Introduction
Tissue hypoxia commonly occurs in tumors and
adaptation to it appears to be one of the important characteristics
of malignant cells. Accordingly, hypoxia-inducible factor-1α
(HIF-1α) plays a key role in adaptation to hypoxia and regulates
the expression of genes responsible for glucose metabolism, cell
proliferation, angiogenesis, aggressive behavior and cancer stem
cell survival (1). HIF-1α and
hypoxia signaling influence a wide variety of pathways including
those related to vascular endothelial growth factor A (VEGF-A)
(2). VEGF-A is the major
angiogenic factor that regulates different aspects of vascular
angiogenesis and lymphangiogenesis (3). While hypoxia has been associated with
various types of solid cancers (4,5),
little is known about its presence and existence in lymphoid
cancer, such as malignant lymphoma.
As a consequence of increased cellularity and
proliferation, as well as enhanced metabolism within a tumor with
relatively abnormal vasculature and blood supply, oxygen
concentration within the tumor is generally lower than in adjacent
non-neoplastic tissue (6). Cancer,
irrespective of its origin, will develop hypoxic regions that
express the HIF-1α and VEGF-A protein (2,4). The
hypothesis is that diffuse large B-cell lymphoma (DLBCL) tissue
would also develop a hypoxic milieu exponentially as the tumor
grows, which causes resistance to chemotherapy, angiogenesis and
maintenance of cancer stem cells (7), as has been demonstrated in several
types of carcinoma, including those of the ovary, breast, prostate,
lung, renal, glial cells, as well as melanoma.
There are numerous published studies concerning
hypoxia in solid tumors. In various types of cancer, HIF-1α and
VEGF-A have prognostic significance. However, there are only a few
studies related to DLBCL, a highly proliferating cancer and
aggressive lymphoma. A previous study by Evens et al
(8,9) reported that HIF-1α was highly
expressed in ~59-70% of patients with DLBCL. In another study,
Pazgal et al (10) revealed
the expression of VEGF and its receptor in non-Hodgkin's lymphoma.
The aim of the present study was to determine the expression of
HIF-1α and VEGF-A in DLBCL, as our preliminary data for future
research.
Materials and methods
Patients and specimens
Using the database from the Division of
Hematology/Medical Oncology and the Department of Anatomical
Pathology of Dr Kariadi Hospital (Semarang, Indonesia), 149
patients who were diagnosed with DLBCL from January to December
2017, were identified. After obtaining approval from the
Institutional Review Board, the following data were recorded
retrospectively from the files: Patient demographic characteristics
(notably age), onset of disease, clinical and pathological results,
serum lactate dehydrogenase (LDH), hemoglobin (Hb) at presentation,
Hb prior to diagnostic procedure, glomerular filtration rate, the
National Comprehensive Cancer Network (NCCN) International
Prognostic Index (IPI) score (11), nodal/extranodal disease, diameter
of the tumor being biopsied, and Ann Arbor staging. No patients had
a history of chemotherapy or radiotherapy before surgical sampling
and DLBCL diagnosis.
The present study involving human tissue samples was
approved by the Medical Ethics Committee of Dr Kariadi Hospital
(reference no. 338/EC/KEPK-RSDK/2019). Written informed consent
from patients was also obtained for the study of the resection
specimens and for the use of their clinical data. However, informed
consent was obtained only from patients with eligible samples
(n=34) for data collection and publication purposes.
Clinical characteristics
Pre-treated DLBCL samples were collected from 34
patients with archived data, from the Division of
Hematology/Medical Oncology and the Department of Anatomical
Pathology patient databases of Dr Kariadi Hospital. The
histological sections were reviewed by two pathologists to verify
the histologic diagnosis. Diagnosis was based on the World Health
Organization Classification of Tumours of Hematopoietic and
Lymphoid Tissue (12).
Characteristics of all patients are shown in Table I. Clinical staging was determined
according to the Ann Arbor system (stage I, involvement limited to
a single lymph node area; stage II, involvement of more than one
lymph node in the regional area; stage III, multiple involvement of
lymph node areas on both sides of the diaphragm; stage IV,
generalized involvement) as well as the presence of ‘B’ symptoms
such as unintentional weight loss, low grade fever and drenching
night sweats (13).
 | Table ICustomized scoring system used in the
present study. |
Table I
Customized scoring system used in the
present study.
| | Positive distribution
score | Intensity score |
|---|
| Score 0 | No cells stained | Negative | Score 0 | Negative |
|---|
| Score 1 | >0 to 10% of cells
stained | Normal | Score 1 | Weak intensity |
| Score 2 | >10 to 30% of
cells stained | Overexpression | Score 2 | Intermediate
intensity |
| Score 3 | >30 to 50% of
cells stained | Overexpression | Score 3 | Strong intensity |
| Score 4 | >50 to 75% of
cells stained | Overexpression | | |
| Score 5 | >75% of cells
stained | Overexpression | | |
Cases preoperatively treated with radiation or
chemotherapy and those with incomplete clinical data were excluded.
