Introduction
Gastrointestinal stromal tumors (GISTs), which
originate from interstitial cells of Cajal, are the most common
submucosal tumors of the gastrointestinal tract. GISTs can occur
anywhere in the gastrointestinal tract, most commonly in the
stomach (50-70%), followed by the jejunum and ileum (25-35%), and
colon (5%); only 5% of GISTs are reported to occur in the duodenum
(1,2). Liu et al (3) reported that the prognosis of patients
with duodenal GISTs (D-GISTs) was significantly worse than that of
patients with gastric GISTs (G-GISTs). Although studies involving
D-GISTs have been published, most included a small number of
samples owing to the infrequency of GISTs. In addition, few studies
have investigated the computed tomography (CT) features of D-GISTs
(4).
Therefore, we conducted this retrospective study to
compare the CT findings between D-GISTs and G-GISTs. We also
performed immunohistochemical staining for CD31 to confirm
microvessel density in the D-GIST and G-GIST specimens.
Materials and methods
Patients
Between June 2006 and October 2018, 10 patients with
D-GISTs and 27 patients with G-GISTs underwent surgery at the
Japanese Red Cross Okayama Hospital. Two patients with G-GISTs who
did not undergo dynamic CT before surgical resection were excluded.
Therefore, a final 35 patients were retrospectively investigated.
GIST was diagnosed based on the histopathological evaluation of the
resected specimen. The degree of recurrence risk of D-GISTs and
G-GISTs was determined according to the modified Fletcher
classification (5). The study
design was approved by the ethics committee of our institute and
adhered to the principles of the Declaration of Helsinki. Informed
consent was obtained from all patients.
CT protocol
Multidetector CT was used to perform triphasic
spiral CT on 35 patients (Aquilion ONE; Canon Medical Systems) with
the following scanning settings: 120 kVp, 360 mA, 2-mm section
collimation, and an 11.0 mm/sec table speed. To capture adjacent
parts, images were reconstructed every 5 mm. Non-ionic iodinated
contrast material (2 ml/kg, iopamidol, Iopamiron 370; Bayer,
Leverkusen, Germany) was administered into the vein using a power
injector at a flow rate of 3.2 ml/sec. At 12, 32, and 180 sec after
the contrast material was injected, arterial, portal, and delayed
phase spiral scans were automatically commenced.
Quantitative evaluation of dynamic
CT
Unenhanced and contrast-enhanced images were
available for each patient during the arterial, portal, and delayed
phases. To determine the tumor enhancement grade, CT attenuation
values of the lesion were measured in Hounsfield units using
