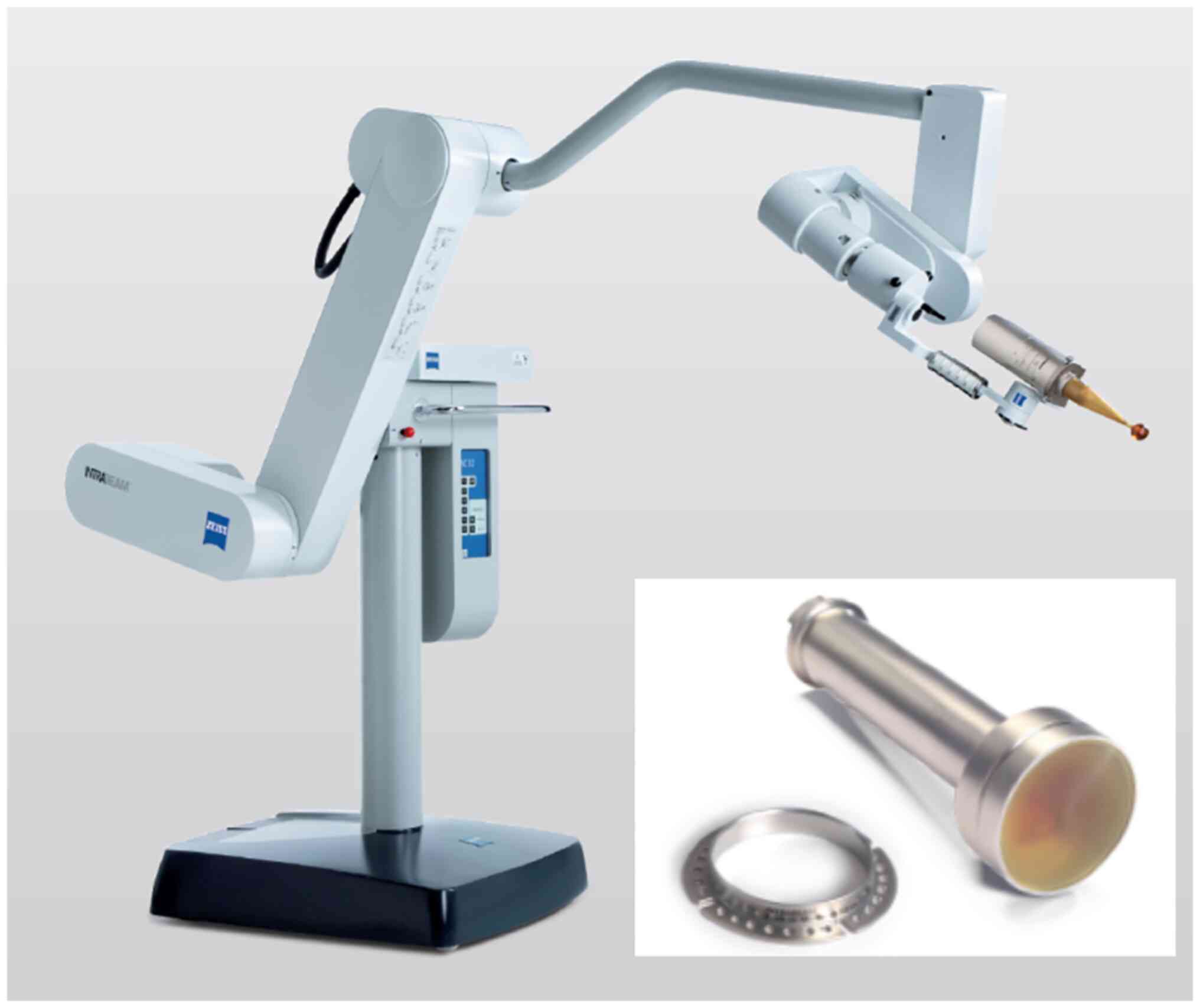
Introduction
A large proportion of head and neck cancer (HNC)
patients present with local or regionally advanced disease, as
signs and symptoms of early disease are often minimal and
nonspecific (1). Despite
technological advances in radiation therapy (RT) techniques, about
40% of local-regionally advanced HNC will persist or recur after
first line treatment, namely surgery and adjuvant or definitive
external beam radiation therapy (EBRT) with or without chemotherapy
(2). Local-regional recurrent HNC
are usually managed with surgery and or reirradiation (3). Reirradiation with EBRT of
local-regionally recurrent HNC comes with risk of treatment-related
toxicity, including death (4).
Alternatively, intraoperative radiation therapy (IORT) has been
shown to improve outcomes from salvage surgery in patients who
previously received EBRT; in the primary setting, IORT can be used
as a boost to optimize local control (5).
IORT generally delivers a single dose of radiation
at the time of resection of HNC (5). There are multiple modalities used for
IORT, including orthovoltage photons, electrons (EIORT), and
brachytherapy. This study includes HNC patients who received IORT
delivered via INTRABEAM (Carl Zeiss, Oberkochen, Germany).
