Introduction
Lung carcinoid (LC) tumors are rare neuroendocrine
neoplasms that represent less than 2% of all lung malignancies
(1,2). However, the incidence of LC is
increasing, likely related to improved imaging and other diagnostic
techniques (2,3). The World Health Organization (WHO)
classifies LC into typical (TC) and atypical (AC) carcinoids
(4). TC accounts for more than 75%
of LC, and presentation with de novo metastatic disease is
rare-less than 10% (5-7).
Surgery is the mainstay of treatment for patients with TC (8-10).
Although non-metastatic TC has excellent postsurgical outcomes,
with a five-year survival rate of over 90%, up to 10% of patients
have recurrences (11). In
numerous solid organ tumors, adjuvant treatment modalities are
rational options for decreasing recurrence and prolonging survival.
Adjuvant cytotoxic chemotherapy in patients with LC remains
controversial due to the lack of prospective randomized controlled
trials (RCT) studying the efficacy of cytotoxic chemotherapy in
this setting (12-14).
Some scientific evidence on this issue has been
provided by several retrospective studies (7,15-18);
however, these studies included both AC and TC, which have
different clinical courses (19).
Studies investigating chemotherapy efficacy in patients with TC and
excluding AC are limited (20).
Therefore, it was aimed to determine the impact of platin-based
chemotherapy on the survival of patients with TC. Potential
prognostic factors, such as clinical characteristics, type of
surgical procedure and pathological features of patients were also
investigated.
Materials and methods
Study population
The electronic medical records of patients admitted
to the Departments of Medical Oncology or Thoracic Surgery at Bursa
Uludag University between January 2002 and December 2020 due to
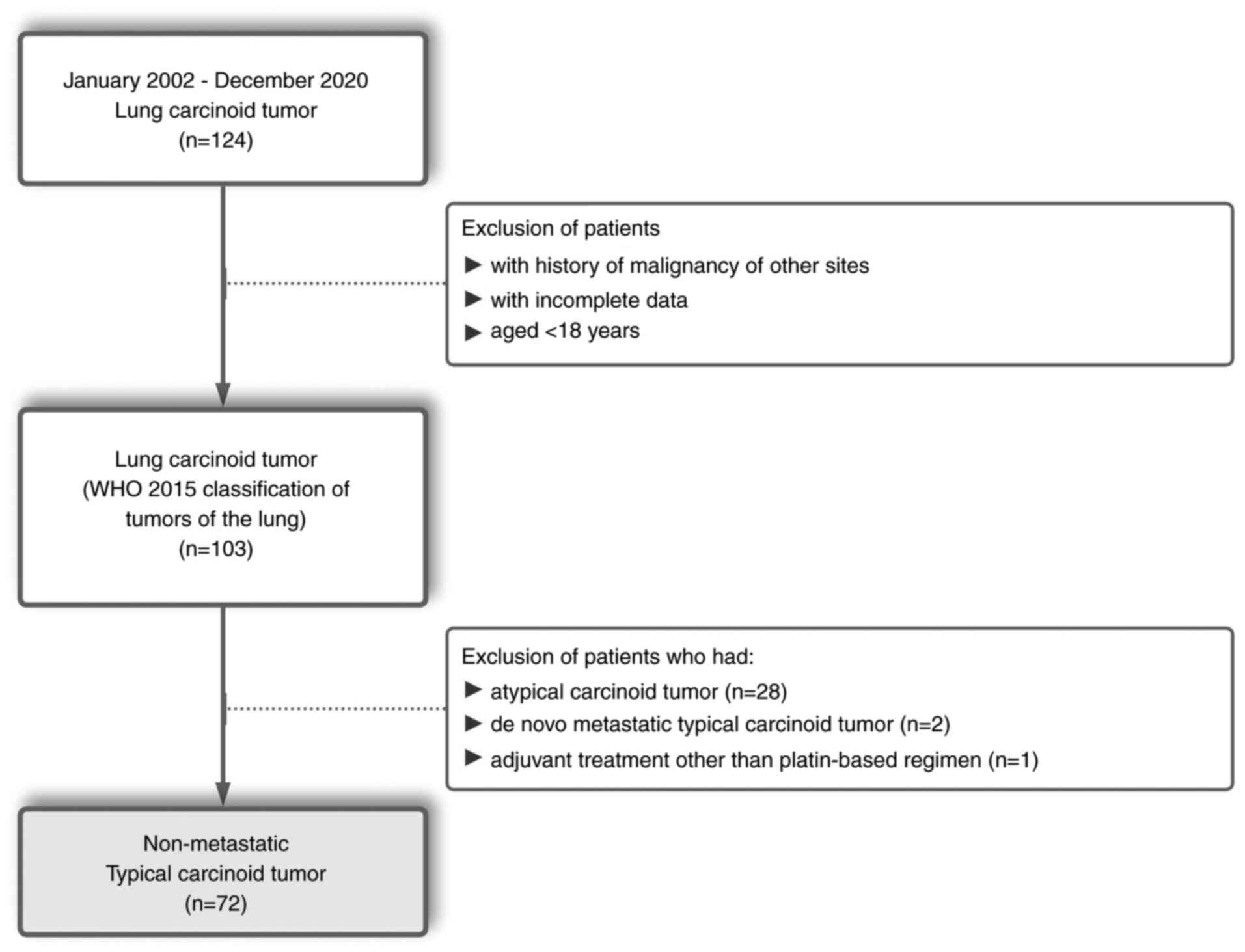
lung carcinoid tumors were retrospectively reviewed. A patient flow
diagram is provided in Fig. 1.
