microvessel-on-a-chip platforms, especially those that are suitable
for advanced imaging, such as transmission electron microscopy and
expansion microscopy, because they enable real-time monitoring of
mediators (29,30).
Although highly aggressive and invasive
neuroblastoma remains an incurable disease, novel therapeutics
could open up new possibilities to reduce mortality.
High-throughput screening and computer-aided drug design should be
applied for screening novel therapeutics because they can provide a
unique insight that can support target validation in future studies
(31,32).
The limitation of our study is the relatively small
number of experiments. Thus, the findings of the present study need
to be further validated in more extensive studies before clinical
use. Only one murine neuroblastoma cell line, Neuro-2a, was
employed in the present study. Given that nafamostat mesylate
inhibits tumor cell proliferation with cell-type specificity
(11), the anticancer effect of
nafamostat mesylate may be different for different cell lines.
Therefore, the anticancer effect should be assessed in human
neuroblastoma cell lines before clinical application. Nevertheless,
nafamostat mesylate is already in clinical use and has minimal
adverse effects than the usual cytotoxic anticancer agents used for
neuroblastoma (11). Thus, it is
concluded that nafamostat mesylate could be a useful therapeutic
modality for treating neuroblastoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MM, HT, and MH conceived and designed the study. MM
and HT performed the experiments and organized all the data. MM,
HT, KN, RH, TO, DN, KA, SI, and MH analyzed and interpreted the
data as well as reviewed and edited the manuscript. HT wrote the
paper. MM, HT, and MH confirm the authenticity of all the raw data.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal procedures and experiments were performed
according to a protocol approved by the Animal Ethics Committee
(Permission number 27-34), Mie University Graduate School of
Medicine, Tsu, Mie, Japan.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Matthay KK, Maris JM, Schleiermacher G,
Nakagawara A, Mackall CL, Diller L and Weiss WA: Neuroblastoma. Nat
Rev Dis Primers. 2(16078)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shankar V, Hori H, Kihira K, Lei Q, Toyoda
H, Iwamoto S and Komada Y: Mesenchymal stromal cell secretome
up-regulates 47 kDa CXCR4 expression, and induce invasiveness in
neuroblastoma cell lines. PLoS One. 10(e0120069)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matthay KK: Chemotherapy for
neuroblastoma: Does it hit the target? Lancet Oncol. 9:195–196.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iwashita K, Kitamura K, Narikiyo T, Adachi
M, Shiraishi N, Miyoshi T, Nagano J, Tuyen DG, Nonoguchi H and
Tomita K: Inhibition of prostasin secretion by serine protease
inhibitors in the kidney. J Am Soc Nephrol. 14:11–16.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fujiwara Y, Furukawa K, Haruki K, Shimada
Y, Iida T, Shiba H, Uwagawa T, Ohashi T and Yanaga K: Nafamostat
mesilate can prevent adhesion, invasion and peritoneal
dissemination of pancreatic cancer thorough nuclear factor kappa-B
inhibition. J Hepatobiliary Pancreat Sci. 18:731–739.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kimura T, Fuchimoto S, Iwagaki H, Hizuta A
and Orita K: Inhibitory effect of nafamostat mesilate on metastasis
into the livers of mice and on invasion of the extracellular matrix
by cancer cells. J Int Med Res. 20:343–352. 1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu YX, Ju HQ, Wang F, Chen LZ, Wu QN,
Sheng H, Mo HY, Pan ZZ, Xie D, Kang TB, et al: Inhibition of the
NF-κB pathway by nafamostat mesilate suppresses colorectal cancer
growth and metastasis. Cancer Lett. 380:87–97. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mander S, You DJ, Park S, Kim DH, Yong HJ,
Kim DS, Ahn C, Kim YH, Seong JY and Hwang JI: Nafamostat mesilate
negatively regulates the metastasis of triple-negative breast
cancer cells. Arch Pharm Res. 41:229–242. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saito N, Uwagawa T, Hamura R, Takada N,
Sugano H, Shirai Y, Shiba H, Ohashi T and Yanaga K: Prevention of
early liver metastasis after pancreatectomy by perioperative
administration of a nuclear factor-κB inhibitor in mice. Surgery.
