Introduction
Acute myeloid leukemia (AML) is a disorder of
hematopoietic stem cells that occurs due to genetic alterations
characterized by overproduction of neoplastic clonal myeloid stem
cells (1). Over the past 28 years,
the global incidence of AML has increased by 87.3% from
63.84x103 cases in 1990 to 119.57x103 cases
in 2017(2), with a mortality rate
of ~3.2% (3). Despite advances in
treatment, therapeutic options for AML are limited to high-dose
cytotoxic chemotherapy. The 5-year survival rate of pediatric acute
myeloid leukemia (AML) with chemotherapy alone remains low
(4). In addition, there is the
problem of limited resources for the frequently complex and costly
associated treatments. Over the past 20 years, high-dose
chemotherapy (HDCT) with cytarabine has been administered to
pediatric patients with AML in the First Affiliated Hospital of
Guangzhou Medical University, and the efficacy with respect to
5-year and longer overall survival (OS) and event-free survival
(EFS) in children with AML has been assessed. The introduction of
this treatment regimen was based on the study by Herzig et
al (5), where high-dose Ara-C
was applied in the maximally tolerated regimen of 3 g/m2
every 12 h for 6 days for refractory leukemia and 70% of these
patients responded, with 51% complete remissions. The present study
aimed to investigate the efficacy of the regimen for pediatric
patients with AML, providing an actionable and effective option for
physicians as well as patients.
Patients and methods
Patients
A total of 15 patients [9 male and 6 female; age
range, 1.2 to 12 years (median 6.7 years)], who had been diagnosed
with AML in the First Affiliated Hospital of Guangzhou Medical
University (Guangzhou, China) were enrolled and administered HDCT
with cytarabine between June 2002 and May 2015 (Table I) after obtaining informed consent
from their parent's or legal guardians. Patients diagnosed with
AML-M3, AML with Down's syndrome or secondary AML were excluded.
All patients presented with a high initial white blood cell count
of ≥100x109/l (normal count range,
4.5-11.0x109/l) and symptoms of bone pain, fever,
localized swelling or weakness. Patients who received complete HDCT
with a dose of 108 g/m2 were included in the final
analysis. The trial was approved by the Ethics Committee of the
First Affiliated Hospital of Guangzhou Medical University and
conducted in compliance with the Declaration of Helsinki.
 | Table ITesting for the 43 fusion genes in 15
patients with acute myeloid leukemia. |
Table I
Testing for the 43 fusion genes in 15
patients with acute myeloid leukemia.
| Case | Sex | Admission date | Onsetage, years |
Classificationa | Immunophenotype | Chromosome
analysis | Fusion gene | High-dose Ara-C,
g/m2 | Outcome at 2022
(>5 years) |
|---|
| 1 | Male | June 2002 | 8 | M2 | Juvenile cells mainly
expressed CD13 73.24%, HLA-DR 64.76%, HLA-DR/CD13 59.40%, CD19/CD13
0.33%, CD19/CD10 0.2%, CD22 0.12% | Unchecked | Negative for
AML1/ETO | 108 | Survival |
| 2 | Male | February 2003 | 5 | M2 | CD19 42.92%, CD13
91.67%, HLA-DR 80.87%, HLA-DR/CD13 77.63%, CD33/CD13 23.99%,
CD15/CD14 0.24%, CD19/CD13 76.32% | Unchecked | Negative for
AML1/ETO | 108 | Survival |
| 3 | Male | May 2003 | 12 | M4EO | Juvenile cells mainly
expressed CD13 99.38%, HLA-DR 56.04%, HLA-DR/CD13 53.6%, CD14
43.07%, CD34 11.28%, CD34/CD14 0.39%, MPO 47.63%, MPO/CD34
21.85% | Negative | Negative for
AML1/ETO | 108 | Survival |
| 4 | Female | November 2005 | 3 | M1 | CD7 71%, HLA-DR
40.21%, CD13 81.22%, MPO/CD13 59.22% | Unchecked | Negative for BCR-ABL,
and AML1/ETO | 108 | Survival |
| 5 | Male | January 2006 | 10 | M2 | Juvenile cells mainly
expressed CD7 51.36%, HLA-DR/CD13 25.71%, HLA-DR 56.53%, CD13
87.76% | Unchecked | Negative for BCR-ABL,
and AML1/ETO | 108 | Survival |
| 6 | Male | February 2006 | 6 | M2 | Juvenile cells mainly