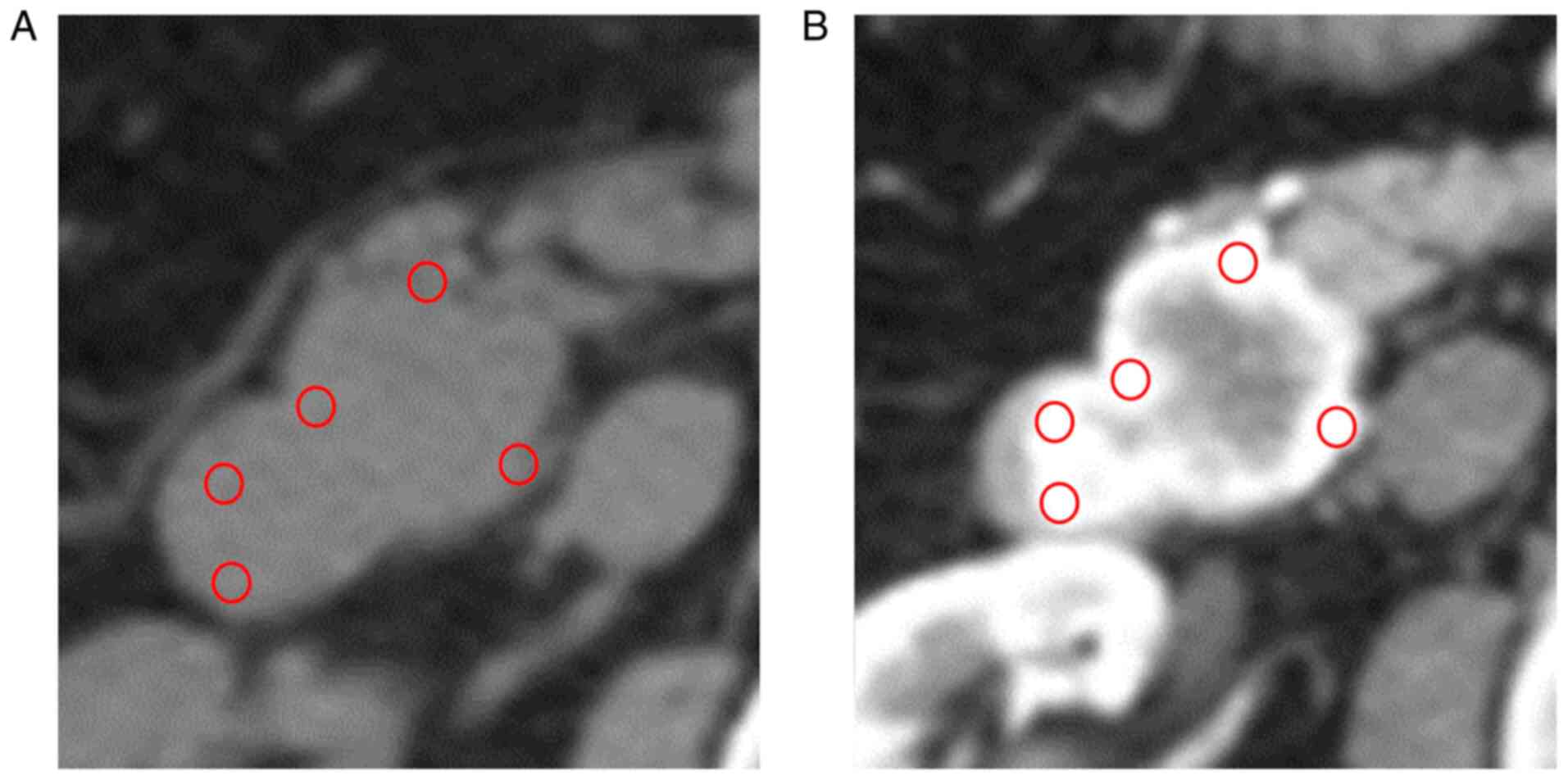
circular regions of interest (ROIs). Five ROIs were placed on the
most strongly enhanced portion of the lesion in each arterial,
portal, and delayed phase. Each ROI was placed in approximately the
same area, and we measured the CT attenuation values of unenhanced
lesions in similar areas (Fig. 1).
Five ROI values for each phase or for the unenhanced phase were
averaged. The contrast ratio was determined by taking the ratio of
the averaged ROI values of lesions in each phase and in the
unenhanced phase.
Assessment of vascularity
Formalin-fixed and paraffin-embedded sections at
4-µm thickness were used for immunohistochemical staining of
surgically resected tissue samples. Immunohistochemistry was
carried out using conventional procedures. Anti-CD31 monoclonal
antibodies (clone JC70A; Novocastra, England) were used to identify
vascular endothelial cells, and hematoxylin was used to
counterstain the sections. Tissue slices with CD31 staining were
inspected under low magnification (x40) to select the three most
vascularized regions inside the tumor. The microvessel densities in
these regions were subsequently quantified as the ratios between
the CD31-stained and -unstained areas within the selected tumor
sections at high magnification (x100) using Adobe Photoshop
Elements (version 14.0; Adobe Systems). The microvessel density
value for the tumor sample was computed using the mean value of the
microvessel densities of the three selected areas. The microvessel
density values of D-GISTs and G-GISTs were compared.
Statistical analysis
JMP software version 12.2.0 was used to perform
statistical calculations (SAS Institute, Inc.). Categorical values
were compared with Fisher's exact test. Continuous values were
compared using the Mann-Whitney U test or the Kruskal-Wallis test
followed by Dunn's test. Correlation coefficients were analyzed
using Pearson's correlation. P<0.05 was considered to indicate a
statistically significant difference. No correction for multiple
hypothesis testing was used because this was an exploratory
study.
Results
Patient characteristics
The backgrounds of the study participants are shown
in Table I. Between these two
groups, no significant differences were observed regarding age,
sex, presence or absence of symptoms, tumor size, presence or
absence of calcification, or risk classification. With respect to
location, D-GISTs were most frequently observed in the 2nd portion
of the duodenum (7/10, 70.0%). G-GISTs were predominantly
identified in the gastric corpus (18/25, 72.0%).
 | Table IBaseline characteristics of the
patients. |
Table I
Baseline characteristics of the
patients.