Patients with a history of other malignancies, patients with
incomplete data and patients aged <18 were excluded. According
to the criteria specified by the WHO 2015 classification of lung
tumors (4), patients with
non-metastatic TC were enrolled in the present study. Patients who
received adjuvant cytotoxic chemotherapy other than platin-based
regimens were also excluded. The present study was approved
(approval no. 2021-5/19) by the Clinical Research Ethics Committee
of Bursa Uludag University Faculty of Medicine (Bursa, Turkey).
Data collection
The following demographic and clinical features of
the participants were extracted from their electronic records: age,
sex, symptoms at presentation, imaging modality, tumor laterality,
tumor localization, clinical stage, surgical procedure and
(neo)adjuvant treatment. A preoperative evaluation was performed as
previously described (21).
Octreotide scintigraphy or Ga68-Dotatate positron emission
tomography-computed tomography was performed in patients with a
preoperative diagnosis of LC (when available). Cranial magnetic
resonance imaging was performed only when clinically indicated.
Mediastinal lymph node (MLN) dissection was performed on all
patients who underwent lobectomy and segmentectomy. In patients who
underwent wedge resection, MLN sampling was performed in the case
of suspicious MLN on imaging. The staging was determined following
the eighth edition of the TNM staging system (22). Our multidisciplinary thoracic
oncology team evaluated the patients and histopathological features
were obtained from the pathology reports of patients, including
tumor size, lymphovascular invasion, Ki-67 percentage and surgical
margins. Patients who received platin-based neoadjuvant or adjuvant
chemotherapy were included in the study. Adverse events were graded
using the National Cancer Institute Common Terminology Criteria for
Adverse Events (version 4.0) (23).
Follow-up, patient outcomes and
statistical analysis
After surgical resection, computed tomography (CT)
was performed every three to six months for up to two years and
then annually. Disease-free survival (DFS) was calculated based on
the amount of time from surgery until disease recurrence, confirmed
by histological examination or imaging modalities, or death for any
reason, whichever occurred first. Overall survival (OS) was
determined as the length of time from diagnosis until death from
any cause. Statistical analyses were performed using SPSS version
22 software (IBM Corp.). Continuous and categorical variables were
expressed as median (minimum-maximum) and frequency values.
Kaplan-Meier analysis was employed for survival rates. Log-rank
testing was used to compare groups of patients according to their
disease stage and whether they received chemotherapy. The possible
factors affecting DFS were examined using Cox regression analysis.
A backward stepwise model was used with parameters with a P-value
<0.25. P<0.05 was considered to indicate a statistically
significant difference.
Results
A total of 72 patients were included in the present
study. The demographic and clinical characteristics of the patients
are provided in Table I. The
median age was 50.2 (18.1-81.1) years. Nearly two-thirds of the
patients were female. Cough was the most common symptom, and
one-third of all patients were asymptomatic at presentation. All
patients were evaluated using CT scans during staging. A total of
48 patients (66%) had centrally located tumors. The biopsies of
three patients were reported as non-small-cell lung cancer. Of the
patients, 68% underwent a lobectomy (Table II). The medians of the tumor size
and the Ki-67 index were 18 mm (5-70) and 2% (0-10), respectively.
The pathological stages of patients were as follows: 73.6% were in
stage I, 15.3% were in stage II and 11.1% were in stage III. All
patients underwent surgery with a negative surgical margin. A total
of 5 patients received platin-based chemotherapy, 2 in the
neoadjuvant setting and 3 in the adjuvant setting. A total of 3
patients were treated with cisplatin (75 mg/m2
intravenous on day 1) and etoposide (100 mg/m2
intravenous on days 1-3) and 2 patients received carboplatin (area
under the curve of five intravenous on day 1) and etoposide. The
regimens were administered every 21 days. The median number of
chemotherapy cycles was 6 (range: 4-6). Of the patients, 93.1%
received no adjuvant treatment. Nausea and hematological toxicity
were observed in patients receiving chemotherapy; the only grade 3
and higher adverse event was grade 3 neutropenia observed in 2
patients.
 | Table IDemographic and clinical
characteristics of the patients. |
Table I
Demographic and clinical
characteristics of the patients.