expressed CD13 91.1%, CD117 4.78%, HLA-DR/CD15 75.23%, MPO
7.8% | Negative | Positive for AML1/
ETO (>60%) | 108 | Survival |
| 7 | Male | February 2007 | 7 | M2 | Juvenile cells
(65.57% of karyocytes) mainly expressed CD7 91.1%, CD33 4.78%,
HLA-DR/ CD7 75.23%, CD33/CD13 0.98% | Negative | Negative for CBFB,
and positive for AML1/ETO | 108 | Survival |
| 8 | Female | May 2008 | 8 | M2 | CD34/CD19 2.2%, CD34
5.9%, CD19 2.7%, HLA-DR/CD13 3.6%, CD15 1.5% | Negative | Negative for 43
fusion genes | 108 | Survival |
| 9 | Male | February 2009 | 12 | M4 | Juvenile cells 64.1%,
CD9 35.3%, CD11b 3.6%, CD34 57.4%, HLA-DR/CD13 54.5% | Negative | Negative for 43
fusion genes | 108 | Survival |
| 10 | Male | March 2010 | 8 | M1 | CD9 35.3%, CD11b
3.6%, CD34 57.4%, HLA-DR/CD13 54.5% | Negative | Negative for MLL | 108 | Survival |
| 11 | Female | October 2011 | 7 | M4 | CD13 43%, DR/CD13
40%, CD15/CD34 33%, CD34 50%, CD33 20% | Negative | Negative for
PML-RAra, negative for AML1/ETO | 108 | Chemotherapy- related
death |
| 12 | Female | December 2014 | 5 | M4 | One group of
CD45-and one group of dimSSC-low juvenile cells (38.2 and
30.3%) | Negative | Positive for CBF-β/
MYH11 | 108 | Survival |
| 13 | Male | April 2015 | 4 years and 2
months | M2 | CD34 72.5%, CD33
72.5%, CD13 71.7%, CD7 52.4%, HLA-DR7 4.3%, CD117 76.8%, CD15
43% | Negative | Negative for 43
fusion genes | 108 | Recurrence and
death |
| 14 | Male | May 2015 | 4 years and 2
months | M6 | CD15 0.8%, CD33
2.5%, HLA-DR 0.3%, CD34 0.3%, CD33 3.3%, CD15 1.1%, CD117 1.0%,
CD34 1.9%, MPO 0.8%, HLA-DR 1.9%, CD11b 1.0% | Negative | Negative for 43
fusion genes | 108 | Survival |
| 15 | Male | April 2012 | 2 | M5 | DR/CD13 21%,
CD15/CD34 15%, CD34 29%, CD34/CD14 8%, CD33/CD11b 23%, CD33 33%,
CD4 36% | Negative | Negative for 43
fusion genes | 108 | Recurrence and
death |
Treatment
Following standard induction therapy using a DAE
regimen [3 days of daunorubicin (40 mg/m2/day) for 3
consecutive days, cytarabine (100 mg/m2/day) for 7
consecutive days and etoposide (0.05-0.075/m2/day) for
10 consecutive days] (6),
consolidation therapy consisted of Ara-C (3 g/m2 twice
daily) from days 1 to 6 (total cumulative dose, 36
g/m2), followed by consolidation chemotherapy with Ara-C
(3 g/m2, every 12 h) on days 1, 3 and 5 of 3 consecutive
weeks (total cumulative dose, 108 g/m2.) After discharge
from the hospital, the patients regularly received maintenance
treatment for 3 years, with the dosage and intervals (up to every
3-6 months in the first year and every 6-12 months in the second
and third years) based upon the condition of the patient and
identification of remaining leukemia cells (minimal residual
disease). Re-examination was performed at 1 year after the start of
treatment.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Since fusion genes might provide targets for the
treatment and monitoring of myeloid leukemia, and some fusion genes
such as MLL/AF4 and AML1/MTG8 frequently appear in AML (7,8),
RT-PCR was performed in this study and the presence of abnormal
fusion genes was identified. RNA was extracted from tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed to cDNA. The cDNA was subjected to
amplification and qPCR to detect the 43 fusion genes that were
identifiable in leukemia at the time of the study, which was
performed by Kindstar Globalgene Technology Inc.
At the time of writing this study, all cases have
been followed up for 20 years (June 2002-December 2020).
Survival analysis
The survival data were plotted on a Kaplan-Meier
curve. EFS time was defined as the time from diagnosis to the last
follow-up visit without event. OS time was defined as the time from
the diagnosis to the last follow-up visit or the time of death from
any cause. Events included tumor recurrence (n=2), development of a
secondary malignancy (n=1), irreversible complications of
chemotherapy and death.