| Variable | Duodenal GISTs
(n=10) | Gastric GISTs
(n=25) | P-value |
|---|
| Mean age, years
(range) | 60.90
(43.00-87.00) | 68.80
(47.00-82.00) | 0.35 |
| Sex, n
(male/female) | 5/5 | 12/13 | 0.64 |
| Symptom, n | | | |
|
Abdominal
pain | 0 | 2 | |
|
Bleeding | 3 | 2 | |
|
Fatigue | 1 | 1 | |
| Location | 1/7/1/1
(1st/2nd/3rd/4th) | 6/18/1
(fundus/body/antrum) | |
| Median tumor size, mm
(range) | 38.00
(10.00-71.00) | 32.00
(14.00-160.00) | 0.76 |
| Calcification, n | | | 0.74 |
|
Present | 1 | 3 | |
|
Absent | 9 | 22 | |
| Degree of risk,
n | | | 0.23 |
|
Very
low | 2 | 3 | |
|
Low | 6 | 12 | |
|
Intermediate | 0 | 5 | |
|
High | 2 | 5 | |
Contrast ratio in each phase
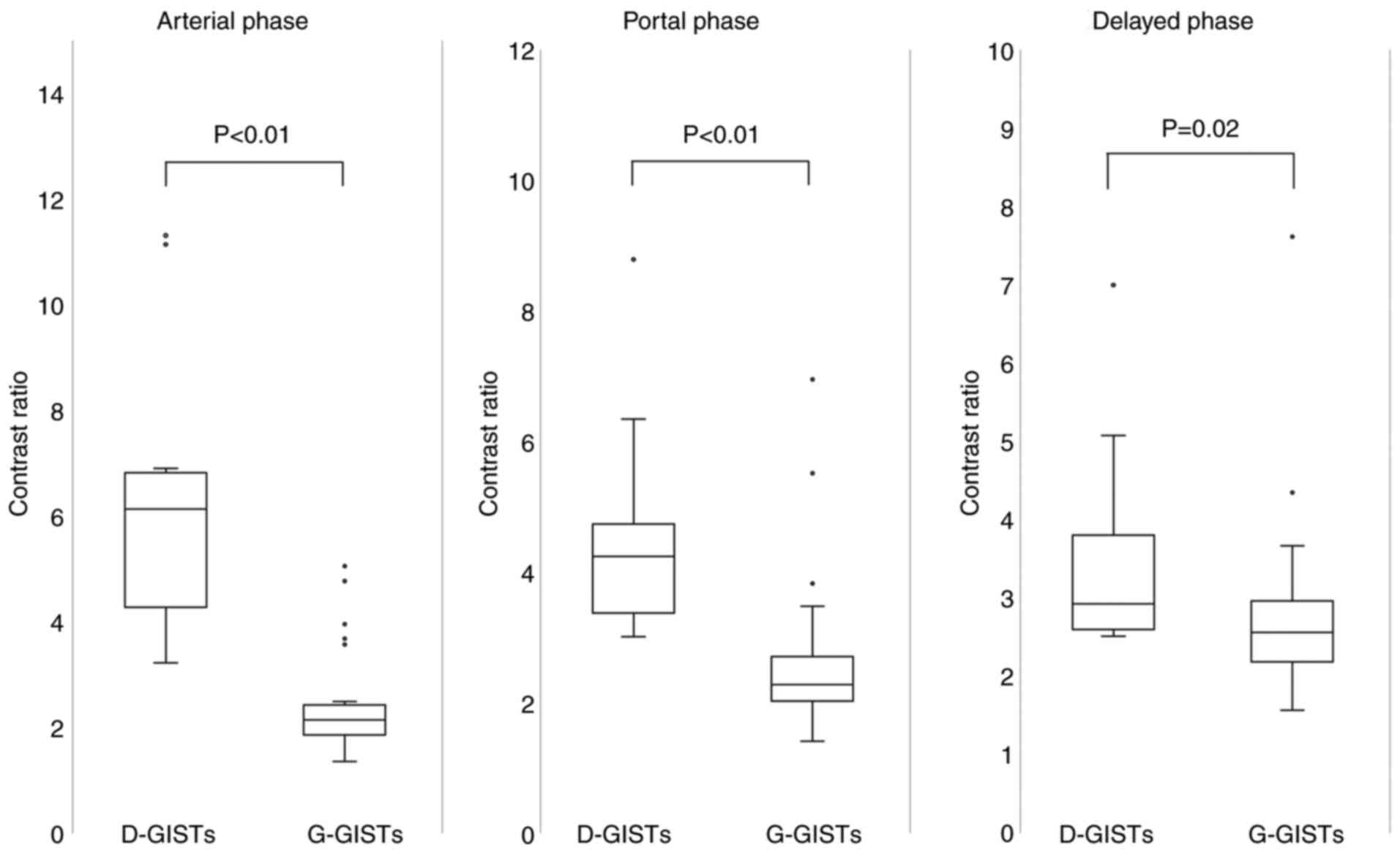
The contrast ratios of D-GISTs and G-GISTs in each
phase are shown in Fig. 2. The
contrast ratio of D-GISTs was significantly higher than that of
G-GISTs in the arterial (6.52±2.77 vs. 2.41±0.98, P<0.01),
portal (4.67±1.74 vs. 2.66±1.21, P<0.01), and delayed phases
(3.64±1.43 vs. 2.81±1.16, P=0.02). The difference in contrast ratio
between D-GISTs and G-GISTs was largest in the arterial phase.