The IORT doses are typically higher than the doses
delivered during individual fractions from EBRT, which reduces
tumor cell survival but may also worsen treatment-related toxicity
of normal tissue (5). However, the
low energy x-ray beam generated with the INTRABEAM source provides
a quick dose drop off which naturally limits the irradiation of
critical structures in an IORT setting. By restricting the
treatment volume to the surgical bed, the dose to surrounding
normal tissue is minimized, and thus the risk of treatment-related
toxicity is reduced. The purpose of this study is to present a
single institution experience with IORT for HNC patients.
Materials and methods
Patients
The present retrospective study was approved by the
institutional review board (IRB #214428) at Loyola University
Chicago. The present study that included all HNC patients treated
consecutively with IORT at Loyola University Medical Center between
January 2014 and December 2018. Patients with benign tumors
confirmed by pathology report were excluded. All patients were
treated per institutional protocols at the discretion of the
treating physician and were not part of any clinical trials.
Treatment
All patients received IORT in the operating room
with the INTRABEAM PRS 500 in a single fraction following the
resection of the tumor volume (6).
The INTRABEAM system consists of a low energy x-ray source (XRS), a
source positioning stand, and a mobile cart holding the XRS control
unit and user interface to set the treatment dose and monitor the
treatment delivery (Fig. 1). The
XRS contains a miniaturized accelerator designed to accelerate
electrons at 50 keV down a thin 10 cm long aluminum drift tube. The
electrons impinge on a thin gold target to emit bremsstrahlung
x-rays isotropically with a mean energy of approximately 20 keV to
provide a uniform dose distribution. The INTRABEAM floor stand
provides 6 degrees of freedom to hold and precisely position the
XRS onto the treatment area. The control unit is used to perform
quality assurance, set the prescription dose and monitor the system
performance during irradiation time.
Patients were initially seen before surgery by the
radiation oncologist to discuss all potential treatment options and
were then consented for IORT. Following the tumor resection by the
otolaryngology team, the radiation oncology team was called to
deliver IORT to the tumor bed. The INTRABEAM stand, XRS and
treatment console were brought into the operating room. First, the
XRS unit producing 50kV x-rays underwent quality assurance to
ensure appropriate straightness of XRS drift tube, and to measure
the daily output of the XRS. All IORT treatments were delivered
with Flat applicators (7). These
applicators are designed for superficial treatments with INTRABEAM
and contain a flattening filter that converts the spherical dose
distribution from the XRS into a homogenous planar dose
distribution at 5-9 mm depths, depending on the applicator size.
The median applicator size for all IORT treatments was 5 cm (range
3-6 cm) and was selected by the treating radiation oncologist to
fully cover the treatment target, a high-risk area within the tumor
bed that was highlighted with clips. Wet gauze was employed to
protect organs at risk (blood vessels and nerves) when applicable.
The INTRABEAM machine was draped and placed under sterile
conditions. The flat applicator was attached to the XRS unit on the
INTRABEAM floor stand. Finally, the applicator was placed against
the highlighted area at risk.
Dose conversion
It is common and accepted practice to assume a
relative biological effectiveness (RBE) value of 1 for high-energy
electrons in the range used in standard linear accelerators and the
Mobetron IORT system, with cell survival assays comparing high
energy electrons to reference photon beams of 6 MV and Co-60
showing no expectation for elevated RBE. In the case of the 50 kVp
X-rays produced by the INTRABEAM system, the low energy photons
would be expected to have an elevated RBE. While clinical data are
not available for elevated RBE of low energy photons in the
intraoperative setting, pre-clinical experiments using the
INTRABEAM system, low energy electrons, and radiobiology-based
computer simulations have been performed demonstrating an increased
expectation for elevated RBE. For 50 kV x-rays from INTRABEAM in
water-equivalent phantom at 8.1 mm from the applicator surface, the
RBE in reference to 6-MV photons ranges from 1.26 to 1.42 for 4
different cell lines (8).
Experiments and simulations evaluating the impact of low energy
electrons and photons show a clear increase in the damage potential
as the photon and electron energy is reduced (9-11).
While consensus data are premature, owing to the large difference
in beam quality between clinical IORT systems, a direct biological
equivalence of similar dose between platforms is unlikely. As such,
it is practical to assume a high likelihood for the INTRABEAM
system to have a higher RBE value than other IORT platforms. For
this report, all prescribed IORT doses were converted according to
the ‘TARGIT to V4.0’ calibration factors provided by Zeiss to
provide a more accurate estimation of the delivered dose to the
treatment targets as suggested by recent publications (12).
Analysis
Endpoints analyzed were overall survival,
local-regional recurrence, and distant metastasis. Recurrence was
defined as radiographic, clinical, or pathologic evidence of
recurrence. If recurrence was found at the IORT site, it was
considered local recurrence; if recurrence involved lymph nodes of
the neck, it was considered regional recurrence. Rates of overall
survival, local-regional recurrence and incidence of distant
metastases were calculated with the Kaplan-Meier method using IBM
SPSS Statistics 27 (IBM, Armonk, NY). The comparison between
upfront and salvage IORT groups and original site of disease was
performed with a two-sided log-rank test, where statistical
significance was defined for P<0.05.
