Introduction
Myoepitheliomas and mixed tumors of the head and
neck are well known, particularly in the salivary glands (1,2).
Myoepithelial tumors of soft tissue were first described in 1997
and have since been increasingly reported (1). Although myoepitheliomas were
initially thought to contain spindle or plasmacytoid cells growing
in solid sheets, it is now believed that myoepithelial and mixed
tumors are part of a spectrum of tumors with overlapping histologic
appearance and similar clinical behavior (2). For example, soft tissue mixed tumors
with ductal differentiation have rearrangements of PLAG1
(encoded on chromosome 8q12), which are characteristic of salivary
pleomorphic adenoma and carcinoma ex pleomorphic adenoma (3). In addition, rearrangement of the
EWSR1 gene (encoded on chromosome 22q), which occurs in
nearly half of all myoepithelial tumors of soft tissue, skin, and
bone, has been reported in up to 39% of primary salivary
myoepithelial carcinomas (MECs) exhibiting clear cell morphology
(3).
Soft tissue MEC is an extremely rare malignant
neoplasm demonstrating myoepithelial differentiation, cords or
nests of epithelioid, ovoid, or spindle cells with moderate or
severe atypia, and a variably reticular architecture with
chondromyxoid or collagenous/hyalinized stroma (2,4-6).
The mainstay of treatment for localized disease has been surgical
resection with adjuvant cytotoxic chemotherapy and/or radiation
therapy (RT) (2,4,5,7).
However, the relapse rate is approximately 30-45% (2,3).
Furthermore, without appropriate chemotherapy, the 5-year overall
survival rate was 14.6% in patients with locally advanced or
metastatic disease who received systemic therapy (4).
To our knowledge, the case reported herein is the
first case of MEC deeply seated in the trunk that was successfully
treated by chemotherapy with cytotoxic agents and proton beam
therapy (PBT).
Case report
A 38-year-old woman was referred to our department
with a 4-year history of low back pain. Examination revealed
tenderness of the L2 spinous process and left paravertebral
muscles. Kemp's test was positive on the left side, but there was
no sensory disturbance or muscle weakness in the lower extremities.
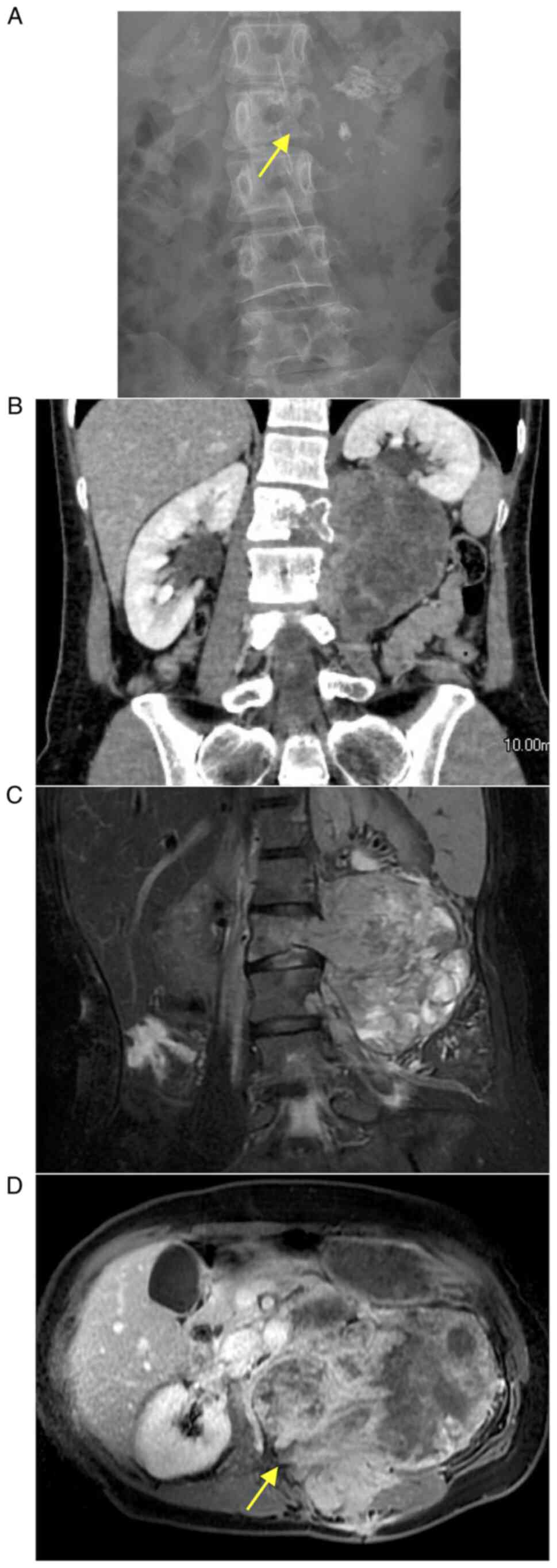
Plain radiographs obtained at the first visit showed the canonical
pedicle sign on the left side at L2 (Fig. 1A). Computed tomography (CT)
revealed a massive neoplasm mainly in the left paraspinal area at
L2-L3 with L2 vertebral destruction (Fig. 1B). Contrast-enhanced CT and
magnetic resonance images showed a soft tissue mass measuring
118x101x83 mm with areas of heterogenous intensity and spread into
the spinal canal (Fig. 1C and
D). Incisional biopsy confirmed
MEC (Fig. 2). Histologically, the
tumor was a poorly differentiated malignant neoplasm composed
mainly of cells having nuclear atypia with easily discernible
nucleoli and an epithelioid morphology with abundant clear or pale
cytoplasm associated with a prominent myxoid matrix.