Correlation between microvessel
density and CT contrast ratio
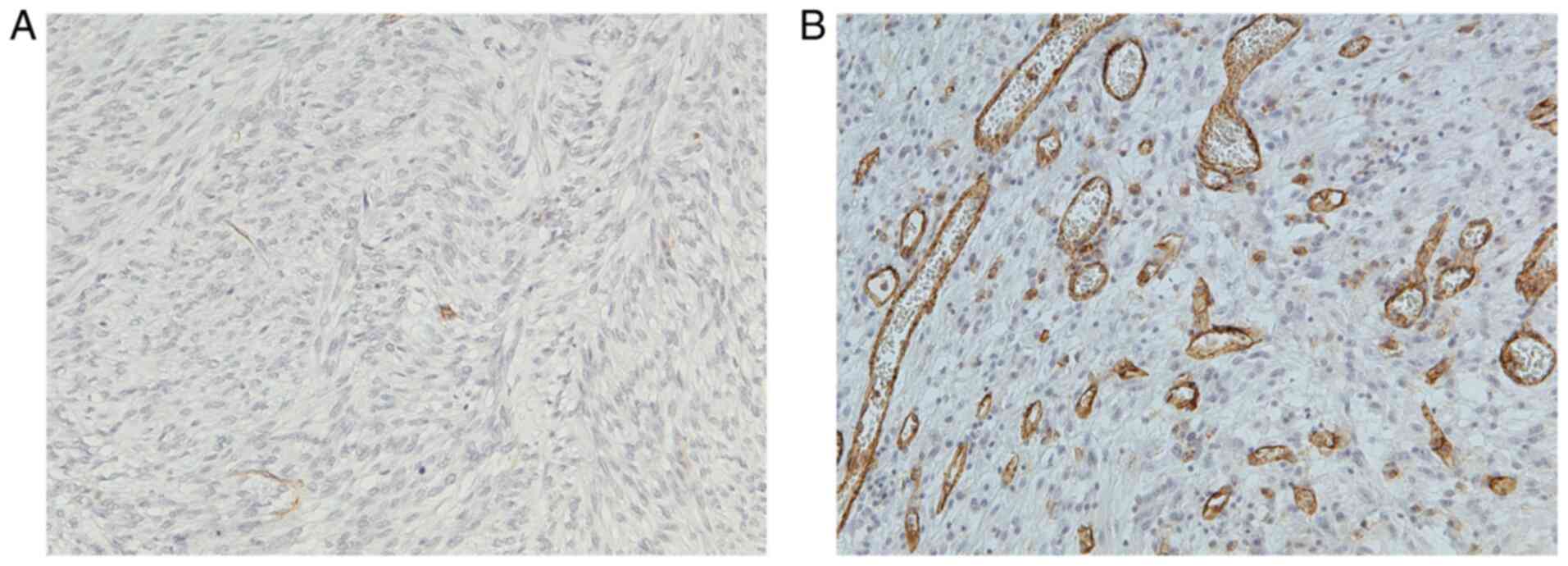
Representative images of CD31 immunostaining in
G-GISTs and D-GISTs are presented in Fig. 3A and B, respectively. CD31-positive
microvessels scarcely existed in G-GISTs, whereas there were plenty