Results
The majority of cases were negative for the 43
fusion genes identifiable in leukemia at the time of the study,
with only two cases positive for the fusion protein AML1/ETO.
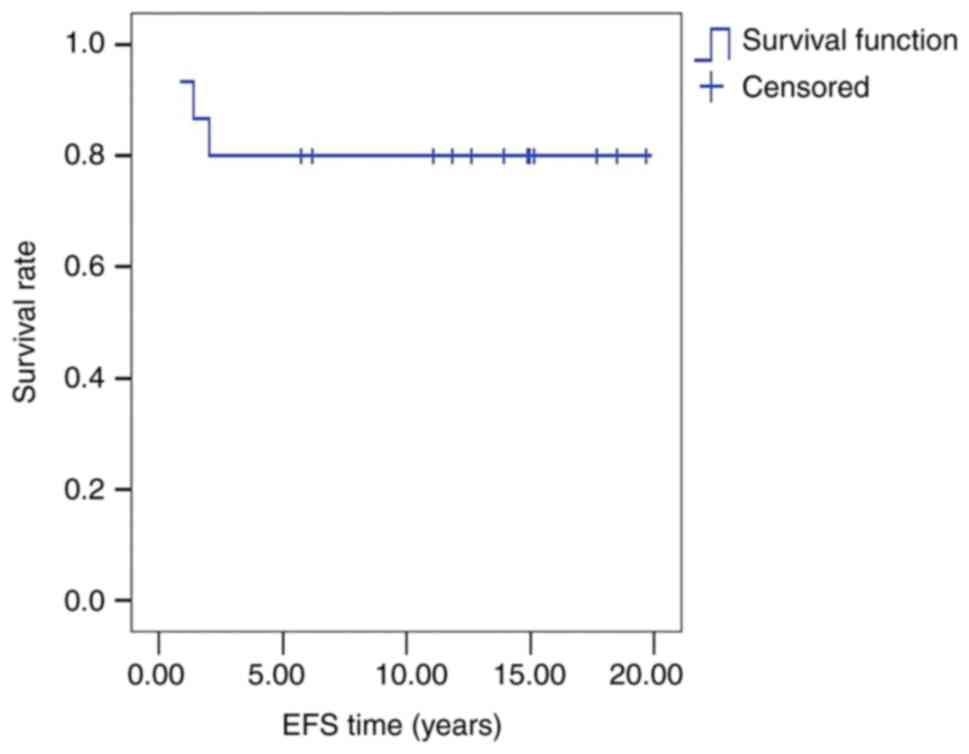
The 5-year OS and EFS rates of the 15 cases were
each 80±10% (Fig. 1). There were 2
cases of recurrence (13.3%) and 1 case of chemotherapy-related
death (6.7%) (Table I).
All cases had varying degrees of myelosuppression
after HDCT, as expected. Approximately one-half had fever, myalgia
and bone pain, and occasionally chest pain, maculopapular rash,
conjunctivitis and other symptoms 6-12 h after administration. The
general condition of the patients was satisfactory, although
antibiotics were not effective for patients with a high fever
(n=2), suggesting they had the rare post-cytarabine syndrome, which
is characterized by a fever, malaise, myalgia, arthralgia and/or a
rash (9). In this case,
corticosteroids were used for prevention and treatment.
Discussion
The prognosis of pediatric AML has been improving
over the years; however, in most collaborative groups, the 5-year
OS rate after chemotherapy alone remains at ~75% (10). Schweitzer et al (11) found that treatment using the
AML-Berlin-Frankfurt-Münster (BFM) 2004 protocol (not with
chemotherapy alone) yielded a 5-year OS rate as high as 70% in
patients with acute megakaryoblastic leukemia, where patients
received a randomized induction therapy of Ara-C, liposomal
daunorubicin (L-DNR) and etoposide. Similarly, Rubnitz et al
(12) reported 3-year OS and EFS
rates of 75 and 66%, respectively, in children aged 0 to 9 years
who underwent AML-02 treatment [high-dose cytarabine (3
g/m2 every 12 h on days 1, 3 and 5) or low-dose
cytarabine (100 mg/m2 every 12 h on days 1-10) plus
daunorubicin (50 mg/m2 on days 2, 4, and 6) and
etoposide (100 mg/m2 on days 2-6)]. The primary cause of
treatment failure in AML is the high risk of recurrence, and thus
increasing the intensity of consolidation treatment after remission
might be beneficial (13).