| Characteristic | Total (n=72) | Percentage (%) |
|---|
| Age, years [Median,
(range)] | 50.2 | (18.1-81.1) |
| Sex | | |
|
Male | 44 | (61.1) |
|
Female | 28 | (38.9) |
| Presentation at
diagnosis | | |
|
Cough | 34 | (47.2) |
|
Dyspnea | 10 | (13.9) |
|
Hemoptysis | 5 | (6.9) |
|
Pneumonia | 3 | (4.2) |
|
Carcinoid
Syndrome | 3 | (4.2) |
|
Asymptomatic | 24 | (33.3) |
| Imaging | | |
|
Computed
tomography | 72 | (100.0) |
|
Octreotide
scintigraphy | 20 | (27.8) |
|
Ga68-Dotatate
PET CT | 18 | (25.0) |
| Tumor
laterality | | |
|
Right | 53 | (73.6) |
|
Left | 19 | (26.4) |
| Localization | | |
|
Central | 48 | (66.7) |
|
Peripheral | 24 | (33.3) |
| Clinical Stage | | |
|
I | 45 | (62.5) |
|
II | 15 | (20.8) |
|
III | 12 | (16.7) |
 | Table IIPathological features and adjuvant
treatment of the patients with non-metastatic disease. |
Table II
Pathological features and adjuvant
treatment of the patients with non-metastatic disease.
| Characteristic | Total (n=72) | Percentage (%) |
|---|
| Surgery | | |
|
Lobectomy | 49 | (68.1) |
|
Wedge | 18 | (25.0) |
|
Bronchoplasty | 3 | (4.2) |
|
Segmentectomy | 2 | (2.7) |
| Tumor size, mm
[Median, (range)] | | 18 (5-70) |
| Ki-67 index, %
[Median, (range)] | | 2 (0-10) |
| Lymphovascular
invasion | | |
|
Present | 8 | (11.1) |
|
Absent | 64 | (88.9) |
| Pathological T
stage | | |
|
T1 | 50 | (69.4) |
|
T2 | 12 | (16.7) |
|
T3 | 4 | (5.6) |
|
T4 | 6 | (8.3) |
| Pathological N
stage | | |
|
N0 | 48 | (66.7) |
|
N1 | 7 | (9.7) |
|
N2 | 2 | (2.8) |
|
Nx | 15 | (20.8) |
| Pathological
stage | | |
|
I | 53 | (73.6) |
|
II | 11 | (15.3) |
|
III | 8 | (11.1) |
| (Neo)Adjuvant
treatment | | |
|
Chemotherapy | 5 | (6.9) |
|
Median
(range), cycles | | 6 (4-6) |
|
Cisplatin
plus etoposide | 3 | (4.2) |
|
Carboplatin
plus etoposide | 2 | (2.7) |
| Radiotherapy | 2 | (2,7) |
| Observation | 67 | (93.1) |
The median amount of time from diagnosis to the
final visit was 68.5 (0.7-210.9) months. A total of 6 patients
(8.3%) had recurrences. Half of these patients presented with
distant metastasis. The 12-, 36- and 60-month DFS rates were 98.5,
95.1 and 92.5%, respectively. The 12-, 36- and 60-month OS rates