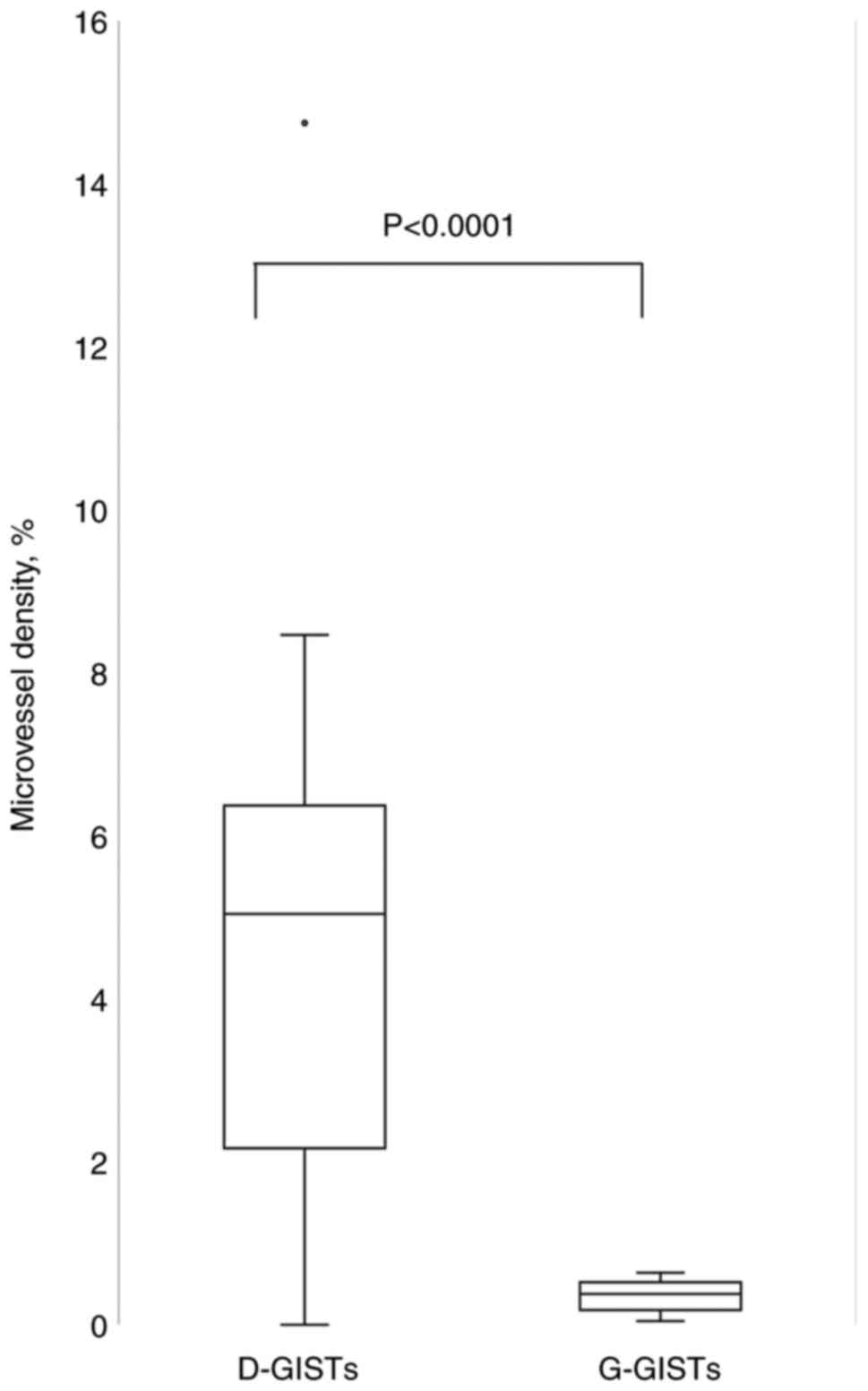
of microvessels in D-GISTs. As shown in Fig. 4, D-GISTs had a higher microvessel
density than G-GISTs (5.52±3.98 vs. 0.35±0.19, P<0.0001).
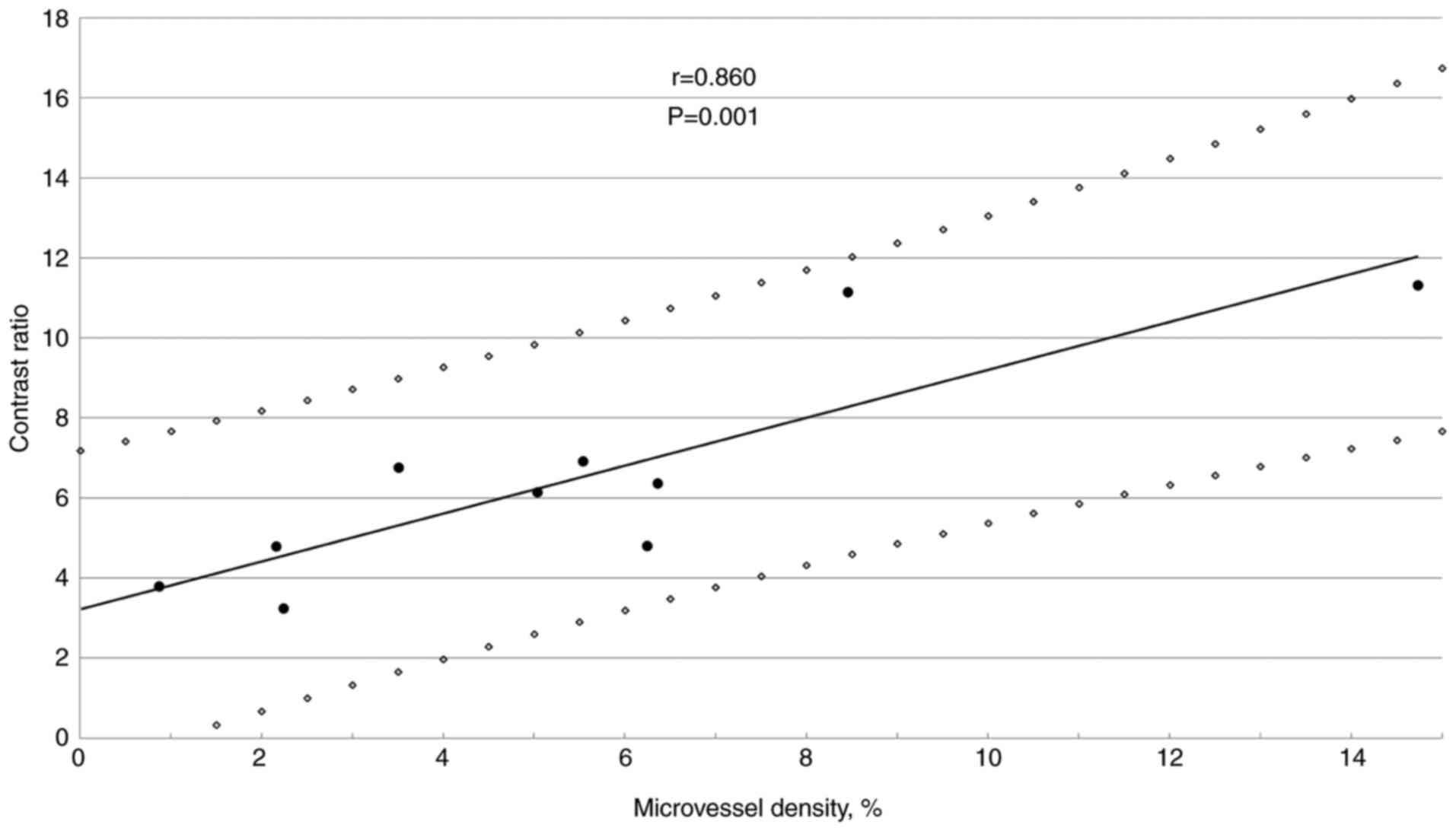
There was a significant correlation between
microvessel density and the contrast ratio in D-GISTs (Fig. 5).