A total of 34 samples were eventually evaluated in the clinical and
histological study. There was a similar proportion of male and
female patients, and all patients had not undergone any treatment
at the time of biopsy. Only samples measuring >2 cm from the
tumor excision were considered. Immunohistochemical staining for
CD10, MUM-1, BCL-6 to germinal center B-cell-like (GCB) and non-GCB
subtyping and Ki-67 were available for all specimens as per
respective regular practice for DLBCL diagnosis and assessment of
proliferation index of Dr Kariadi Hospital.
Histological assessment
All archival specimens were fixed in 10%
neutral-buffered formalin for 24 h at room temperature and embedded
in paraffin using the routine method. For this study, the specimens
were cut into 4-µm-thick sections and subjected to
immunohistochemical analysis conducted via the
avidin-biotin-peroxidase complex method.
Both the H&E slides and the immunohistochemical
stains were evaluated in a blinded fashion separately by two
pathologists (HI and DP). In the case where there was a
discrepancy, the slides were reviewed together on a double-headed
scope. For all immunohistochemical markers, the percentage of cell
staining was recorded. Tumors were considered positive if >10%
of cells evaluated expressed the antibody. Overexpression was
further categorized into groups by the percentage and intensity of
cells stained.
Immunohistochemistry (IHC)
Immunohistochemical techniques were used to evaluate
the expression of HIF-1α and VEGF-A using specific antibodies:
Ηuman anti-HIF-1α (cat. no. MAB1536-SP; R&D Systems, Inc.) and
human anti-VEGF (cat. no. 298-VS; R&D Systems, Inc.), both at a
1:100 dilution and their respective IgG isotype control (cat. no.
BZ-0840590F-AP; Bioenzy, Inc.). After recovering the antigen from
the slides, they were briefly placed in a 0.01 M sodium citrate
solution and incubated in a 40-50˚C water bath for 20 min. The
sections were then blocked with 5% pig serum (Biocare Medical) for
2 h at room temperature and the antibodies of interest were added.
The preparation was incubated with the primary antibody overnight
at 4˚C in a humid chamber. The universal biotinylated link (JAN
code 4987582002362; Dako; Agilent Technologies, Inc.) and
streptavidin-conjugated with horseradish peroxidase (HRP) (cat. no.
HP604; Biocare Medical) were used as secondary antibodies,
incubated also at room temperature for at least 30 min. Color was
generated by adding the substrate diaminobenzidine (DAB) for 1 to 2
min and counterstaining was performed with hematoxylin for 5 min.
Finally, the cells were dehydrated and covered with resins.
All immunohistochemical reactions were conducted
using formalin-fixed and paraffin-embedded samples. Specific
immunoreactivity was observed in the cytoplasm and the nuclei of
the tumor cells. Expression of both hypoxia markers was assessed by
analyzing at least 1,000 tumor cells from representative tumor
fields using a microscope at a magnification of x250, and the
labeling index was calculated as the percentage of labeled nuclei
of the total number of tumor cells counted.
Scoring criteria
The extent of staining was categorized into six
semiquantitative scales based on the percentage of positive tumor
cells: 0 (<1% positive cells), 1 (1-10% positive cells), 2
(11-24% positive cells), 3 (25-49% positive cells), 4 (50-74%
positive cells), and 5 (≥75% positive cells). The cut-off for
HIF-1α percentage staining with IHC was based on a previous study
by Evens et al (8,9), where a 10% value was defined as
overexpression of a DLBCL tumor. The intensity of staining was also
determined semi-quantitatively on a scale 0 to 3 as follows: 0
(negative), 1 (weakly positive), 2 (moderately positive) and 3
(strongly positive). Multiplication of the percentage score and
intensity gave rise to the final score of a maximum of 8 points
(Table I). For statistical
analysis, tumors were categorized according to their final staining
score as negative or normal or low expression (score, +2), mild
overexpression (score, 3-4), moderate overexpression (score, 5-6),
and high overexpression (score, 7-8).
Statistical analysis
Differences were evaluated using SPSS v.21 (IBM
Corp.). The association between staining intensity and
clinicopathological patterns was assessed. Spearman correlation
test was used to examine the correlation between clinical
characteristics, tumor size, and IHC. Spearman rank test was used
to investigate whether the scores of HIF-1α and VEGF-A
immunohistochemical labelling were correlated with age, tumor
diameter, and serum LDH. Statistical tests were two-sided and
correlation was considered significant for a P-value of
<0.05.