Immunohistochemistry revealed extensive positivity for epithelial
membrane antigen (EMA) and focal positivity for S-100 protein with
negativity for AE1/AE3 and glial fibrillary acidic protein (GFAP).
Staining for INI-1 was negative with appropriate staining of
vascular endothelial cells serving as the internal control,
indicating that the product of this gene on the long arm of
chromosome 22 was lost or deleted. The appearance and
immunophenotype fit well with high-grade MEC, which was confirmed
by outside consultation. FDG PET/CT showed that the tumor had a
maximum standardized uptake value of 5.2. We anticipated that
radical resection would result in considerable morbidity, so the
patient was treated with a combination of doxorubicin and
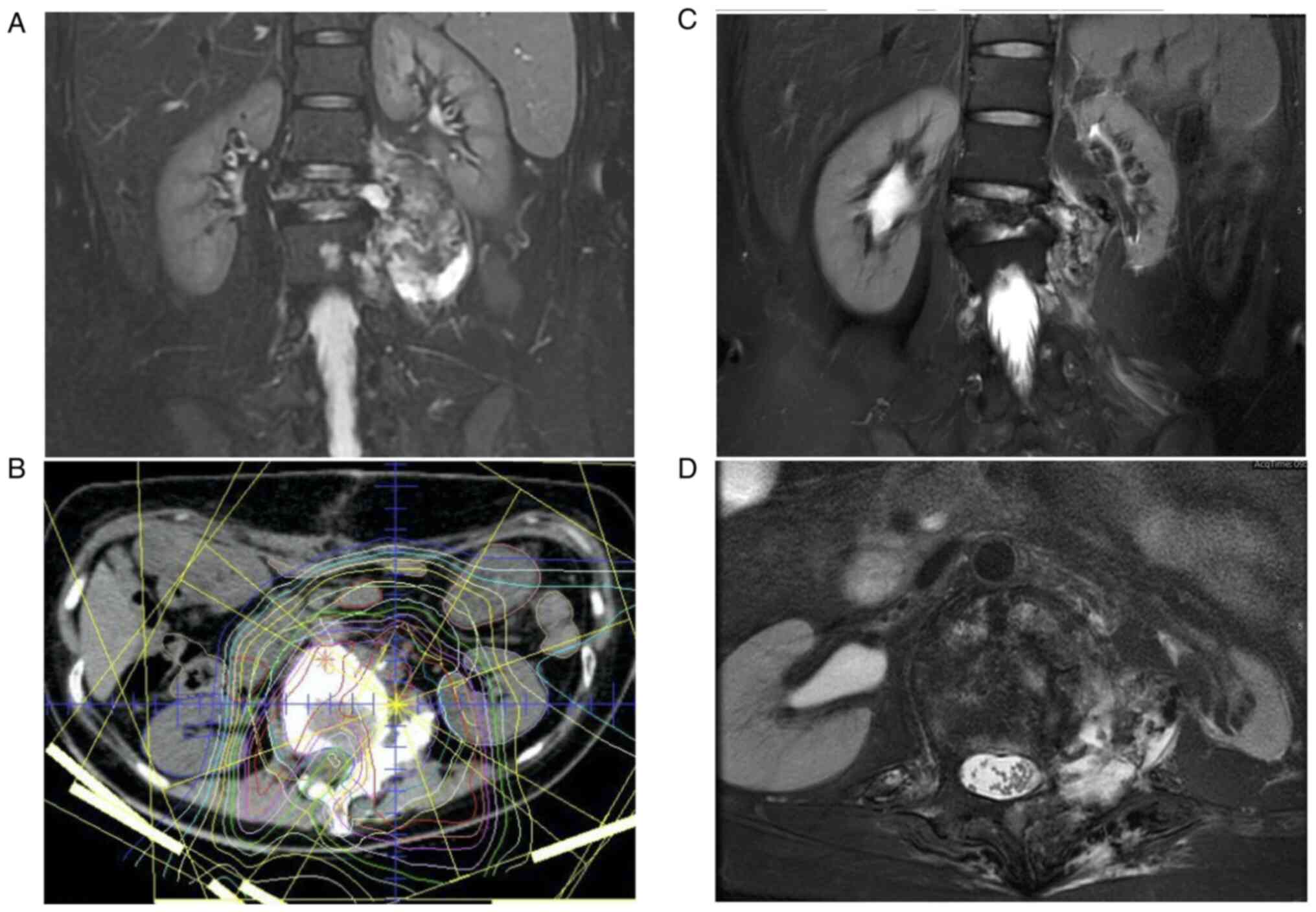
ifosfamide. After 4 courses, she showed a partial response based on
Response Evaluation Criteria in Solid Tumors (RECIST) (Fig. 3A). Despite the tumor size reduction
by chemotherapy, wide resection with clear margins was still
difficult. Therefore, definitive local PBT of 70.4 Gy (relative
biological effectiveness [RBE]) in 32 fractions was performed after
4 additional courses of systemic therapy (Fig. 3B), followed by another course of
the same regimen. In total, 507 mg/mm2 doxorubicin and
84.5 g/mm2 ifosfamide were administered. The patient
developed left L2-L3 nerve palsy 4.8 years later as late toxicity
of PBT but could still walk independently. Retrospectively, we