Comparison of contrast ratios between
risk classifications
According to the modified Fletcher classification,
D-GISTs were classified as very low risk (n=2), low risk (n=6), or
high risk (n=2). G-GISTs were classified as very low risk (n=3),
low risk (n=12), intermediate risk (n=5), or high risk (n=5).
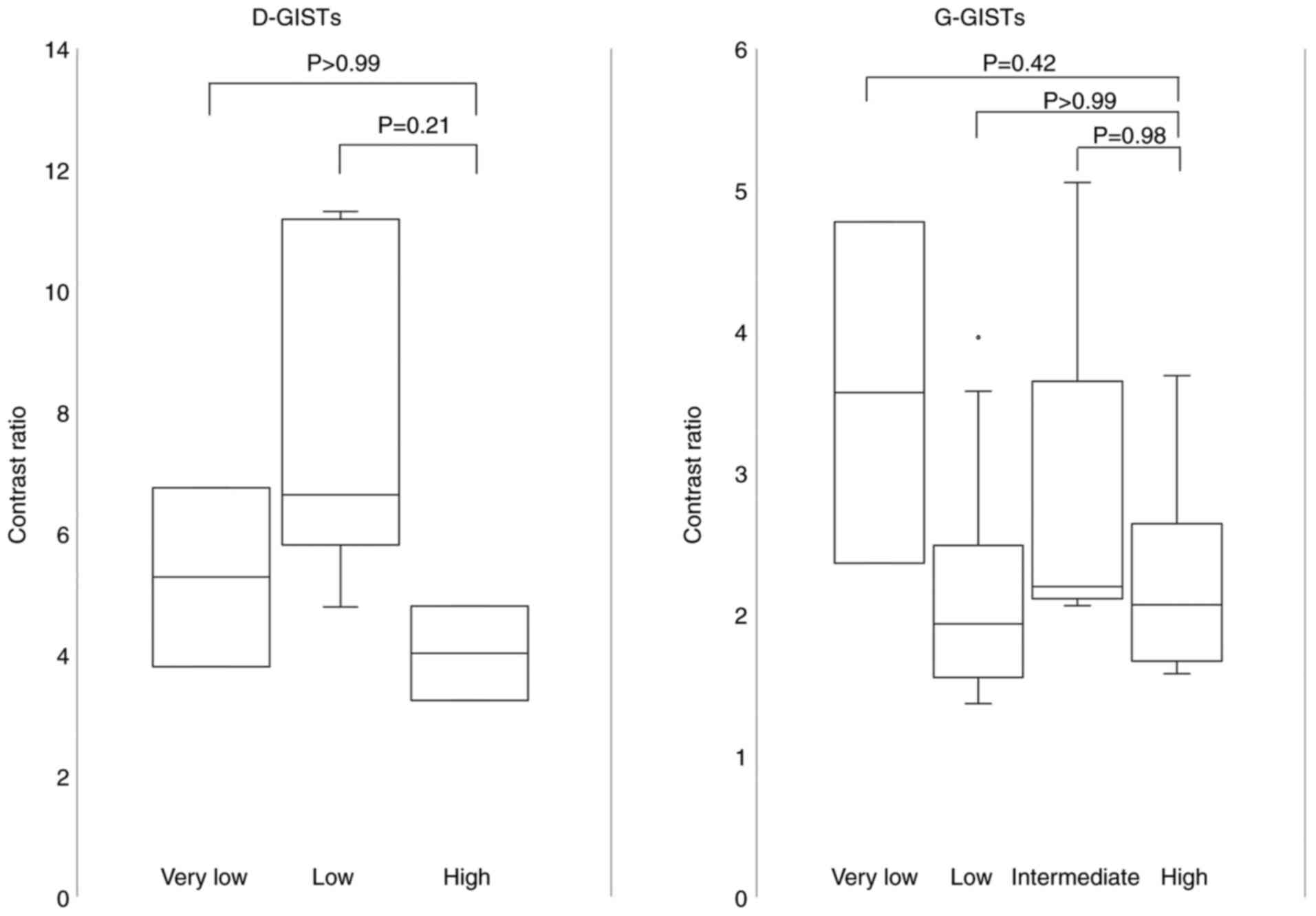
Fig. 6 shows the correlation
between the contrast ratio in the arterial phase and risk
classification in D-GISTs and G-GISTs. The contrast ratio did not
differ between the risk grades.