Results
Patients
Between January 2014 and December 2018, 24 HNC
patients were treated consecutively with IORT at our institution.
This study included 23 of these HNC patients, as 1 patient was
excluded for a pathology report significant for a benign Warthin
tumor. Patients' median age at time of IORT was 66 years (range:
34-91 years). Per the surgical pathology report, the most common
histology was squamous cell carcinoma (SCC) (70%, 16 patients). Two
patients (9%) had salivary duct carcinoma, and one patient (4%) had
adenocarcinoma, favoring salivary duct carcinoma. One patient (4%)
had poorly differentiated carcinoma, possibly SCC. One patient (4%)
had acinic cell carcinoma. One patient (4%) had pleomorphic adenoma
(carcinoma in situ). One patient (4%) had mammary analogue
secretory carcinoma. Median size of the tumor on surgical pathology
was 2.7 cm (range 0.3-4.5 cm) (Table
I). 17% of patients were female, 83% were male. 13% were active
smokers, while 87% were former smokers. 9% had a greater-than-40
pack-year smoking history, 30% had a 20-40 pack-year smoking
history, 30% had an under-20 pack-year smoking history, and 30% did
not disclose a pack-year smoking history. 96% of patients
identified as white, and 4% (1 patient) identified as Asian
(Table II). 48% of patients (11
patients) received IORT upfront with or without postoperative
adjuvant external beam radiation therapy (EBRT), while 52% (12
patients) received salvage IORT after local tumor recurrence.
Patient tumor sites included parotid gland (10 individuals, 43%),
lymph nodes (10, 43%), oral tongue (2, 9%), and ear (1, 4%)
(Table I). Of the 23 patients, 6
patients had primary parotid disease, 1 patient had acinic cell
carcinoma, 1 patient had primary left temporal skin cancer, 5
patients had skin HNC with local-regional metastasis, 4 patients
had primary oropharyngeal/tongue disease, 3 patients had cancer to
the supraglottis, 1 patient had left supraclavicular and posterior
pharyngeal disease, 1 patient had right tragus disease, and 1
patient had left neck squamous cell carcinoma with unknown primary.
No patients had distant metastatic disease at the time of
presentation.
 | Table ITumor characteristics, treatment and
outcomes. |
Table I
Tumor characteristics, treatment and
outcomes.
| Patient no. | Tumor site | Histology | Tum or size, cm | Age at IORT,
years | IORT dose, Gy | Upfront vs.
salvage | Follow up
(months) | Time to local-
regional recurrence, months | Time to distant
metastasis, months |
|---|
| 1 | Right cervical
neck | SCC | 4 | 54 | 7.5 | Salvage | 22 | 11.00 | 11.00 |
| 2 | Right parotid | High grade salivary
duct carcinoma | 2.3 | 66 | 8 | Upfront | 70 | | |
| 3 | Oral tongue | Adenocarcinoma, favor
low grade, well- differentiated salivary duct carcinoma | 1.4 | 81 | 10 | Salvage | 38 | | 40.00 |
| 4 | Right parotid | Well-differentiated
SCC | 2 | 48 | 7.5 | Upfront | 50 | | |
| 5 | Left parotid | SCC | 4.2 | 91 | 8 | Upfront | 12 | | |
| 6 | Head and neck | SCC | 2.6 | 74 | 5 | Upfront | 11 | | |
| 7 | Left parotid | Poorly-differentiated
SCC | 3.1 | 80 | 5 | Upfront | 81 | | |
| 8 | Right neck node | Poorly-differentiated
SCC | 2.8 | 70 | 14 | Salvage | 28 | 26.00 | |
| 9 | Left parotid |
Moderately-differentiated SCC | 3.4 | 66 | 5 | Salvage | 36 | | |
| 10 | Left parotid | SCC | 4.5 | 59 | 5 | Upfront | 72 | | |
| 11 | Right parotid |
Poorly-differentiated carcinoma, possibly
SCC | 1.1 | 71 | 5 | Upfront | 76 | | |
| 12 | Right neck | SCC | 0.3 | 43 | 10 | Salvage | 6 | 4.00 | |
| 13 | Neck | SCC | 1.5 | 34 | 12 | Salvage | 2 | 1.00 | |
| 14 | Right neck
node | SCC | 5.6 | 64 | 12 | Salvage | 26 | | 26.00 |
| 15 | Left Neck | SCC | 3.1 | 72 | 12 | Salvage | 51 | | |
| 16 | Right neck | Acinic cell
carcinoma | 2.6 | 53 | 5 | Upfront | 7 | | 4.00 |
| 17 | Left
subclavian |
Poorly-differentiated SCC | 4.1 | 60 | 5 | Salvage | 6 | 6.00 | 6.00 |
| 18 | Oral tongue |
Moderately-differentiated SCC | 3 | 66 | 10 | Salvage | 5 | 5.00 | 5.00 |
| 19 | Left parotid | Salivary duct
carcinoma, poorly- differentiated, high grade | 2.6 | 66 | 5 | Upfront | 39 | 14.00 | 21.00 |
| 20 | Left parotid
bed | Pleomorphic
adenoma, carcinoma in situ | 1.8 | 66 | 5 | Upfront | 52 | | |
| 21 | Right parotid
bed | Mammary analogue
secretory carcinoma | 4.1 | 66 | 5 | Upfront | 56 | 34.00 | |
| 22 | Right ear | Well-differentiated
SCC | 1.2 | 80 | 14 | Salvage | 40 | | |
| 23 | Right neck | SCC | 2.9 | 86 | 12 | Salvage | 25 | 23.00 | 23.00 |
 | Table IIPatient demographics. |
Table II
Patient demographics.