noted that collapse of the L2 vertebral body progressed during the
first 6 months of chemotherapy, but no serious neurological
disturbance occurred because it was possible to avoid the spinal
cord and cauda equina as much as possible in PBT due to adequate
dose distribution. As of this writing, she remains disease-free at
9 years after the initial diagnosis with an International Society
Of Limb Salvage (ISOLS) score of 77% and a Toronto Extremity
Salvage Score (TESS) of 67.2% (Fig.
3C and D).
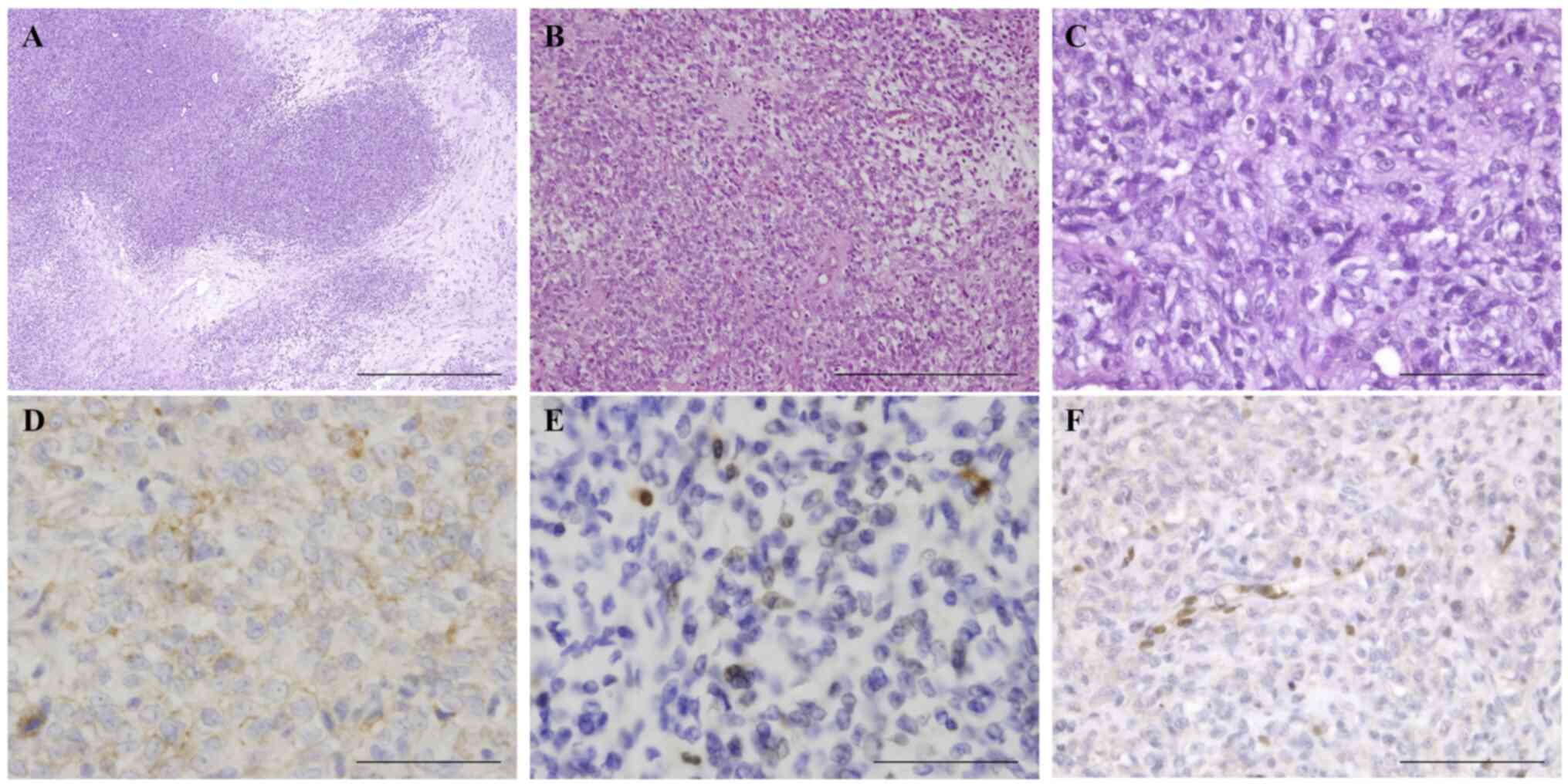 | Figure 2Histology and immunohistochemical
staining of the myoepithelial carcinoma. (A-C) Hematoxylin-eosin
staining reveals tumor cells showing a solid growth pattern with
focal myxoid stroma (A, scale bar, 1,000 mm; B, scale bar, 500 mm;
C, scale bar, 100 mm). Tumor cells show extensive positivity for
(D) epithelial membrane antigen (scale bar, 100 mm), (E) strong
focal positivity for S-100 protein (scale bar, 100 mm), (F) and
loss of INI-1 immunoreactivity (scale bar, 100 mm). |
Discussion
We report herein the first known case of MEC
originating in the trunk treated by a combination of canonical
cytotoxic chemotherapy and definitive PBT, which resulted in
long-term survival for at least 9 years. To our knowledge, only 2
cases of paraspinal MEC with follow-up after surgery have been
reported (6). In both of those
cases, the margin status was microscopically positive (R1),
suggesting that complete resection of paraspinal MEC would be