Discussion
In the present study, we showed that D-GISTs had a
larger CD31-positive area than G-GISTs. This result was consistent
with the fact that D-GISTs may appear as hypervascular lesions. The
duodenum is divided into four portions; it has been reported that
the feeding arteries of the first and second portions of the
duodenum are different from those of the third and fourth portions
(6). The first and second portions
of the duodenum are nourished by the posterior superior
pancreaticoduodenal artery (PSPDA), which branches from the
gastroduodenal artery. The PSPDA and the inferior
pancreaticoduodenal artery form an arcade called the
pancreaticoduodenal arcade. The third and fourth portions are
nourished by the first jejunal artery, which branches from the
superior mesenteric artery (SMA). The vasculature of G-GISTs has
been reported in two cases (7).
Cai et al (8) reported on
the vasculature of D-GISTs, especially the importance of the supply
arteries and drainage veins in differential diagnosis. According to
their report, the vasculature of D-GISTs from each portion was
consistent with that of the normal duodenum, and it was difficult
to find the obvious supply arteries for duodenal adenocarcinoma or
lymphoma. Furthermore, 50% of D-GISTs drain directly into the
portal venous trunk, which is not detected in other duodenal tumors
(8). Futo et al (9) reported that D-GISTs arising from the
second or third portions of the duodenum may be incorrectly
diagnosed as pancreatic neuroendocrine tumors. Thus, some studies
have shown the features of D-GIST imaging findings; however, the
reason why D-GISTs show hypervascularity remains unexplained. We
hypothesized that one of the reasons why D-GISTs display
hypervascularity is due to the large number and complexity of the
duodenal feeding arteries.
Using immunohistochemical staining samples, we found
that microvessel density in D-GISTs was greater than that in
G-GISTs, and there was a significant correlation between the
contrast ratio and microvessel density in D-GISTs. The correlation
between dynamic CT findings and microvessel density has been
reported in pancreatic ductal adenocarcinoma (10), pancreatic neuroendocrine neoplasm
(11), and prostate disease
(12). A correlation between
microvessel density and clinical characteristics has been reported
for GISTs. However, to the best of our knowledge, no previous study
has investigated the correlation between microvessel density and
dynamic CT findings. We believe that the degree of vascularity has
a significant relationship with microvessel density in D-GISTs.
A previous report showed that high-grade GISTs had
higher CT attenuation values than intermediate-, low-, or very
low-grade GISTs in the arterial phase (13). However, we found no significant
difference between the contrast ratio and degree of risk. We used
the modified Fletcher classification system to evaluate the risk of
recurrence. The modified Fletcher classification is defined by
tumor size and mitotic index (4).
We discovered a significant correlation between the contrast ratio
and microvessel density in D-GISTs. Therefore, if there is a
correlation between microvessel density and the mitotic index,
there may also be a correlation between the contrast ratio and risk
classification. Microvessel density has a strong positive
correlation with the degree of malignancy of intraductal papillary
mucinous neoplasms of the pancreas (14) and colorectal tumors (15). In the case of GISTs, high CD31
values, in other words, high microvessel density, were reported to
be related to poor prognosis (16). Meanwhile, other researchers have
reported that microvessel density was not significantly correlated
with mitosis (17). The fact that
80% of the D-GISTs in this study were in the very low or low risk
group despite their poor prognosis may be related to the fact that
there was no significant correlation between contrast ratio and
risk classification. At this stage, we cannot assert a correlation
between the microvessel density and the mitotic index. Therefore,
we investigated the correlation between mitosis as a risk
classification factor and microvessel density.
Imamura et al (17) reported that there was significant
correlation between microvessel density and vascular endothelial
growth factor (VEGF) expression in GISTs. VEGF plays a major role
in promoting tumor angiogenesis (18). The authors also found that
intestinal GISTs had greater VEGF expression and microvessel
density than G-GISTs (17). This
may explain why D-GISTs are more hypervascular than G-GISTs.
Further research on VEGF, other angiogenic factors, and genetic
abnormalities of GISTs may lead to treatments targeting them.
Our study had some limitations. First, this was a
retrospective case study with a small cohort, and all dynamic CT
scans were performed at a single institution. D-GISTs are
relatively rare, and we could only analyze 10 D-GIST cases in our
institution. Further investigation with more cases from multiple
institutions is needed to reveal the imaging and pathological
features of D-GISTs. Second, we attempted to perform microvessel
analysis at the most vascularized region inside the tumor. However,
the region of GISTs where we analyzed the microvessel density and
CT ratio may be slightly different.