166:991–996. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Uwagawa T, Misawa T, Tsutsui N, Ito R,
Gocho T, Hirohara S, Sadaoka S and Yanaga K: Phase II study of
gemcitabine in combination with regional arterial infusion of
nafamostat mesilate for advanced pancreatic cancer. Am J Clin
Oncol. 36:44–48. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamashita Y, Ishiguro Y, Sano D, Kimura M,
Fujita K, Yoshida T, Horiuchi C, Taguchi T, Matsuda H, Mikami Y and
Tsukuda M: Antitumor effects of Nafamostat mesilate on head and
neck squamous cell carcinoma. Auris Nasus Larynx. 34:487–491.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shan D, Chen L, Njardarson JT, Gaul C, Ma
X, Danishefsky SJ and Huang XY: Synthetic analogues of migrastatin
that inhibit mammary tumor metastasis in mice. Proc Natl Acad Sci
USA. 102:3772–3776. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qi L, Toyoda H, Shankar V, Sakurai N,
Amano K, Kihira K, Iwasa T, Deguchi T, Hori H, Azuma E, et al:
Heterogeneity of neuroblastoma cell lines in insulin-like growth
factor 1 receptor/Akt pathway-mediated cell proliferative
responses. Cancer Sci. 104:1162–1171. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Toyoda H, Wimmer E and Cello J: Oncolytic
poliovirus therapy and immunization with poliovirus-infected cell
lysate induces potent antitumor immunity against neuroblastoma in
vivo. Int J Oncol. 38:81–87. 2011.PubMed/NCBI
|
|
18
|
Yu JL, Chan S, Fung MK and Chan GC:
Mesenchymal stem cells accelerated growth and metastasis of
neuroblastoma and preferentially homed towards both primary and
metastatic loci in orthotopic neuroblastoma model. BMC Cancer.
21(393)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Toyoda H, Ido M, Hayashi T, Gabazza EC,
Suzuki K, Kisenge RR, Kang J, Hori H and Komada Y: Experimental
treatment of human neuroblastoma using live-attenuated poliovirus.
Int J Oncol. 24:49–58. 2004.PubMed/NCBI
|
|
20
|
Kitchen P, Salman MM, Halsey AM,
Clarke-Bland C, MacDonald JA, Ishida H, Vogel HJ, Almutiri S, Logan
A, Kreida S, et al: Targeting aquaporin-4 subcellular localization
to treat central nervous system edema. Cell. 181:784–799.e19.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sylvain NJ, Salman MM, Pushie MJ, Hou H,
Meher V, Herlo R, Peeling L and Kelly ME: The effects of
trifluoperazine on brain edema, aquaporin-4 expression and
metabolic markers during the acute phase of stroke using
photothrombotic mouse model. Biochim Biophys Acta Biomembr.
1863(183573)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Markou A, Unger L, Abir-Awan M, Saadallah
A, Halsey A, Balklava Z, Conner M, Törnroth-Horsefield S, Greenhill
SD, Conner A, et al: Molecular mechanisms governing aquaporin
relocalisation. Biochim Biophys Acta Biomembr.
1864(183853)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Salman MM, Kitchen P, Yool AJ and Bill RM:
Recent breakthroughs and future directions in drugging aquaporins.
Trends Pharmacol Sci. 43:30–42. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wagner K, Unger L, Salman MM, Kitchen P,
Bill RM and Yool AJ: Signaling mechanisms and pharmacological
modulators governing diverse aquaporin functions in human health
and disease. Int J Mol Sci. 23(1388)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cao X, Geradts J, Dewhirst MW and Lo HW:
Upregulation of VEGF-A and CD24 gene expression by the tGLI1
transcription factor contributes to the aggressive behavior of
breast cancer cells. Oncogene. 31:104–115. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fallah J and Rini BI: HIF Inhibitors:
Status of current clinical development. Curr Oncol Rep.
21(6)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Su F, Geng J, Li X, Qiao C, Luo L, Feng J,
Dong X and Lv M: SP1 promotes tumor angiogenesis and invasion by
activating VEGF expression in an acquired trastuzumab-resistant
ovarian cancer model. Oncol Rep. 38:2677–2684. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL and Xie K: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Raimondi I, Izzo L, Tunesi M, Comar M,
Albani D and Giordano C: Organ-On-A-Chip in vitro models of the
brain and the blood-brain barrier and their value to study the
microbiota-gut-brain axis in neurodegeneration. Front Bioeng
Biotechnol. 7(435)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Salman MM, Marsh G, Kusters I, Delince M,
Di Caprio G, Upadhyayula S, de Nola G, Hunt R, Ohashi KG, Gray T,
et al: Design and validation of a human brain endothelial
microvessel-on-a-chip open microfluidic model enabling advanced
optical imaging. Front Bioeng Biotechnol. 8(573775)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aldewachi H, Al-Zidan RN, Conner MT and
Salman MM: High-Throughput screening platforms in the discovery of
novel drugs for neurodegenerative diseases. Bioengineering (Basel).
8(30)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Salman MM, Al-Obaidi Z, Kitchen P, Loreto
A, Bill RM and Wade-Martins R: Advances in applying computer-aided
drug design for neurodegenerative diseases. Int J Mol Sci.
22(4688)2021.PubMed/NCBI View Article : Google Scholar
|