| Demographic | Number (%) |
|---|
| Sex | |
|
Male | 19(83) |
|
Female | 4(17) |
| Smoking status | |
|
Active
smoker | 3(13) |
|
Former
smoker | 20(87) |
| Smoking history of
active and former smokers | |
|
>40-pack-years | 2(9) |
|
20-40
pack-years | 7(30) |
|
<20
pack-years | 7(30) |
|
Unknown | 7(30) |
| Race | |
|
White | 22(96) |
|
Asian | 1(4) |
Of the 23 patients, 8 patients (35%) had treatment
of unilateral parotid gland, 2 patients had treatment of unilateral
parotid bed, 5 were treated at unilateral neck, 1 at bilateral
neck, 2 at a singular right neck node, 1 at left supraclavicular
region, 1 patient received treatment to the head and neck, 2 at the
oral tongue, and 1 at right ear.
The median prescribed dose was 7.5 Gy (range: 5-14
Gy) prescribed to a depth of 5 mm. The corresponding median beam-on
time was 30.25 min (range: 12.02-54.80 min). The median resulting
dose at the surface of the treatment site was 13.68 Gy (range:
5.95-29.88 Gy). After the radiation was delivered, the flat
applicator was removed promptly.
Using ‘TARGIT to V4.0’ calibration factors resulted
in converted dose values averaging 15% higher (σ=0.7%), with a
median recalculated dose of 8.57 Gy (range 5.73-16.18 Gy). The
converted surface doses were 17% higher, with a median recalculated
dose of 15.87 Gy (range: 6.93-35.26 Gy).
After surgery, patients were hospitalized for
postoperative observation. Median duration of hospital stay was 2
days (range 1-9 days). Median follow up was 36 months (range 2-81
months), with follow up reported as the last office visit with any
physician.
Treatment outcomes
Twelve patients (52%) were alive at last follow up,
though four of these patients were considered lost to follow up
with last appointment dated more than two years prior to time of
analysis. Three patients (13%) had a status of alive with disease
(AWD) at last follow up (two of these patients were lost to follow
up), whereas nine patients (39%) were alive with no evidence of HNC
(NED) at last follow up (two of these patients were lost to follow
up). Two patients (9%) died with a status of NED (one patient was
diagnosed with primary bile duct carcinoma and declined further
treatment, and the other patient died from acute respiratory
failure due to COVID pneumonia). Nine patients (39%) died with
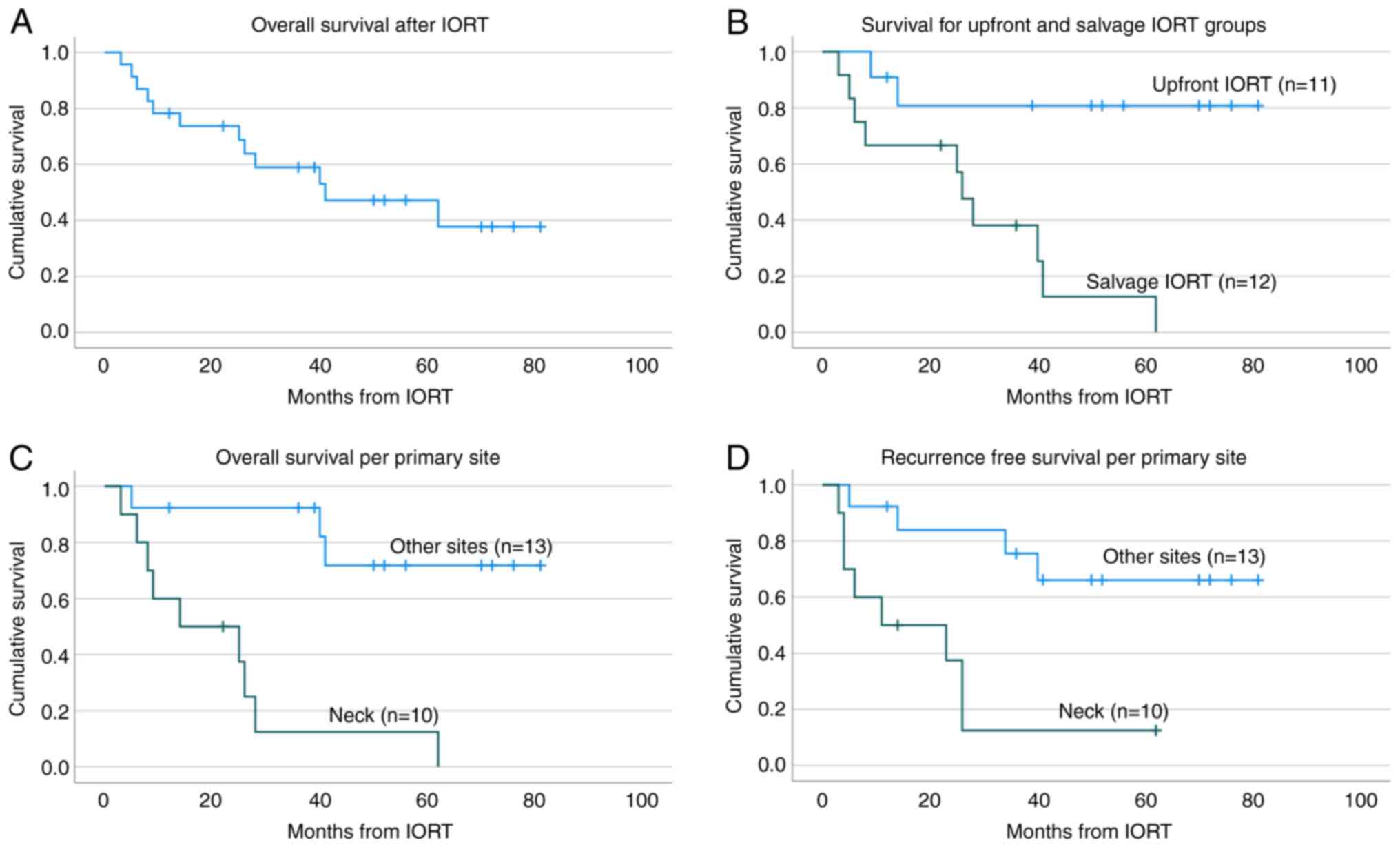
either persistent or recurrent disease (DWD). Overall survival is