difficult.
Although not necessary in MEC, RT is often used in
an adjuvant setting. A systematic review found that 163 (32.3%) of
505 cases of MEC (including cases originating in a salivary gland)
received RT, which was administered as neoadjuvant therapy in 3
cases, adjuvant therapy (median dose, 60 Gy) in 110, and radical
treatment (median dose, 62 Gy) in 10(7). According to a literature review of
soft tissue MECs, 19 of 58 patients (32.8%) were treated with RT as
initial therapy (8). An analysis
of the Surveillance, Epidemiology, and End Results (SEER) registry
data showed an overall survival benefit with adjuvant RT in
high-grade MEC cases (9). However,
to our knowledge, there has been only 1 reported case in which ion
beam RT was used for MEC (10).
That patient received PBT at a total dose of 79.2 GyE in 36
fractions to treat a recurrent tumor in the maxillary sinus after
initial maxillectomy and survived for 30 months without relapse.
Given that report and our present case, PBT of over 70 Gy (RBE)
could be a promising definitive local treatment for inoperable
MEC.
There are limitations to systemic anticancer
chemotherapy as treatment for MEC. Chamberlain et al
reported a case series including 24 soft tissue MECs in adults
treated by a multidisciplinary team at a single institution
(4). Nine cases (37.5%) underwent
chemotherapy, of which 5 cases (55.6%) were treated with
doxorubicin alone or in combination as first-line treatment.