Notably, the application of high-dose Ara-C in the consolidation
phase has resulted in better outcomes (14).
In the present study, HDCT therapy was improved upon
by increasing the dosage of Ara-C and combining consolidation and
maintenance therapy. In 2002, treatment with Ara-C was started for
pediatric AML, using standard induction therapy followed by HDCT as
aforementioned. The cumulative dose of 108 g/m2
cytarabine is 3 times the maximum dosage suggested by Herzig et
al (1983) (5). A complete
regimen of HDCT achieved 5-year EFS and OS rates of 80%.
In recent years, the application of high-dose
cytarabine in the consolidation phase has resulted in better AML
treatment outcomes worldwide. The treatment regimens used by the
various collaborative groups are basically similar, and these have
been found to achieve a remission rate of ~90% (15).
Although only a small number of patients were
enrolled in the present study, the follow-up time was long at up to
20 years. This allowed a long-term assessment of efficacy, proving
the durability of the curative effect. One patient (male, 4 years
old, M2 type) was admitted to the hospital and received only one
course of treatment, in January 2001. The patient was withdrawn
from the therapy in the early intensive phase for economic reasons
and therefore was excluded from the analysis.
AML is still known as a threat to humans, and in
China, investigators keep exploring better therapeutic approaches
for the disorder. In recent years, some significant advances have
occurred in the treatment for AML, such as the addition of
gemtuzumab ozogamicin (GO) or bortezomib to traditional
chemotherapy, which have improved the prognosis (EFS, 48% with GO
vs. 29% without; OS, 63% with GO vs. 53% without) (16), but the outcomes are still not
satisfactory. Cytarabine, daunorubicin and etoposide (ADE) is an
effective induction regimen for pediatric patients with relapsed
AML, and a study by Garg et al obtained 2-year EFS and OS
rates of 29% (±7%) and 34% (±7%) at the first relapse, with a
complete remission rate of 66% (17).
HDAC improves OS and relapse-free survival rates in
induction therapy while reducing the relapse rate in consolidation
therapy, especially for the favorable-risk group. In one study,
patients randomly assigned HD cytarabine treatment obtained 6-year
OS rates of 42.5%, compared with 38.7% for those receiving standard
cytarabine (18). The median OS
time of patients with AML in a study also using high-dose
cytarabine in Brazil was 23.5 months for the M0, M1 and M2
subtypes, 97.7 months for M3, and 7.4 months for M4, M5, M6 and M7,
with a poor prognosis in most patients (19).
It has been noted that Ara-C in a dosage of 3
g/m2 twice daily provides a maximal therapeutic effect
in consolidation chemotherapy for adult patients with AML,
associated with grade 3-4 non-hematological toxicity (20), but the optimal dosage for pediatric
patients with AML is still controversial. Studies have evaluated
high-dose cytarabine in induction therapy for children with de
novo AML in Japan, adopting HD-ADE therapy, and suggested that
Ara-C in a dosage of 3 g/m2 twice daily obtains a good
outcome with improved disease-free survival rates (21). In Saudi Arabia, the 5-year OS rates
for the low-risk, intermediate-risk and high-risk groups were
72.0±6.9, 59.8±6.2 and 45.1±7.4%, and the EFS rates were 50.5±8.0,
46.3±6.4 and 23.3±6.4% (22).
Notably, the HDCT regimen developed in this study is
clinically safe and effective, with relatively low cost, as it
successfully reached 5-year EFS and OS rates of 80%. It should be
emphasized that this regimen not only has great therapeutic value,
but that also its EFS and OS rates for pediatric AML reach the
current international leading level, and even the international
leading academic level for hematological malignancies. HDCT seems
an optimal option for childhood leukemia with chemotherapy
alone.
According to a summary of treatments for AML from 8
international organizations involving the Children's Oncology
Group, Berlin-Frankfurt-Munster, the Italian Association of
Pediatric Hematology Oncology, and the European Organization for
Research and Treatment of Cancer, the 5-year EFS rate ranges from
55.5 to 77.7% (23-26).
This indicates that in the world, the highest 5-year EFS rate
reaches ~77%, which is the result of not only chemotherapy, but
also immune and targeted therapy. Thus similarly high or slightly
higher rates of long-term remission are achieved by more complex
regimens. However, these may not be available in numerous parts of
China, for various reasons, including difficulties in finding a
fully matched donor for transplantation, expense of medical care
and the presence of a number of complications. Another issue is
that AML is prone to relapse. As in most developing countries, most
Chinese people usually only accept chemotherapy for leukemia, so it
is imperative to seek a low-cost and efficient chemotherapy
regimen.