were 100, 98.5 and 96.0%, respectively.
The results of the univariate and multivariate Cox
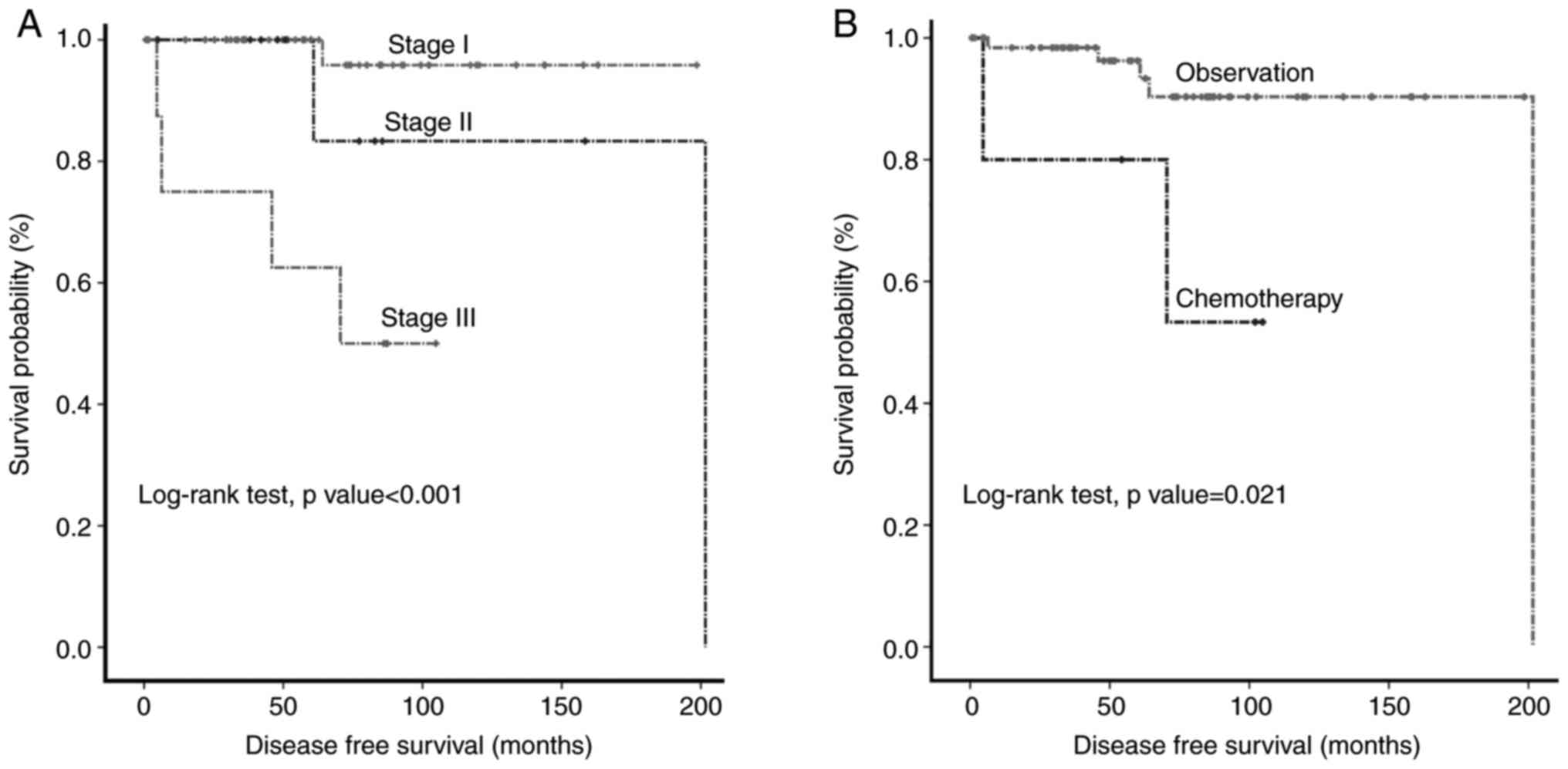
regression analyses of DFS are presented in Table III. Kaplan-Meier curves of DFS
according to pathological stage and adjuvant chemotherapy (Fig. 2A and B). Log-rank testing showed that patients
with stage III disease and patients who received chemotherapy had
significantly worse survival rates (P<0.001 and P=0.021,
respectively). Although univariate analyses displayed that patients
who did not receive chemotherapy had improved DFS than those who
received it, the multivariate Cox regression analysis revealed that
the pathological stage was the only statistically significant
factor affecting DFS (P=0.016).
 | Table IIIUnivariate and multivariate cox
regression analysis of the predictors for recurrence. |
Table III
Univariate and multivariate cox
regression analysis of the predictors for recurrence.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 1.042 | 0.982-1.107 | 0.176 | | | |
| Sex [male (R) vs.
female] | 2.666 | 0.310-22.945 | 0.372 | | | |
| Tumor laterality
[right (R) vs. left] | 0.602 | 0.066-4.832 | 0.5564 | | | |
| Localization
[central (R) vs. peripheral] | 3.167 | 0.369-27.201 | 0.293 | | | |
| Surgery [lobectomy
(R) vs. sublobar resection] | 1.221 | 0.223-6.694 | 0.818 | | | |
| Tumor size, mm | 1.010 | 0.963-1.058 | 0.690 | | | |
| Ki-67 index, % | 1.318 | 0.895-1.941 | 0.162 | | | |
| Lymphovascular
invasion [absent (R) vs. present] | 2.271 | 0.264-19.497 | 0.455 | | | |
| Pathological
stage | | | | | | |
|
I(R) | 1 | | 0.015 | 1 | | 0.016 |
|
II | 4.567 | 0.286-73.037 | 0.283 | 4.367 | 0.273-68.839 | 0.297 |
|
III | 22.188 | 2.472-199.188 | 0.006 | 21.216 | 2.363-190.462 | 0.006 |
| Chemotherapy [no
(R) vs. yes] | 5.824 | 1.063-31.918 | 0.042 | | | |
Discussion
In the present retrospective study, the effect of
platin-based chemotherapy and other clinicopathologic parameters on
DFS in patients with non-metastatic resected TC were investigated.
It was observed that chemotherapy did not improve DFS and that
pathological stage was the only independent risk factor for
DFS.