In summary, the results of our analyses demonstrated
that D-GISTs were more hypervascular than G-GISTs on imaging and
pathological examination. The reason why D-GISTs are more
hypervascular than G-GISTs remains uncertain. However, we speculate
that this involves two components: Anatomical and molecular
biology. In other words, D-GISTs have large feeding arteries and
drainage veins that are sufficient for detection and greater VEGF
expression than G-GISTs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS wrote the manuscript and made substantial
contributions to conception and design, and acquisition of data. RH
and KH made substantial contributions to analysis and
interpretation of data. TT, NH, MIn and HK assisted in the
statistical procedures. MM made substantial contributions to the
analysis and interpretation of data of CT imaging. MT, AH and MIw
made substantial contributions to the analysis and interpretation
of data of the pathological examinations. RS and RH confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study design was approved by the ethics
committee of Japanese Red Cross Okayama Hospital (Okayama, Japan)
and adhered to the principles of the Declaration of Helsinki.
Written informed consent was obtained from all patients.
Patient consent for publication
Written informed consent was obtained from the
patients for publication of this study and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joensuu H: Gastrointestinal stromal tumor
(GIST). Ann Oncol. 17:x280–x286. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Scola D, Bahoura L, Copelan A, Shirkhoda A
and Sokhandon F: Getting the GIST: A pictorial review of the
various patterns of presentation of gastrointestinal stromal tumors
on imaging. Abdom Radiol (NY). 42:1350–1364. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu Z, Zheng G, Liu J, Liu S, Xu G, Wang
Q, Guo M, Lian X, Zhang H and Feng F: Clinicopathological features,
surgical strategy and prognosis of duodenal gastrointestinal
stromal tumors: A series of 300 patients. BMC Cancer.
18(563)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sandrasegaran K, Rajesh A, Rushing DA,
Rydberg J, Akisik FM and Henley JD: Gastrointestinal stromal
tumors: CT and MRI findings. Eur Radiol. 15:1407–1414.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C,
et al: Risk of recurrence of gastrointestinal stromal tumour after
surgery: An analysis of pooled population-based cohorts. Lancet
Oncol. 13:265–274. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Desai GS and Pande PM: Gastroduodenal
artery: Single key for many locks. J Hepatobiliary Pancreat Sci.
26:281–291. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Teoh WC, Teo SY and Ong CL:
Gastrointestinal stromal tumors presenting as gynecological masses:
Usefulness of multidetector computed tomography. Ultrasound Obstet
Gynecol. 37:107–109. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Cai PQ, Lv XF, Tian L, Luo ZP, Mitteer RA
Jr, Fan Y and Wu YP: CT characterization of duodenal
gastrointestinal stromal tumors. AJR Am J Roentgenol. 204:988–993.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Futo Y, Saito S, Miyato H, Sadatomo A,
Kaneko Y, Kono Y, Matsubara D, Horie H, Lefor AK and Sata N:
Duodenal gastrointestinal stromal tumors appear similar to
pancreatic neuroendocrine tumors: A case report. Int J Surg Case
Rep. 53:358–361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang ZQ, Li JS, Lu GM, Zhang XH, Chen ZQ
and Meng K: Correlation of CT enhancement, tumor angiogenesis and
pathologic grading of pancreatic carcinoma. World J Gastroenterol.
9:2100–2104. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Horiguchi S, Kato H, Shiraha H, Tsutsumi
K, Yamamoto N, Matsumoto K, Tomoda T, Uchida D, Akimoto Y, Mizukawa
S, et al: Dynamic computed tomography is useful for prediction of
pathological grade in pancreatic neuroendocrine neoplasm. J
Gastroenterol Hepatol. 32:925–931. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wilson NM, Masoud AM, Barsoum HB, Refaat
MM, Moustafa MI and Kamal TA: Correlation of power Doppler with
microvessel density in assessing prostate needle biopsy. Clin
Radiol. 59:946–950. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wei SC, Xu L, Li WH, Li Y, Guo SF, Sun XR
and Li WW: Risk stratification in GIST: Shape quantification with
CT is a predictive factor. Eur Radiol. 30:1856–1865.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamamoto N, Kato H, Tomoda T, Matsumoto K,
Sakakihara I, Noma Y, Horiguchi S, Harada R, Tsutsumi K, Hori K, et
al: Contrast-enhanced harmonic endoscopic ultrasonography with
time-intensity curve analysis for intraductal papillary mucinous
neoplasms of the pancreas. Endoscopy. 48:26–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhuang H, Yang ZG, Chen HJ, Peng YL and Li
L: Time-intensity curve parameters in colorectal tumours measured
using double contrast-enhanced ultrasound: Correlations with tumour
angiogenesis. Colorectal Dis. 14:181–187. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Basilio-de Oliveira RP and Pannai VLN:
Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell
proliferation (Ki67) for gastrointestinal stromal tumors. World J
Gastroenterol. 21:6924–6930. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Imamura M, Yamamoto H, Nakamura N, Oda Y,
Yao T, Kakeji Y, Baba H, Maehara Y and Tsuneyoshi M: Prognostic
significance of angiogenesis in gastrointestinal stromal tumor. Mod
Pathol. 20:529–537. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992.PubMed/NCBI View
Article : Google Scholar
|