Results
Patient and tumor sample
characteristics
A total of 34 samples were included in the current
analyses. The clinical and pathological characteristics of patients
with DLBCL are shown in Table II.
There were 17 men and women with a mean age of 51.2 years (ranging
from 24 to 77 years old). The histological diagnosis in all
patients was DLBCL, based on the WHO classification (12). The mean time from onset of disease
to first diagnostic revelation was 5.1 months. There was more
limited-stage DLBCL compared to advanced-stage disease. However,
the majority of samples had high-intermediate and high prognostic
risk according to the National Comprehensive Cancer Network
(NCCN)-IPI score (11). The
majority of tumors were sampled based on excisional biopsy from
nodal disease (n=25) while the rest were examined from extranodal
tumors from the gastrointestinal tract (n=5), nasopharyngeal (n=3),
and central nervous system (n=1). With regard to cell-of-origin
subtype, this study involved both GCB and non-GCB in a relatively
comparable proportion, that is 47.1 and 52.9%, respectively.
 | Table IICharacteristics of patients with
DLBCL. |
Table II
Characteristics of patients with
DLBCL.
| Variables | No. of patients
(%) |
|---|
| Age (years) | 51.2±14.2 |
| Age, min-max
(years) | 24-77 |
| Sex
(male/female) | 17/17 |
| From onset to
hospital admission, months | 5.1±2.2 |
| Presence of B
symptoms | 21 (61.8%) |
| Laboratory
values | |
|
Hb value at
presentation (g/dl) | 12.1±2.2 |
|
Pre-operative
Hb (g/dl) | 12.6±1.4 |
|
eGFR
(ml/min) | 83.1±26.7 |
|
Pre-operative
LDH (IU/l) | 829.5±551.5 |
|
LDH range,
min-max (IU/l) | 277-2,322 |
| Disease
information | |
|
Ann Arbor
staging | |
|
Limited
stage (Ann Arbor I + II) | 22 (64.7%) |
|
Advanced
stage (Ann Arbor III + IV) | 12 (35,3%) |
|
NCCN-IPI
Score | |
|
Low