The guidelines provided by the European Society for
Medical Oncology (ESMO) and the National Comprehensive Cancer
Network (NCCN) suggested considering adjuvant platin-based
chemotherapy in LC patients with aggressive clinicopathological
features such as AC, N2 disease and a high proliferative index in
multidisciplinary councils (8,9). In
contrast to ESMO and NCCN, the North American Neuroendocrine Tumor
Society does not recommend any adjuvant treatment modality
(24). These recommendations are
based on retrospective studies, as no previous randomized clinical
trials have been conducted to study adjuvant therapy modalities in
LC. Table IV shows previous
studies in the international literature that have investigated the
impact of chemotherapy on survival in non-metastatic disease.
 | Table IVStudies investigating the efficacy of
chemotherapy in non-metastatic typical carcinoid. |
Table IV
Studies investigating the efficacy of
chemotherapy in non-metastatic typical carcinoid.
| Authors | Year | Patients | Chemotherapy | Analysis | Results |
|---|
| Our study | 2021 | TC Single-center
study, 2002-2020 72 non-metastatic TC | 6.9% received
platin plus etoposide | Multivariate | Although patients
receiving CT had worse DFS in univariate analyses, multivariate
analysis revealed that CT was not associated with inferior DFS |
| He et al
(18) | 2021 | TC + AC The SEER
database, 1975-2016, 1702 all staged TC | 5.8% of all
patients received CT, but regimens were not reported | Multivariate | CT was associated
with inferior CSS in all TC patients |
| Girelli et
al (25) | 2020 | TC + AC + LCNEC
Single-center study, 1998-2016, 21 non-metastatic N+ TC | 14.2% of all
patients received CT, but regimens were not reported | Univariate | (Neo)Adjuvant CT
was associated with inferior OS |
| Gosain et al
(16) | 2019 | TC + AC The NCDB,
2004-2014, 5727 non-metastatic TC | 2.9% of all
patients received CT, but regimens were not reported | Univariate,
Multivariate | CT was associated
with inferior OS in the subgroup analysis of TC |
| Westin et al
(17) | 2017 | TC + AC, The NCDB,
2004-2012, 651 non-metastatic N + TC | 6% of TC patients
received CT, but regimens were not reported | Multivariate | CT was associated
with inferior OS |
| Nussbaum et
al (20) | 2015 | TC, The NCDB,
1998-2006, 4612 non-metastatic TC | 5.9% of TC patients
received CT, but regimens were not reported | Univariate,
PSMA | CT was associated
with inferior OS in univariate analysis. After PSMA, CT was
associated with a trend toward inferior OS, which was not
statistically significant |
| Filosso et
al (15) | 2013 | TC + AC,
Single-center study, 1995-2010, 81 non-metastatic TC | Adjuvant treatment,
including platin-based CT, RT, and SSA, was administered to 7.4% of
TC patients | Multivariate | Adjuvant treatment
was not an independent factor for survival |
In 2013, Filosso et al (15) reported that 7.4% of 81 patients
with non-metastatic TC received adjuvant treatment, including
platin-based chemotherapy, radiotherapy and somatostatin analogs.
Multivariate analysis revealed that adjuvant therapy did not affect
survival. Nussbaum et al (20) conducted one of the most prominent
studies investigating adjuvant chemotherapy, evaluating 4,612
non-metastatic TC patients from the National Cancer Database
(NCDB). It was found that chemotherapy was associated with a trend
toward inferior OS, which was not statistically significant in the
propensity score match analysis. Two other large-scale studies in
the NCDB identified that patients receiving chemotherapy had worse
survival rates than those who did not receive it (16,17).
In a single-center study evaluating patients with node-positive TC,
univariate analysis revealed that (neo)adjuvant chemotherapy was
associated with inferior OS (25).
Recently, He et al (18)
published a study that included 1,702 TC patients from the
Surveillance, Epidemiology, and End Results database. It was
identified that patients who received chemotherapy had shorter
cancer-specific survival than those who did not receive it
(18). These studies support the
present findings that chemotherapy does not improve survival, even
though it may be harmful in non-metastatic disease. Although the
aforementioned studies provided the scientific evidence that
underlies the recommendations contained in international treatment
guidelines, a significant limitation to these studies is that they
do not report the regimens used or the duration of the chemotherapy
administration. In this context, to the best of our knowledge, this
is the first study demonstrating the results of administering a
platin-based chemotherapy regimen to patients.
After entering the cell, platinum compounds interact
with the purine bases of DNA, resulting in interstrand cross-links
(26). Adjuvant platin-based
chemotherapy combinations are standard therapies in numerous
aggressive, rapidly proliferative solid organ tumors, such as lung
cancer. However, TC is a well-differentiated, low-grade tumor,
which can explain the low efficacy of platin agents in this
setting. In addition, genomic alterations in DNA repair pathways,
such as BRCA 1/2, which cause cancer to be more sensitive to platin
agents, were not observed in neuroendocrine tumors (27,28).
Considering these data and the adverse effects of platinum-based
combination regimens, clinicians should offer adjuvant platin-based
chemotherapy only to selected patients, such as patients with
recurrent or N3 disease.