presented in Fig. 2A.
For individuals receiving upfront vs. salvage IORT,
the cumulative proportion surviving at 1, 2, 3 and 4 years is 91,
81, 81, 81% respectively for upfront IORT and 67, 67, 38 and 16%
respectively for salvage IORT. Patients who received upfront IORT
had significantly improved overall survival compared to those who
received IORT as salvage therapy (Fig.
2B) (P=0.01). Of the eleven patients who received upfront IORT,
eight patients were NED and two patients were AWD at the time of
last follow up, though two of these patients were lost to follow
up. Of the twelve patients who received salvage IORT, one patient
was AWD and two patients were NED at the time of last follow up;
however, both of these patients were subsequently lost to follow
up.
For individuals receiving IORT at the primary site
(i.e., parotid, parotid bed, oral tongue) vs. the neck, the
cumulative proportion surviving at 1, 2, 3, and 4 years is 92, 92,
92, and 74% respectively for primary site IORT and 60, 49, 12 and
12% respectively for IORT involving the neck. Patients who received
IORT to primary site also had significantly improved overall
survival compared to those who received IORT to the neck, as shown
in (Fig. 2C) (P<0.001). The
distribution of origination sites was as follow: ten received IORT
to the neck (44%), eight to the parotid (35%), two to the parotid
bed (9%), two to the oral tongue (9%) and one to the ear (4%).
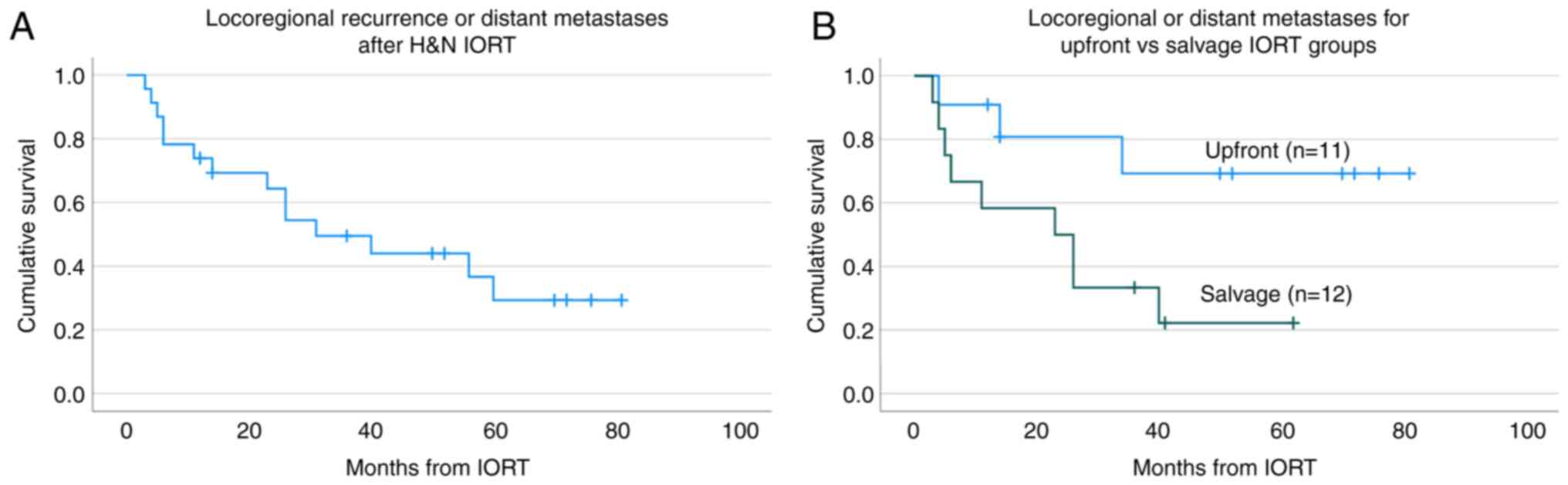
Fig. 3A presents
the incidence of local recurrence and of distant metastases. The
local-regional recurrence rate was 39% (median time to local
recurrence was 11 months, range 1-34 months), compared to a lower
35% rate of distant metastasis (median time to distant metastasis
was 16 months, range 4-40 months). 21% of the patient cohort had
both local-regional recurrence and distant metastases. Of the 8
patients with distant metastasis, 3 had bony metastasis, 5 had lung
metastasis. The percent of local-regional recurrence and distant
metastases among patients receiving salvage IORT was 58 and 50%
respectively, compared to 18, and 18% respectively in those
receiving upfront IORT with or without adjuvant EBRT. The incidence
rates of loco-regional recurrence and distant metastases was higher
for patients receiving salvage IORT compared to those receiving
IORT upfront (P=0.021), as shown in Fig. 3B.