and low-intermediate risk (0-2) | 10 (29.4%) |
|
High-intermediate
and high risk (3-5) | 24 (70.6%) |
|
Tumor
characteristics | |
|
Tumor
diameter (min-max), sampled (in cm) | 2.9±0.8
(2.0-5.4) |
|
DLBCL
subtype | |
|
GCB | 16 (47.1%) |
|
Non-GCB | 18 (52.9%) |
|
Ki-67
expression | 49.1±13.2 |
|
Ki-67,
min-max | 30-77.5 |
|
HIF-1α
expression | |
|
Normal | 4 (11.8%) |
|
Overexpression | 30 (88.2%) |
|
VEGF-A
expression | |
|
Normal | 4 (11.8%) |
|
Overexpression | 30 (88.2%) |
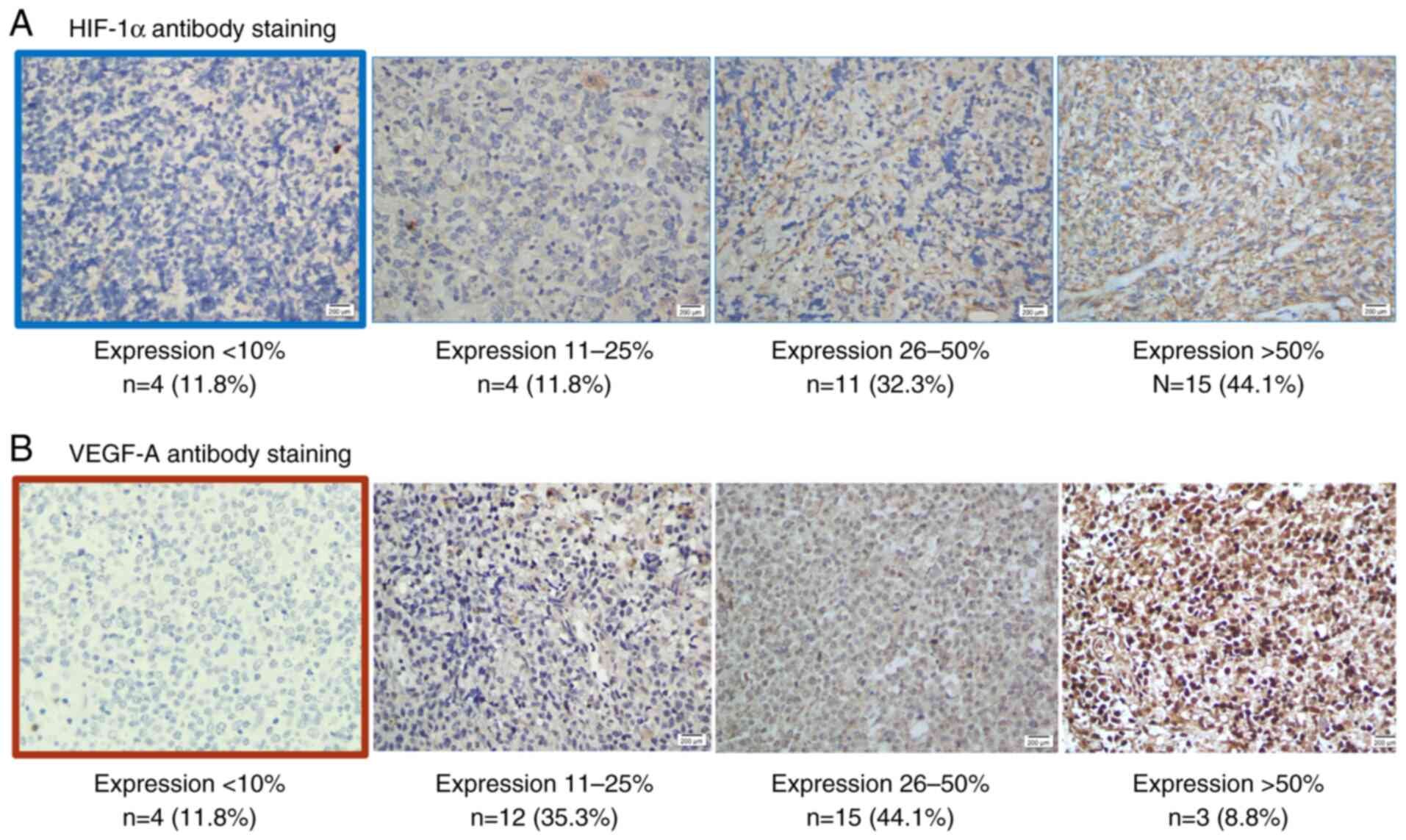
HIF-1α protein expression
Within positive tumors, the extent, intensity,
intracellular location, and distribution of staining observed with
the antibodies were heterogenous. The number of positive tumors did
not correlate with the intensity of staining and ranged from <1%
to 90% of tumor cells. In the cases examined, HIF-1α nuclear
staining was found in <10% of tumor nuclei in 11.8%, between
10-25% in 11.8%, between 26-50% in 35.3%, between 51-75% in 44.1%
and in >75% in 8.8% of tumors. The intensity of nuclear
immunoreactivity for each antigen in different tumor cells varied.
To illustrate these points, examples of immunostaining are shown in
Fig. 1A.
VEGF-A protein expression
Hypoxia upregulates the expression of a variety of
genes important in cancer biology, including VEGF (3,10).
Immunohistochemical staining was also performed to determine
whether the pattern of HIF-1α protein expression observed was
correlated with the distribution of VEGF-A protein. Signals were
predominantly observed at non-necrotic and viable tumor margins
(Fig. 1B), as has been previously
reported in solid tumors (14).
Approximately 88.2% of DLBCL samples had
overexpression of VEGF-A with 32.4% of the neoplastic cells
exhibiting high immunoreactivity (score of 7 or 8). The pattern of
labelling was diffuse and cytoplasmic. Similar proportions of the