According to RECIST, the best response to these doxorubicin-based
regimens was a partial response in 1 patient, stable disease in 3,
and progressive disease in 1. Review articles have shown that 18.8
to 36.2% of patients with MEC received chemotherapy, though it did
not significantly decrease distant metastasis or local recurrence
(7,8). Even among 11 children who received
chemotherapy for metastatic or unresectable disease, a clinical
response was seen in only 1 case (5).
Our literature review clearly identified only 8
cases of MECs in soft tissue, bone, skin, or organs excluding the
salivary glands which showed a partial or complete response to some
form of cytotoxic chemotherapy (Table
I). Noronha et al reported a case with metastatic MEC
primarily originating in the vulva that had a complete response to
carboplatin and paclitaxel (11).
Rastrelli et al similarly reported a case of metastatic MEC
in which a complete response was achieved after locoregional and
systemic therapy using continuous infusion of ifosfamide (12). Another man with a soft tissue MEC
of the neck showed a partial response to 6 cycles of doxorubicin
after progression on carboplatin and capecitabine (4). Two children who showed a partial
response to combination chemotherapy were described by Bisogno
et al (13). Furthermore,
Mourtzoukou et al reported a 36-year-old man with metastatic
MEC arising as a primary tumor within the soft tissue of the neck
(14). Immunohistochemically, the
tumor showed loss of INI1 with no rearrangement of either
EWSR1 or FUS on fluorescence in situ
hybridization, and partial response was achieved by systemic
administration of doxorubicin. Hoggard et al also reported a
case of metastatic MEC showing partial response to carboplatin and
paclitaxel with a disease-free interval of more than 3 years
(15). In that case, molecular
analysis of the tumor was notably negative for rearrangement of the
EWSR1 (22q12) locus. On the other hand, high-grade MEC
harboring EWSR1-POU5F1 fusion showed chemosensitivity to the
VDC/IE regimen (vincristine, doxorubicin, cyclophosphamide
alternating with ifosfamide and etoposide) based on the protocol
for Ewing sarcoma (16).
 | Table ISummary of reported adult soft tissue
myoepithelial carcinoma cases showing response for cytotoxic
chemotherapy. |
Table I
Summary of reported adult soft tissue
myoepithelial carcinoma cases showing response for cytotoxic
chemotherapy.
| First author,
year | Age, y | Sex | Primary site |
Immunohistochemistry | Molecular
analysis | Systemic
treatment | Surgery | Radiotherapy | Outcome | (Refs.) |
|---|
| Noronha, 2006 | 37 | F | Vulva | S100(+; few),
CAM5.2(+), AE1/AE3(+; few), SMA(-), desmin(-), calponin(+;
few) | (Unknown) | CDDP (PD) > CBDCA
+ PTX + GEM > CBDCA + PTX (CR) | Yes | Yes; Adj., 45 Gy/
10.8 Gy | NED; 3.5 y | (11) |
| Rastrelli, 2013 | 61 | M | Toe | (Unknown) | (Unknown) | CDDP + DXR (PD) >
IFO (CR) | Yes | Yes; 50.4/ 64.8
Gy | NED; 3.0 y | (12) |
| Bisogno, 2014 | 7.8 | F | Orbit | Cytokeratins(+),
SMA(-), S100(-), INI1 (intact) | FISH (rearrangement):
EWSR1(+) | ICpE + IVE (PR) | Yes | Yes; 41 Gy | NED; 5.1 y | (13) |
| Bisogno, 2014 | 0.5 | M | Orbit | Cytokeratins(+),
SMA(-), S100(-), INI1 (intact) | FISH (rearrangement):
EWSR1(-) | ICpE + IVE (PR) | No | Yes; 36 Gy | NED; 0.9 y | (13) |
| Mourtzoukou,
2016 | 36 | M | Neck | INI1(loss), EMA(+),
S100(+), AE1/AE3(+), SMA(+), calponin(+) | FISH (rearrangement):
EWSR1(-), FUS(-) | CBDCA + Cape. (PD)
> DXR (PR) | Yes | Yes; Adj. | AWD; 3.4 y | (14) |
| Hoggard, 2017 | 34 | M | Knee | EMA(+), S100(-),
CAM5.2(+), AE1/AE3(+), desmin(+), GFAP(-) | WES: EWSR1 (22q12)
locus rearrangement(-) | CBDCA + PTX (PR) | Yes | Yes; Adj., 66 Gy | CDF; >3.0
ya DOD; 4.1 y | (15) |
| Chamberlain,
2019 | 33 | M | Neck | (Unknown) | (Unknown) | CBDCA + Cape. (PD)
> DXR (PR) | Yes | Yes; Adj. | AWD; 10 mo | (4) |
| Shenoy, 2020 | 21 | M | Kidney | (Unknown) | FISH: EWSR1-
POU5F1 | VDC/IE (PR) | No | No | CDF; 7.8 y | (16) |
| Present case | 38 | F | Paraspinal | INI1(loss), EMA(+),
S100(+), AE1/AE3(-), SMA(+), GFAP(-) | (Unknown) | DXR + IFO (PR) | No | Yes; PBT, radical,
70.4 Gy (RBE) | | |