In conclusion, HDCT therapy is clinically safe,
effective and relatively low cost, and thus should be strongly
considered in limited access (remote) or underserved areas.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the privacy of the
patients, but are available from the corresponding author on
reasonable request.
Authors' contributions
ZiW designed the HDCT for pediatric AML and
organized this team to implement the regimen among patients with
AML hospitalized in the First Affiliated Hospital of Guangzhou
Medical University (Guangzhou, China), and wrote the draft of the
manuscript. ZeW is also an important contributor to the manuscript
and was responsible for some of the 20-year follow-up and data
analysis. As great contributors, YZ, JG, SW and DC applied HDCT in
clinical practice for over a decade and contributed to the
conception of the study. All authors read and approved the final
manuscript. ZiW, ZeW, YZ, JG, SW and DC confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
This study protocol was approved by the Ethics
Committee of the First Affiliated Hospital of Guangzhou Medical
University. Informed consent was obtained from the patients
parent's/legal guardians before initiation of the regimen, as this
study followed the Declaration of Helsinki on medical protocol and
ethics.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pelcovits A and Niroula R: Acute myeloid
leukemia: A review. R I Med J (2013). 103:38–40. 2020.PubMed/NCBI
|
|
2
|
Yi M, Li A, Zhou L, Chu Q, Song Y and Wu
K: The global burden and attributable risk factor analysis of acute
myeloid leukemia in 195 countries and territories from 1990 to
2017: Estimates based on the global burden of disease study 2017. J
Hematol Oncol. 13(72)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chennamadhavuni A, Lyengar V and
Shimanovsky A: Leukemia. In: StatPearls [Internet]. Treasure Island
(FL), StatPearls Publishing; 2022.
|
|
4
|
Song TY, Lee SH, Kim G, Baek HJ, Hwang TJ
and Kook H: Improvement of treatment outcome over 2 decades in
children with acute myeloid leukemia. Blood Res. 53:25–34.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Herzig RH, Wolff SN, Lazarus HM, Phillips
GL, Karanes C and Herzig GP: High-dose cytosine arabinoside therapy
for refractory leukemia. Blood. 62:361–369. 1983.PubMed/NCBI
|
|
6
|
Hwang WL, Young JH, Gau JP, Hu HT and Tsai
YT: DAE (daunorubicin, Ara-C, and etoposide) and intermediate dose
Ara-C for remission induction and consolidation treatment of adult
patients with acute myeloid leukemia. Am J Clin Oncol. 15:531–534.
1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gessner A, Thomas M, Castro PG, Büchler L,
Scholz A, Brümmendorf TH, Soria NM, Vormoor J, Greil J and
Heidenreich O: Leukemic fusion genes MLL/AF4 and AML1/MTG8 support
leukemic self-renewal by controlling expression of the telomerase
subunit TERT. Leukemia. 24:1751–1759. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakamura H, Kuriyama K, Sadamori N, Mine
M, Itoyama T, Sasagawa I, Matsumoto K, Tsuji Y, Asou N, Kageyama
SI, et al: Morphological subtyping of acute myeloid leukemia with
maturation (AML-M2): Homogeneous pink-colored cytoplasm of mature
neutrophils is most characteristic of AML-M2 with t(8;21).
Leukemia. 11:651–655. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jirasek MA and Herrington JD: Cytarabine
syndrome despite corticosteroid premedication in an adult
undergoing induction treatment for acute myelogenous leukemia. J
Oncol Pharm Pract. 22:795–800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Moore AS, Kearns PR, Knapper S, Pearson AD
and Zwaan CM: Novel therapies for children with acute myeloid
leukaemia. Leukemia. 27:1451–1460. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schweitzer J, Zimmermann M, Rasche M, von
Neuhoff C, Creutzig U, Dworzak M, Reinhardt D and Klusmann JH:
Improved outcome of pediatric patients with acute megakaryoblastic
leukemia in the AML-BFM 04 trial. Ann Hematol. 94:1327–1336.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rubnitz JE, Pounds S, Cao X, Jenkins L,
Dahl G, Bowman WP, Taub JW, Pui CH, Ribeiro RC, Campana D and Inaba
H: Treatment outcome in older patients with childhood acute myeloid
leukemia. Cancer. 118:6253–6259. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kaspers GJ, Zimmermann M, Reinhardt D,
Gibson BE, Tamminga RY, Aleinikova O, Armendariz H, Dworzak M, Ha
SY, Hasle H, et al: Improved outcome in pediatric relapsed acute
myeloid leukemia: Results of a randomized trial on liposomal
daunorubicin by the International BFM Study Group. J Clin Oncol.