tumor cells also showed HIF-1α expression; a mild overexpression
(score, 3-4) was recorded in 35.3%, a moderate overexpression
(score, 5-6) in 44.1%, and high overexpression in 8.8% of the
samples. The pattern of labelling was primarily diffuse and
cytoplasmic, and, in one case, perinuclear. The proportion of
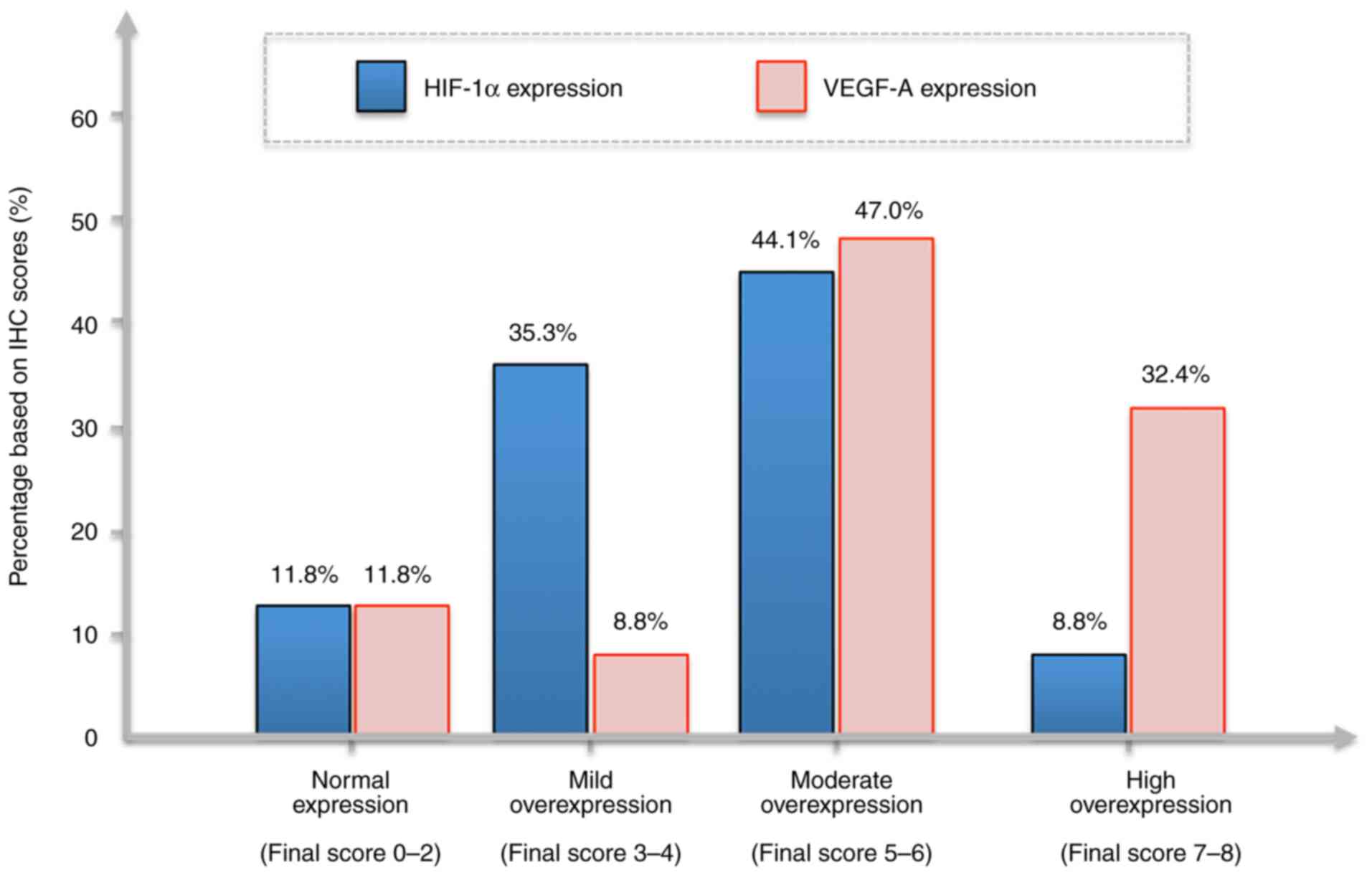
HIF-1α and VEGF-A immunohistochemical scores in DLBCL are presented
in Fig. 2.
HIF-1α and VEGF-A correlation with
clinical characteristics
In univariate analysis, the patterns of HIF-1α and
VEGF-A expression were analyzed with the following clinical
parameters: Age, tumor diameter, Hb values, serum LDH, and tumor
Ki-67 (Table III). The following
results were obtained: i) HIF-1α and VEGF-A: Based on the IHC total
score (the sum of the percentage of stained cells and intensity),
HIF-1α and VEGF-A were revealed to reflect the degree of
overexpression and a moderate positive correlation was observed
between them; ii) age of the patients: Both HIF-1α and VEGF-A
expression did not appear to be affected by age; tumor diameter: A
significant positive correlation between HIF-1α and VEGF-A with
tumor size was noted (the larger the DLBCL tumor was, the higher
both HIF-1α and VEGF-A expression levels were); Hb level: HIF-1α
was negatively correlated with the Hb value (P=0.165) while VEGF-A
appeared to less likely be associated with the Hb value; serum LDH:
LDH was significantly correlated with HIF-1α and VEGF-A expression
as well as the tumor diameter; and Ki-67 tumor: This proliferation
index was not correlated with HIF-1α VEGF-A, age, tumor diameter,
Hb values, and serum LDH.
 | Table IIICross correlation between certain
clinical variables, laboratory value and hypoxia markers. |
Table III
Cross correlation between certain
clinical variables, laboratory value and hypoxia markers.