Recent studies have reported that older age, left
side and high ki-67 index were poor prognostic factors (29-31).
In addition, female patients are expected to have improved outcomes
due to the protective effect of progesterone and estrogen via
regulating immune cell response and suppressing tumor growth in
mainly low-grade neuroendocrine neoplasms (32,33).
The low recurrence risk in our study group, possibly due to a lower
median age than is found in the literature, more right-side tumors,
and female predominance, may be the reasons that no benefits of
adjuvant chemotherapy were found.
Although chemotherapy is not suggested for patients
with resected non-metastatic TC according to the current
guidelines, a clinician survey conducted by Mansoor et al
(34) indicated that 11% of
respondents considered offering adjuvant treatment after surgical
resection in patients with node-positive non-metastatic TC.
Therefore, RCTs should be conducted to investigate the efficacy of
new agents, including immunotherapy and targeted therapy, by
detecting the genomic alterations that underlie the disease.
LC is the only neuroendocrine neoplasm that does not
have a specific staging system (9). Nevertheless, the number of
publications asserting the limitations of TNM staging in LC has
been increasing (12,35-37).
Combining TNM staging and histopathological features, such as
grade, tumor size and Ki-67 index, was reported to improve the
prediction of cancer-specific survival (37-40).
In addition, nomograms and prognostic scores have been developed to
predict survival more accurately (5,18,41).
However, updated international guidelines recommend using the
eighth TNM staging system (8,9,24)
since it is the most important prognostic parameter after
histological grade (12). Several
recent studies support the present findings, indicating the
prognostic value of TNM staging (42,43).
Surgery is the primary treatment modality for TC
(8,9,24).
The surgical approach aims to achieve complete resection of the
tumor with parenchymal-preserving anatomic lung resection and lymph
node dissection (44). Surgeons
may select different surgical procedures according to the type,
stage and localization of the tumor and performance status of the
patient. The treatment guidelines for non-small-cell lung cancer
resection should be followed if a diagnosis of TC cannot be made
preoperatively or intraoperatively (44). Negative surgical margins should be
examined during surgery. The present findings support the idea that
patients who undergo optimal resection that achieves negative
surgical margins have excellent clinical outcomes.
There are several limitations to the present study,
including its retrospective design and the fact that it was
conducted in a single center. In addition, the number of patients
receiving chemotherapy was low due to the recommendations of
international treatment guidelines. Moreover, the effect of
chemotherapy on OS could not be analyzed due to the limited number
of patients who succumbed.
In conclusion, the administration of platin-based
chemotherapy may not provide a survival benefit to patients with
non-metastatic TC. Therefore, clinicians should offer chemotherapy
only to carefully selected patients with a high recurrence risk. In
addition, the eighth edition of the TNM staging has prognostic
value in this population. Although optimal surgery has satisfying
long-term outcomes, RCTs studying new agents are needed in the
adjuvant setting to decrease the recurrence rate, particularly in
stage III disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ABS and HM designed the study. ABS, BO, SOO, AD, BC
and BE collected the data. ABS performed the analysis. TE, ASB, EC,
HM, BO and EUA made contributions to the interpretation of data.
ABS wrote the manuscript, while HM was a major contributor in
writing the manuscript. TE, ASB, EC, EUA, AD, SOO and BC edited the
manuscript. ABS, HM, BO, SOO and BE confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2021-5/19) by the Clinical Research Ethics Committee of Bursa
Uludag University Faculty of Medicine (Bursa, Turkey) and was
conducted in accordance with the 1964 Declaration of Helsinki.
Informed consent was not necessary due to the retrospective nature
of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hauso O, Gustafsson BI, Kidd M, Waldum HL,
Drozdov I, Chan AK and Modlin IM: Neuroendocrine tumor
epidemiology: Contrasting Norway and North America. Cancer.
113:2655–2664. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walters SL, Canavan ME, Salazar MC, Resio
BJ, Blasberg JD, Mase V and Boffa DJ: A National study of
surgically managed atypical pulmonary carcinoid tumors. Ann Thorac
Surg. 112:921–927. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG (eds): WHO Classification of Tumours of the Lung,
Pleura, Thymus and Heart. 4th edition. IARC Press, Lyon, 2015.
|
|
5
|
Dong S, Liang J, Zhai W and Yu Z:
Development and validation of an individualized nomogram for
predicting overall survival in patients with typical lung carcinoid
tumors. Am J Clin Oncol. 43:607–614. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Petursdottir A, Sigurdardottir J,
Fridriksson BM, Johnsen A, Isaksson HJ, Hardardottir H, Jonsson S
and Gudbjartsson T: Pulmonary carcinoid tumours: Incidence,
histology, and surgical outcome. A population-based study. Gen
Thorac Cardiovasc Surg. 68:523–529. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chong CR, Wirth LJ, Nishino M, Chen AB,
Sholl LM, Kulke MH, McNamee CJ, Jänne PA and Johnson BE:
Chemotherapy for locally advanced and metastatic pulmonary
carcinoid tumors. Lung Cancer. 86:241–246. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baudin E, Caplin M, Garcia-Carbonero R,
Fazio N, Ferolla P, Filosso PL, Frilling A, de Herder WW, Hörsch D,
Knigge U, et al: Lung and thymic carcinoids: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up*. Ann Oncol.