31:599–607. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abrahamsson J, Forestier E, Heldrup J,
Jahnukainen K, Jónsson OG, Lausen B, Palle J, Zeller B and Hasle H:
Response-guided induction therapy in pediatric acute myeloid
leukemia with excellent remission rate. J Clin Oncol. 29:310–315.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rubnitz JE: Current management of
childhood acute myeloid leukemia. Paediatr Drugs. 19:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pollard JA, Guest E, Alonzo TA, Gerbing
RB, Loken MR, Brodersen LE, Kolb EA, Aplenc R, Meshinchi S,
Raimondi SC, et al: Gemtuzumab ozogamicin improves event-free
survival and reduces relapse in pediatric KMT2A-Rearranged AML:
Results from the phase III Children's Oncology Group Trial
AAML0531. J Clin Oncol. 39:3149–3160. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garg A, Ganguly S, Vishnubhatla S, Chopra
A and Bakhshi S: Outpatient ADE (cytarabine, daunorubicin, and
etoposide) is feasible and effective for the first relapse of
pediatric acute myeloid leukemia: A prospective, phase II study.
Pediatr Blood Cancer. 67(e28404)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Willemze R, Suciu S, Meloni G, Labar B,
Marie JP, Halkes CJ, Muus P, Mistrik M, Amadori S, Specchia G, et
al: High-dose cytarabine in induction treatment improves the
outcome of adult patients younger than age 46 years with acute
myeloid leukemia: Results of the EORTC-GIMEMA AML-12 trial. J Clin
Oncol. 32:219–228. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li W, Gong X, Sun M, Zhao X, Gong B, Wei
H, Mi Y and Wang J: High-dose cytarabine in acute myeloid leukemia
treatment: A systematic review and meta-analysis. PLoS One.
9(e110153)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Padilha SL, Souza EJ, Matos MC and Domino
NR: Acute myeloid leukemia: Survival analysis of patients at a
university hospital of Parana. Rev Bras Hematol Hemoter. 37:21–27.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu D, Duan C, Chen L and Chen S: Efficacy
and safety of different doses of cytarabine in consolidation
therapy for adult acute myeloid leukemia patients: A network
meta-analysis. Sci Rep. 7(9509)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jastaniah W, Al Ghemlas I, Al Daama S,
Ballourah W, Bayoumy M, Al-Anzi F, Al Shareef O, Alsultan A, Abrar
MB and Al Sudairy R: Clinical characteristics and outcome of
childhood de novo acute myeloid leukemia in Saudi Arabia: A
multicenter SAPHOS leukemia group study. Leuk Res. 49:66–72.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pession A, Masetti R, Rizzari C, Putti MC,
Casale F, Fagioli F, Luciani M, Lo Nigro L, Menna G, Micalizzi C,
et al: Results of the AIEOP AML 2002/01 multicenter prospective
trial for the treatment of children with acute myeloid leukemia.
Blood. 122:170–178. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Creutzig U, Zimmermann M, Dworzak MN,
Ritter J, Schellong G and Reinhardt D: Development of a curative
treatment within the AML-BFM studies. Klin Padiatr. 225 (Suppl
1):S79–S86. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Creutzig U, Zimmermann M, Bourquin JP,
Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B,
Lehrnbecher T, von Neuhoff C, et al: Randomized trial comparing
liposomal daunorubicin with idarubicin as induction for pediatric
acute myeloid leukemia: Results from Study AML-BFM 2004. Blood.
122:37–43. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cooper TM, Franklin J, Gerbing RB, Alonzo
TA, Hurwitz C, Raimondi SC, Hirsch B, Smith FO, Mathew P, Arceci
RJ, et al: AAML03P1, a pilot study of the safety of gemtuzumab
ozogamicin in combination with chemotherapy for newly diagnosed
childhood acute myeloid leukemia: A report from the Children's
Oncology Group. Cancer. 118:761–769. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arber DA: The 2016 WHO classification of
acute myeloid leukemia: What the practicing clinician needs to
know. Semin Hematol. 56:90–95. 2019.PubMed/NCBI View Article : Google Scholar
|