| | HIF-1α | VEGF-A | Age | Tumor diameter | Hb value | Serum LDH | Ki-67 |
|---|
| HIF-1α | N/A | r=0.475;
P=0.005a | r=0.085;
P=0.631 | r=0.388;
P=0.023a | r=-0.244;
P=0.165 | r=0.662;
P<0.001a | r=0.220;
P=0.211 |
| VEGF-A | r=0.475;
P=0.005a | N/A | r=0.131;
P=0.461 | r=0.341;
P=0.049a | r=0.020;
P=0.910 | r=0.498;
P=0.003a | r=0.182;
P=0.304 |
| Age | r=0.085;
P=0.631 | r=0.131;
P=0.461 | N/A | r=0.256;
P=0.145 | r=0.212;
P=0.229 | r=0.089;
P=0.617 | r=0.172;
P=0.332 |
| Tumor diameter | r=0.388;
P=0.023a | r=0.341;
P=0.049a | r=0.256;
P=0.145 | N/A | r=-0.127; P=
0.475 | r=0.364;
P=0.034a | r=0.301; P=
0.085 |
| | | | | | | | |
| Hb value | r=-0.244;
P=0.165 | r=0.020;
P=0.910 | r=0.212;
P=0.229 | r=-0.127;
P=0.475 | N/A | r=-0.266;
P=0.128 | r=-0.138;
P=0.436 |
| Serum LDH | r=0.662;
P<0.001a | r=0.498;
P=0.003a | r=0.089;
P=0.617 | r=0.364;
P=0.034a | r=-0.266; P=
0.128 | N/A | r=0.055;
P=0.756 |
| Ki-67 | r=0.220;
P=0.211 | r=0.182;
P=0.304 | r=0.172;
P=0.332 | r=0.301;
P=0.085 | r=-0.138;
P=0.436 | r=0.055;
P=0.756 | N/A |
Discussion
Since DLBCL is the most common subtype of aggressive
lymphomas in humans, previous research has attempted to identify
early markers of this condition (12), which would be helpful to detect the
aggressiveness of the cancer. The presence of hypoxic regions
within tumors as the result of tumor growth and imbalance of
vasculature has long been reported to be associated with a poor
survival (14). Because hypoxia
stabilizes HIF-1α, which then triggers the expression of target
genes (1,4,7), the
present study focused on HIF-1α and VEGF-A (one of its downstream
products). Previous studies by Evens et al (8,9) and
Pazgal et al (10) have
already addressed this topic, but results have been largely
controversial. This is the first time, to the best of our
knowledge, that the impact of both markers were evaluated in
DLBCL.
The hypothesis is that DLBCL tissue develop a
hypoxic milieu exponentially as the tumor grows, which causes
resistance to chemotherapy, angiogenesis and maintenance of cancer
stem cells, as has been shown in several types of carcinomas
including those of the ovary, breast, prostate, lung, renal, glial,
as well as melanomas (7). The
association between protein expression levels in this ‘solid-like
tumor’ hematological malignancy and its various clinicopathological
features were therefore examined.
A major finding in the present study of high-grade
malignant lymphoma was the up-regulation of HIF-1α and VEGF-A
protein levels in tumors. Since the HIF-1α subunit is unstable in
oxygenated tissue, it should be kept in mind that these findings in
fixed tissue will represent the in vivo situation for every
detail. The present study extends these findings in demonstrated
upregulation of HIF-1α as well as VEGF-A, which raises an important
question about both the mechanisms of protein upregulation and its
consequences for the tumor, and ultimately the impact to overall
medical management of DLBCL.
The present study revealed that 88.2% of DLBCL tumor
samples exhibited VEGF-A overexpression. With regard to VEGF-A
expression, Shahini et al (15) demonstrated a high proportion of
VEGF-A in DLBCL from low positivity to high positivity with only 3%
staining negative (n=30). Notably, the level of VEGF-A expression
was correlated with IPI prognostic score and microvascular density.
In another study, overexpression of VEGF, its VEGF-receptor, and
microvessel density were reported to be correlated with a poorer
response related to systemic chemotherapy (16). A systematic meta-analysis also
concluded that tumor VEGF expression was associated with worse
survival in non-Hodgkin's lymphoma (17).
The main stimulus for VEGF expression is hypoxia
through the HIF-1α pathway (1,3). The
results of the present study were similar to those previously
described for DLBCL, with moderate to strong cytoplasmic and
nucleoplasmic staining of HIF-1α antibody observed in DLBCL cells
(10). In previous studies, the
HIF-1α positivity rate ranged from 56.0 to 67.3% (8,9). The
present study revealed a higher amount (>80%) of HIF-1α
overexpression in DLBCL. The present study also revealed that the
expression of HIF-1α may differ according to age and Hb value
(P>0.05). The association between HIF-1α and VEGF was examined
in the present study and it was determined that both
transcriptional factors were significantly correlated with a higher
tumor diameter and more advanced stage.