Adverse events
There were no perioperative fatalities. One patient
(4%) developed postoperative acute thromboembolic stroke, and one
patient (4%) experienced protracted wound healing. 92% of patients
did not experience wound healing complications. No patients
developed evidence of carotid blowout, osteonecrosis or bone
fracture.
Discussion
In select HNC patients who are surgical candidates
with local-regional recurrence of a resectable tumor, salvage
surgery with or without reirradiation is a good option (13). Despite multiple available treatment
modalities for recurrent squamous cell HNC, the prognosis remains
generally poor (14). This is
consistent with our data, which showed significantly higher overall
survival in patients undergoing upfront IORT compared to those
receiving salvage IORT. Furthermore, our data reflected that
overall survival was improved in IORT to primary sites compared to
IORT involving the neck nodes, which translates to poorer prognosis
compared to patients with local disease. For patients deemed good
candidates for salvage therapy, previously irradiated tissue poses
a challenge in treatment planning. Surgery is preferred for tumors
that are away from at-risk organs and are amenable to surgical
resection. Adverse events from reirradiation are a major concern,
however, reirradiation may be recommended in situations where
benefit outweighs risk (e.g., positive margins, perineural
invasion, lymphovascular invasion, or extranodal extension), or if
patients have unresectable, locally-recurrent HNC (15).
Hilal et al included 15 retrospective studies
in their review of IORT as a treatment for HNC, concluding that
while IORT seems to be a promising treatment modality for HNC, most
available literature remains from single institutions (5). IORT is considered in patients with
HNC recurrences for its advantage of decreasing treatment volume to
the site directly observed in the operating room and avoiding
organs at risk. This advantage is particularly valuable for neck
recurrences in which tumors close to critical structures or
surrounded by fibrosis from previous irradiation can be difficult
to resect.
Two of the 15 studies reviewed by Hilal et al
investigated electron IORT (EIORT) in the treatment of recurrent
HNC (5). One such study by Zeidan
et al reviewed 46 patients with parotid disease receiving a
single dose of 15 or 20 Gy, median dose 15-20 Gy, with median
follow up of 5.6 years and 3-year OS of 48% (16). This 3-year OS is greater than that
of our study 3-year OS of 38% for those receiving salvage IORT.
However, Zeidan et al also reported toxicity in 27% of
individuals, including 7% with vascular toxicity, 6% with
osteoradionecrosis, 4% with fistulas, 4% with flap necrosis, 2%
with wound dehiscence, and 1% with neuropathy (16). Our study reported only 9% of
patients with toxicity and no cases of vascular toxicity or
osteoradionecrosis. Differences in IORT modality (Mobetron EIORT
vs. INTRABEAM 50 kV x-rays) and in IORT dose (median dose 7.5 Gy
vs. 15-20 Gy) are possible factors contributing to differences in
toxicity between the Zeidan et al study and our study. Small
population size and variety of disease sites in our study also
could have affected outcomes.
Chen et al shared a retrospective study of a
single institution experience using IORT as salvage therapy for
recurrent HN cancer in 137 patients, revealing 3-year overall
survival rate of 36%, with acceptable rates of complications
(17). They concluded that IORT
was a promising treatment modality for salvage therapy of HNC. Our
study has 12 patients who underwent salvage IORT with 3-year
overall survival rate of 38%, a similar OS rate to that of Chen
et al but with minimal complications. Chen et al
report median IORT dose of 15 Gy (range 10-18 Gy) whereas our study
reports a median dose of 12 Gy (range 5-14 Gy) for those receiving
salvage IORT. While the accurate dosimetry of the INTRABEAM source
is still an active field of investigation, converting the
prescribed TARGIT dose to the recommended ‘V4.0’ dose provided a
more accurate estimate of the dose received by our patients' cohort
(12). Overall, the median
recalculated dose for those receiving IORT was increased by 15% to
13.68 Gy (range 5.73-16.18 Gy), which is a similar dose to that of
the Chen et al study. To our knowledge, this is the first
study to convert INTRABEAM IORT doses using the recommended ‘TARGIT
to V4.0’ calibration factors. Future prescription of IORT treatment
for HNC and retrospective analysis of HNC patients treated with
INTRABEAM should report treatment doses using the V4.0 dose
calibration protocol to allow for a meaningful comparison of
outcomes vs. treatment modalities for IORT, such as soft x-rays and
electron. The Chen et al study population was prescribed
EIORT with 6-18 MeV electron beams delivered with a modified linear
accelerator or 4-12 MeV of electron therapy with a Mobetron EIORT
unit (71% received 6 MeV), whereas our population was treated with
50 kV x-rays via INTRABEAM (6,18).