32:439–451. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
National Comprehensive Cancer Network.
Neuroendocrine and Adrenal Tumors. Version 4.2021. 2021. Available
from: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf.
|
|
10
|
Thomas C: Lung Neuroendocrine (carcinoid)
Tumors: Treatment and Prognosis. UpToDate. 2021. Available from:
https://www.uptodate.com/contents/lung-neuroendocrine-carcinoid-tumors-treatment-and-prognosis.
|
|
11
|
Bini A, Brandolini J, Cassanelli N, Davoli
F, Dolci G, Sellitri F and Stella F: Typical and atypical pulmonary
carcinoids: Our institutional experience. Interact Cardiovasc
Thorac Surg. 7:415–418. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baudin E, Hayes AR, Scoazec JY, Filosso
PL, Lim E, Kaltsas G, Frilling A, Chen J, Kos-Kudła B, Gorbunova V,
et al: Unmet medical needs in pulmonary neuroendocrine (Carcinoid)
neoplasms. Neuroendocrinology. 108:7–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Das S, Al-Toubah T and Strosberg J:
Chemotherapy in neuroendocrine tumors. Cancers (Basel).
13(4872)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Espinosa-Olarte P, La Salvia A,
Riesco-Martinez MC, Anton-Pascual B and Garcia-Carbonero R:
Chemotherapy in NEN: Still has a role? Rev Endocr Metab Disord.
22:595–614. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Filosso PL, Oliaro A, Ruffini E, Bora G,
Lyberis P, Asioli S, Delsedime L, Sandri A and Guerrera F: Outcome
and prognostic factors in bronchial carcinoids: A single-center
experience. J Thorac Oncol. 8:1282–1288. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gosain R, Groman A, Yendamuri SS, Iyer R
and Mukherjee S: Role of adjuvant chemotherapy in pulmonary
carcinoids: An NCDB analysis. Anticancer Res. 39:6835–6842.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Westin GF, Alsidawi S, Leventakos K,
Halfdanarson TR and Molina JR: Impact of adjuvant chemotherapy in
non-metastatic node positive bronchial neuroendocrine tumors
(BNET). J Clin Oncol. 35 (Suppl 15)(S8533)2017.
|
|
18
|
He Y, Zhao F, Han Q, Zhou Y and Zhao S:
Prognostic nomogram for predicting long-term cancer-specific
survival in patients with lung carcinoid tumors. BMC Cancer.
21(141)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vansteenkiste J: Pulmonary carcinoid: A
rare thoracic malignancy, a high need for better defined systemic
therapy. Ann Oncol. 26:1527–1529. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nussbaum DP, Speicher PJ, Gulack BC,
Hartwig MG, Onaitis MW, D'Amico TA and Berry MF: Defining the role
of adjuvant chemotherapy after lobectomy for typical
bronchopulmonary carcinoid tumors. Ann Thorac Surg. 99:428–434.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Melek H, Çetinkaya G, Özer E, Yentürk E,
Sevinç TE, Bayram AS and Gebitekin C: Pathological complete
response after neoadjuvant/induction treatment: Where is its place
in the lung cancer staging system? Eur J Cardiothorac Surg.
56:604–611. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (eighth) edition of the TNM Classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events (CTCAE) Version 5.0
[Internet]. 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
|
|
24
|
Singh S, Bergsland EK, Card CM, Hope TA,
Kunz PL, Laidley DT, Lawrence B, Leyden S, Metz DC, Michael M, et
al: Commonwealth neuroendocrine tumour research collaboration and
the North American neuroendocrine tumor society guidelines for the
diagnosis and management of patients with lung neuroendocrine
tumors: An International collaborative endorsement and update of
the 2015 European Neuroendocrine Tumor Society Expert Consensus
Guidelines. J Thorac Oncol. 15:1577–1598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Girelli L, Casiraghi M, Sandri A, Petrella
F, Galetta D, Gasparri R, Maisonneuve P, Fazio N and Spaggiari L:
Results of surgical resection of locally advanced pulmonary
neuroendocrine tumors. Ann Thorac Surg. 112:405–414.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Johnstone TC, Park GY and Lippard SJ:
Understanding and improving platinum anticancer
drugs-Phenanthriplatin. Anticancer Res. 34:471–476. 2014.PubMed/NCBI
|
|
27
|
Rottenberg S, Disler C and Perego P: The
rediscovery of platinum-based cancer therapy. Nat Rev Cancer.