The HIF-1α transcriptional factor plays an essential
role in oxygen homeostasis and high expression of HIF-1α protein
has been found to be associated with both tumor aggressiveness and
unfavorable prognosis of various types of cancers. In contrast to
VEGF-A data, HIF-1α overexpression was found to be an important
independent favorable prognostic factor for survival in patients
with DLBCL treated with standard chemotherapy R-CHOP (9,10).
It is now understood that the metabolic
reprogramming in cancer is driven by several oncogenes and tumor
suppressors (18). Some of the
identified oncogenes, namely protein kinase B (PKB/Akt), Ras, and
von Hippel-Lindau (VHL), act via the HIF-1α protein, resulting in
non-hypoxic expression of HIF-1α. In normal cells, HIF-1α becomes
stabilized in a hypoxic environment (1,4).
Hypoxia is also a condition that almost certainly occurs in the
tumor microenvironment when there is tumor expansion leading to an
imbalance between vascular growth and increased oxygen demands.
This finding is now accepted as a universal finding in numerous
types of solid cancers (2,4,5,19),
and is also characteristic of highly proliferative cancers such as
DLCBL (20).
Further research incorporating HIF-1a and/or VEGF-A
or some other factor in the hypoxia pathway will be undertaken in a
future study. The primary hypothesis is nonetheless that an
additional therapeutic target in combating tumor hypoxia,
angiogenesis and progression will ultimately lead to improvement of
the outcome of DLBCL.
The VEGF gene and several other genes involved in
the homeostatic response to oxygen levels are under the control of
HIF-1α, a transcriptional activator mediating changes in gene
expression in response to changes in cellular oxygen concentrations
(3,6). HIF-1α expression in numerous human
cancers has been demonstrated to be correlated with tumorigenicity
and angiogenesis (5,21). With all samples taken into account,
Table III revealed that the IHC
HIF-1α score was correlated with VEGF-A. Notably, the degree of
overexpression as depicted in Fig.
2 indicated that perhaps at some point, the regulation of both
markers was different and independent of each other. An explanation
for this finding cannot be provided; however, the degree of tumor
diameter, the proportion of nodal vs. extranodal involvement,
disease stage, and patient performance status may be considered to
modify each marker expression and the relationship between
them.
Immunohistochemistry is inherently a subjective
assessment method to quantify tumor proteins or markers. The
scoring system used in the present study delineates the expression
and intensity against both HIF-1α and VEGF-A antibodies in tumors,
aiming to enhance its objectivity. Another possible objective tool
used for assessment is RT-qPCR. However, such technology was
unavailable, and along with our small sample size, were limitations
of the present study. No attempt was made to correlate HIF-1α with
VEGF-A due to the nature of this semiquantitative data. The number
of samples in the present study was also relatively small and this
was a single center study, thus it may not sufficiently be
representative of DLBCL tumors, especially since tumor size was not
taken into account in HIF-1α and VEGF-A quantification due to
technical issues related to diagnostic sample availability.
In conclusion, HIF-1α and VEGF-A expression levels
were elevated in patients with DLBCL in a significant proportion of
the samples obtained in January 2017 to December 2017 at Dr Kariadi
Hospital. These findings may have implications for the
understanding of DLBCL biology and potential treatment strategies
with regard to the hypoxic milieu and either HIF-1α and/or VEGF-A
expression. The present study ultimately provides preliminary data
to confirm the clinical significance of HIF-1α and VEGF-A
expression for routine application. Further investigations of this
pathway should be performed both in vivo and in vitro
to determine whether the HIF-1α/VEGF-A pathway is clinically useful
for either prognosis or as a therapeutic target for an improved
approach in patients with DLBCL.
Acknowledgements
We would like to thank and acknowledge the
Department of Anatomical Pathology at Dr Kariadi Hospital
(Semarang, Indonesia) for allowing the use of the archived tissues,
access to the hospital's medical records to obtain the patient
clinical data from the computerized database of the Department of
Hematology/Medical Oncology. The authors also acknowledge the
excellent technical assistance of Mr. Beny Iswahyudi (Associate
Health Analyst) from the Department of Pathological Anatomy of Dr
Kariadi Hospital. We likewise extend our gratitude to all the staff
of Dr Kariadi Hospital Central Laboratory, particularly Mr Handit
for providing us with administrative help during the study, and Dr
Dharminto M. Kes for his valuable help in the statistical analysis
of the results.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
EAP and PW designed of the study, and performed
patient inclusion and follow-up. RMN helped write the first draft
of the manuscript, and organized and searched for the relevant
literature. HI and DP participated in the immunohistochemical
staining, interpreting and analyzing the results, and then
providing their expertise in pathological evaluation. BS and DS
were involved in reviewing and critically revising the article for
important intellectual content. CS also designed the study,
analyzed and interpreted the results and supervised the course of
the study. EAP and PW confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Human samples used in the present study were
obtained from patients who had provided written informed consent.