Chen et al stratified their rate of distant metastasis-free
survival by patients treated with salvage IORT to the primary tumor
site vs. IORT to disease sites in the neck, revealing 3-year
distant metastasis-free survival of 61 and 30%, respectively. While
65% of the total patients in our study were metastasis-free for the
duration of their follow up, due to small population size, we were
unable to perform statistical analysis for distant metastasis-free
survival by patients treated with salvage IORT stratified by site
of delivery. There are recent studies investigating the potential
immunotherapy role of IORT in preventing recurrence in breast and
pancreatic cancers (19,20), thus larger prospective studies
would be useful for analyzing locoregional recurrence and distant
metastasis in HNC patients who received IORT in the salvage
setting.
Due to the small population size and the fact that
six patient pathology reports were missing surgical margin data, we
were unable to analyze outcomes as they pertain to surgical margin
status. This limitation is relevant because multiple studies found
that positive microscopic margins decreased in-field control when
compared to negative margins (5,17).
For instance, Chen et al found that in-field control was
improved with negative margins compared to positive margins at 82%
vs. 48% at 3 years (17).
Our study analyzed data from patients who received
relatively low doses of IORT compared to that of other studies
discussed, even after applying dose-rate correction factors (median
dose 7.5 Gy, 8.57 Gy with dose-rate correction, compared to median
dose range of 15-20 Gy, respectively) (5,17).
Zeidan et al reported on data from a cohort of individuals
who received 15 or 20 Gy IORT doses in the salvage setting and had
a greater 3-year OS survival compared to our study (48% vs. 38%,
respectively), but had 27% of study participants with toxicity, 7%
with vascular toxicity and 6% with osteoradionecrosis, whereas our
study had 9% toxicity, none with vascular toxicity or
osteoradionecrosis. Chen et al reported a relatively lower
median dose of 15 Gy, which is similar to that of our study median
dose for those receiving salvage IORT, and they concluded that they
had acceptable rates of toxicities. Toita et al report
severe late complications with single IORT doses greater than 20 Gy
(21). Further data are needed to
explore this pattern suggestive of dose-dependent IORT-related
toxicity.
Since most patients in the study had received
radiation therapy prior to IORT, there is no meaningful way to
analyze adverse effects from IORT. However, only one patient
experienced protracted wound healing, and none of the patients in
the study developed evidence of adverse events at the IORT site
such as osteoradionecrosis, bone fracture or carotid blowout after
receiving IORT. The fact that this study population experienced
minimal complications after IORT points toward IORT at median dose
of 7.5 Gy (8.57 Gy with correction) being a safe modality for
treating individuals with HNC, regardless of prior radiation to the
site.
In conclusion, while overall survival for locally
advanced or recurrent HNC is limited, the results of our
retrospective study on patients with locally advanced and recurrent
HNC treated with IORT show that IORT using INTRABEAM 50kV x-ray is
a safe modality in this population with overall survival data
comparable to that of published IORT studies. Prospective
multi-center studies would be needed to further assess effect of
IORT on overall survival, locoregional recurrence, and distant
metastasis in the salvage setting.
Acknowledgements
The authors thank Professor Jim Sinacore (Department
of Public Health Sciences at Loyola University Chicago) for his
assistance with the statistical analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CC and TR designed the study and created the outline
for the manuscript. CC and SG performed the statistical analysis.
CC and SG confirm the authenticity of all the raw data. CC
generated Tables I and II. SG generated Fig. 1, Fig.
2 and Fig. 3. CC, SG, BL, AB
and TR analyzed and interpreted the data. BE, AS and WS acquired
and interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The institutional review board (IRB #214428) at
Loyola University Chicago approved the present study. No consent
was required as this was a retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vokes EE, Weichselbaum RR, Lippman SM and
Hong WK: Head and neck cancer. N Engl J Med. 328:184–194.
1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Denaro N, Merlano MC and Russi EG:
Follow-up in head and neck cancer: Do more does it mean do better?
A systematic review and our proposal based on our experience. Clin
Exp Otorhinolaryngol. 9:287–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vargo JA, Ferris RL, Ohr J, Clump DA,
Davis KS, Duvvuri U, Kim S, Johnson JT, Bauman JE, Gibson MK, et
al: A prospective phase 2 trial of reirradiation with stereotactic
body radiation therapy plus cetuximab in patients with previously
irradiated recurrent squamous cell carcinoma of the head and neck.