21:37–50. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pulvirenti A, Raj N, Cingarlini S, Pea A,
Tang LH, Luchini C, Chou JF, Grego E, Marinova I, Capanu M, et al:
Platinum-based treatment for well- and poorly differentiated
pancreatic neuroendocrine neoplasms. Pancreas. 50:138–146.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Patané AK, Guma G, Rayá M, Rosales A,
Astorino W and Rosenberg M: Pulmonary neuroendocrine carcinoid
tumors : Is there a predictive role to the Ki 67 index? Ann Thorac
Med. 16:274–279. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
La Salvia A, Persano I, Siciliani A,
Verrico M, Bassi M, Modica R, Audisio A, Zanata I, Trabalza
Marinucci B, Trevisi E, et al: Prognostic significance of
laterality in lung neuroendocrine tumors. Endocrine. 76:733–746.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Naheed S, Holden C, Tanno L, Pattini L,
Pearce NW, Green B, Jaynes E, Cave J, Ottensmeier CH and Pelosi G:
Utility of KI-67 as a prognostic biomarker in pulmonary
neuroendocrine neoplasms: A systematic review and meta-analysis.
BMJ Open. 12(e041961)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Muscogiuri G, Barrea L, Feola T, Gallo M,
Messina E, Venneri MA, Faggiano A and Colao A: NIKE (Neuroendocrine
Tumors, Innovation inKnowledge, Education) Group. Pancreatic
neuroendocrine neoplasms: Does sex matter? Trends Endocrinol Metab.
31:631–641. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abdel-Rahman O, Ghosh S and Fazio N:
Sex-based differences in the outcomes of patients with lung
carcinoids. J Comp Eff Res. 11:523–531. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mansoor W, Ferguson S, Ross V and Talbot
D: Diagnostic and management pathways for pulmonary carcinoid
tumours in the United Kingdom: Results from the National lung
neuroendocrine tumour pathway project. Int J Endocrinol.
2020(9287536)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Melosky B: Advanced typical and atypical
carcinoid tumours of the lung: Management recommendations. Curr
Oncol. 25 (Suppl 1):S86–S93. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gagliardi I, Tarquini M, Ambrosio MR,
Giannetta E, Borges de Souza P, Gafà R, Carnevale A, Franceschetti
P and Zatelli MC: NEP-Score thresholds predict survival of patients
with bronchial carcinoids. Front Endocrinol (Lausanne).
11(621557)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cattoni M, Vallières E, Brown LM,
Sarkeshik AA, Margaritora S, Siciliani A, Filosso PL, Guerrera F,
Imperatori A, Rotolo N, et al: Improvement in TNM staging of
pulmonary neuroendocrine tumors requires histology and regrouping
of tumor size. J Thorac Cardiovasc Surg. 155:405–413.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yi C, Dai J, Song N, Wu C, Zhang L, Zhu Y,
Jiang G, Zhang H and Zhang P: Improvement of pathological staging
system for neuroendocrine tumors of the lung. Ann Transl Med.
9(447)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dermawan JKT and Farver CF: The role of
histologic grading and Ki-67 index in predicting outcomes in
pulmonary carcinoid tumors. Am J Surg Pathol. 44:224–231.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Moran CA, Lindholm KE, Brunnström H,
Langman G, Jang SJ, Spagnolo D, Chai SM, Laycock A, Falconieri G,
Pizzolitto S, et al: Typical and atypical carcinoid tumors of the
lung: A clinicopathological correlation of 783 cases with emphasis
on histological features. Hum Pathol. 98:98–109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chiappetta M, Sperduti I, Ciavarella LP,
Leuzzi G, Bria E, Mucilli F, Lococo F, Filosso P, Ratto G,
Spaggiari L, et al: Prognostic score for survival with pulmonary
carcinoids: The importance of associating clinical with
pathological characteristics. Interact Cardiovasc Thorac Surg.
31:315–323. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Georgakopoulou VE, Zygouris E, Nikokiris
C, Damaskos C, Pierrakou A, Garmpis N, Garmpi A, Sklapani P,
Aravantinou A, Trakas N, et al: Predictive indicators of survival
in patients with surgically resected lung carcinoid tumors at a
greek medical center. Cureus. 12(e10300)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
He H, Guo W, Song P, Liu L, Zhang G, Wang
Y, Qiu B, Tan F, Xue Q and Gao S: Preoperative systemic
immune-inflammation index and prognostic nutritional index predict
prognosis of patients with pulmonary neuroendocrine tumors after
surgical resection. Ann Transl Med. 8(630)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Caplin ME, Baudin E, Ferolla P, Filosso P,
Garcia-Yuste M, Lim E, Oberg K, Pelosi G, Perren A, Rossi RE, et
al: Pulmonary neuroendocrine (carcinoid) tumors: European
neuroendocrine tumor society expert consensus and recommendations
for best practice for typical and atypical pulmonary carcinoids.
Ann Oncol. 26:1604–1620. 2015.PubMed/NCBI View Article : Google Scholar
|