The study was approved by the Ethics Committee of Dr Kariadi
Hospital (Semarang, Indonesia), and was conducted according to The
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koh MY and Powis G: Passing the baton: The
HIF switch. Trends Biochem Sci. 37:364–372. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Walsh JC, Lebedev A, Aten E, Madsen K,
Marciano L and Kolb HC: The clinical importance of assessing tumor
hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic
opportunities. Antiox Redox Signal. 21:1516–1554. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69 (Suuppl 3):S4–S10.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hockel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dhani N, Fyles A, Hedley D and Milosevic
M: The clinical significance of hypoxia in human cancers. Semin
Nucl Med. 45:110–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vaupel P and Mayer A: Tumor hypoxia:
Causative mechanisms, microregional heterogeneities, and the role
of tissue-based hypoxia markers. Adv Exp Med Biol. 923:77–86.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Keith B and Simon MC: Hypoxia indicible
factors, stem cells and cancer. Cell. 129:465–472. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Evens AM, Schumacker PT, Helenowski IB,
Singh AT, Dokic D, Keswani A, Kordeluk E, Raji A, Winter JN,
Jovanovic BD, et al: Hypoxia inducible factor-alpha activation in
lymphoma and relationship to the thioredoxin family. Br J Haematol.
141:676–680. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Evens AM, Sehn LH, Farinha P, Nelson BP,
Raji A, Lu Y, Brakman A, Parimi V, Winter JN, Schumacker PT, et al:
Hypoxia-inducible factor-1α expression predicts superior survival
in patients with diffuse large B-cell lymphoma treated with R-CHOP.
J Clin Oncol. 28:1017–1024. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pazgal I, Boycov O, Shpilberg O, Okon E
and Bairey O: Expression of VEGF-C, VEGF-D and their receptor
VEGFR-3 in diffuse large B-cell lymphomas. Leuk Lymphoma.
48:2213–2220. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ruppert A, Dixon JG, Salles G, Wall A,
Cunningham D, Poeschel V, Haioun C, Tilly H, Ghesquieres H, Ziepert
M, et al: International prognostic indices in diffuse large B-cell
lymphoma: A comparison of IPI, R-IPI, and. NCCN-IPI. Blood.
135:2041–2048. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO Classification of Tumours of
Haematopoietic and Lymphoid Tissues. International Agency for
Research on Cancer, Lyon, 2017.
|
|
13
|
Armitage JO: Staging non-Hodgkin lymphoma.
CA Cancer J Clin. 55:368–376. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brown LF, Berse B, Jackman RW, Tognazzi K,
Guidi AJ, Dvorak HF, Senger DR, Connolly JL and Schnitt SJ:
Expression of vascular permeability factor (vascular endothelial
growth factor) and its receptors in breast cancer. Hum Pathol.
26:86–91. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shahini L, Gasparov S, Petrusevska G,
Manxhuka Kerliu S, Veselaj F, Kurshumliu F and Kavaja F: Clinical
significance of VEGF-A and microvessel density in diffuse large
B-cell lymphoma and low-grade follicular lymphoma. Acta Clin Croat.
56:588–593. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gratzinger D, Zhao S, Tibshirani RJ, His
ED, Hans CP, Pohlman B, Bast M, Avigdor A, Schiby G, Nagler A, et
al: Prognostic significance of VEGF, VEGF receptors, and.
Microvessel density in diffuse large B cell lymphoma treated with
anthracycline-based chemotherapy. Lab Investig. 88:38–47.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang J, Li W, He X, Zhang G, Yue L and
Chai Y: VEGF overexpression is a valuable prognostic factor for
non-Hodgkin's lymphoma: Evidence from a systematic meta-analysis.
Dis Markers. 2015(786790)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nagao A, Kobayashi M, Koyasu S, Chow CCT
and Harada H: HIF-1 dependent reprogramming of glucose metabolic
pathway of cancer cells and its therapeutic significance. Int J Mol
Sci. 20(238)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia inducible factor 1 alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
20
|
Wu X, Pertovaara H, Korkola P, Vornanen M,
Eskola H and Kellokumpu-Lehtinen PL: Glucose metabolism correlated
with cellular proliferation in diffuse large B-cell lymphoma. Leuk
Lymphoma. 53:400–405. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Maxwell PH, Dachs GU, Gleadle JM, Nicholls
LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW and Ratcliffe PJ:
Hypoxia-inducible factor 1 modulates gene expression in solid
tumors and influences both angiogenesis and tumor growth. Proc Natl
Acad Sci USA. 94:8104–8109. 1997.PubMed/NCBI View Article : Google Scholar
|