Int J Radiat Oncol Biol Phys. 91:480–488. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wong SJ and Spencer S: Reirradiation and
concurrent chemotherapy after salvage surgery: Pay now or pay
later. J Clin Oncol. 26:5500–5501. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hilal L, Al Feghali KA, Ramia P, Abu
Gheida I, Obeid JP, Jalbout W, Youssef B, Geara F and Zeidan YH:
Intraoperative radiation therapy: A promising treatment modality in
head and neck cancer. Front Oncol. 7(148)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sethi A, Emami B, Small W Jr and Thomas
TO: Intraoperative radiotherapy With INTRABEAM: Technical and
dosimetric considerations. Front Oncol. 8(74)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schneider F, Clausen S, Thölking J, Wenz F
and Abo-Madyan Y: A novel approach for superficial intraoperative
radiotherapy (IORT) using a 50 kV X-ray source: A technical and
case report. J Appl Clin Med Phys. 15(4502)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Q, Schneider F, Ma L, Wenz F and
Herskind C: Relative biologic effectiveness (RBE) of 50 kV X-rays
measured in a phantom for intraoperative tumor-bed irradiation. Int
J Radiat Oncol Biol Phys. 85:1127–1133. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sowa MB, Kathmann LE, Holben BA, Thrall BD
and Kimmel GA: Low-LET microbeam investigation of the track-end
dependence of electron-induced damage in normal human diploid
fibroblasts. Radiat Res. 164:677–679. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Raju MR, Carpenter SG, Chmielewski JJ,
Schillaci ME, Wilder ME, Freyer JP, Johnson NF, Schor PL, Sebring
RJ and Goodhead DT: Radiobiology of ultrasoft X Rays. I. cultured
hamster cells (V79). Radiat Res. 110:396–412. 1987.PubMed/NCBI
|
|
11
|
Lee BH: A Monte Carlo-Based Simulation
Study for Assessing Radiation-Induced Dna Damage and Cell Survival
(unpublished PhD thesis). Georg Inst Technol, 2017.
|
|
12
|
Watson PGF, Popovic M, Liang L, Tomic N,
Devic S and Seuntjens J: Clinical implication of dosimetry
formalisms for electronic low-energy photon intraoperative
radiation therapy. Pract Radiat Oncol. 11:e114–e121.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ward MC, Riaz N, Caudell JJ, Dunlap NE,
Isrow D, Zakem SJ, Dault J, Awan MJ, Vargo JA, Heron DE, et al:
Refining patient selection for reirradiation of head and neck
squamous carcinoma in the IMRT Era: A Multi-institution Cohort
Study by the MIRI Collaborative. Int J Radiat Oncol Biol Phys.
100:586–594. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goodwin WJ Jr: Salvage surgery for
patients with recurrent squamous cell carcinoma of the upper
aerodigestive tract: When do the ends justify the means?
Laryngoscope. 110 (3 Pt 2 Suppl 93):1–18. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Merlotti A, Mazzola R, Alterio D, Alongi
F, Bacigalupo A, Bonomo P, Maddalo M, Russi EG and Orlandi E: What
is the role of postoperative re-irradiation in recurrent and second
primary squamous cell cancer of head and neck? A literature review
according to PICO criteria. Crit Rev Oncol Hematol. 111:20–30.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zeidan YH, Shiue K, Weed D, Johnstone PA,
Terry C, Freeman S, Krowiak E, Borrowdale R, Huntley T and Yeh A:
Intraoperative radiotherapy for parotid cancer: A
single-institution experience. Int J Radiat Oncol Biol Phys.
82:1831–1836. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen AM, Bucci MK, Singer MI, Garcia J,
Kaplan MJ, Chan AS and Phillips TL: Intraoperative radiation
therapy for recurrent head-and-neck cancer: The UCSF experience.
Int J Radiat Oncol Biol Phys. 67:122–129. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wootton LS, Meyer J, Kim E and Phillips M:
Commissioning, clinical implementation, and performance of the
Mobetron 2000 for intraoperative radiation therapy. J Appl Clin Med
Phys. 18:230–242. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Linares-Galiana I, Berenguer-Frances MA,
Cañas-Cortés R, Pujol-Canadell M, Comas-Antón S, Martínez E,
Laplana M, Pérez-Montero H, Pla-Farnós MJ, Navarro-Martin A, et al:
Changes in peripheral immune cells after intraoperative radiation
therapy in low-risk breast cancer. J Radiat Res. 62:110–118.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee YS, Kim HS, Cho Y, Lee IJ, Kim HJ, Lee
DE, Kang HW and Park JS: Intraoperative radiation therapy induces
immune response activity after pancreatic surgery. BMC Cancer.
21(1097)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Toita T, Nakano M, Takizawa Y, Sueyama H,
Kakihana Y, Kushi A, Ogawa K, Hara R, Sunakawa H, Arasaki A, et al:
Intraoperative radiation therapy (IORT) for head and neck cancer.
Int J Radiat Oncol Biol Phys. 30:1219–1224. 1994.PubMed/NCBI View Article : Google Scholar
|