Including our case, 5 of the 9 patients shown in
Table I were aged 30-40 years, and
the regimens that proved effective were doxorubicin administered
alone or in combination with ifosfamide (n=3), a combination of
carboplatin and paclitaxel (n=2), and ifosfamide as a continuous
infusion (n=1). MEC is prone to local recurrence, as well as
distant and lymph node metastasis, even after complete surgical
resection. Based on our case and a previous report (17), doxorubicin with an adequate total
dose may provide a good long-term prognosis. Furthermore, the TREP
project (Tumori Rari in Eta Pediatrica) in pediatric
patients recommends the ICpE regimen (ifosfamide, cisplatin, and
etoposide) with RT, which can be used as a clinical reference even
in adolescents and young adults with MEC (13).
Rearrangement of the EWSR1 gene occurs in
nearly half of soft tissue MECs, and a small subset have
alternative FUS rearrangements in lieu of EWSR1
(3). Their fusion partners were
reported to be POU5F1, PBX1, ZNF444,
KLF17, ATF1, PBX3 and KLF15 (3,18-22).
Moreover, among MECs lacking EWSR1 rearrangements, a
considerable subset that show immunohistochemical loss of SMARCB1
(INI1) are characterized by homozygous deletions of SMARCB1
(3). SMARCB1 is a member of the
SWI/SNF complex and is often lost in certain subtypes of sarcomas,
including epithelioid sarcoma, malignant rhabdoid tumor, poorly
differentiated chordoma, epithelioid malignant peripheral nerve
sheath tumor, and MEC. This genetic feature appears in MECs rather
than in benign myoepithelial neoplasms. In our review of MECs that
responded well to treatment, loss of immunostaining for INI1 was
noted in 2 cases and lack of EWSR1 rearrangement in 2 cases.
Although the two gene loci are close on 22q, the association
between EWSR1 fusions and SMARCB1 perturbations
remains unclear in MEC. Further research and elucidation of their
downstream pathways may be helpful for better understanding the
pathogenesis of myoepithelial neoplasms. More basic and clinical
research is warranted to clarify the relationship between molecular
genetic alterations and the clinical response to anti-tumoral
agents in the effort to develop effective therapeutic strategies
for patients with advanced MEC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST made contributions to the acquisition and
interpretation of data for this case, drafted the manuscript, and
reviewed the literature. TN contributed to the concept and design
of the case report, managed the therapeutic strategy, clinically
treated the patient with chemotherapy and helped draft the. RM
clinically treated the patient with chemotherapy and revised the
manuscript critically for important intellectual content. YB
curated the pathological data and revised the manuscript critically
for important intellectual content. MS carried out pathological
diagnosis, including immunohistochemistry, and revised the
manuscript critically for important intellectual content. YD
carried out PBT and revised the manuscript critically for important
intellectual content. TO planned and carried out PBT and revised
the manuscript critically for important intellectual content. KS
made contributions to the conception of the case report and revised
the manuscript critically for important intellectual content. ST
and TN confirm the authenticity of all the raw data. All authors
read and approved the final manuscript, and agreed to be
accountable for all aspects of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kilpatrick SE, Hitchcock MG, Kraus MD,
Calonje E and Fletcher CD: Mixed tumors and myoepitheliomas of soft
tissue: A clinicopathologic study of 19 cases with a unifying
concept. Am J Surg Pathol. 21:13–22. 1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hornick JL and Fletcher CD: Myoepithelial
tumors of soft tissue: A clinicopathologic and immunohistochemical
study of 101 cases with evaluation of prognostic parameters. Am J
Surg Pathol. 27:1183–1196. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jo VY: Soft tissue special issue:
Myoepithelial neoplasms of soft tissue: An updated review with
emphasis on diagnostic considerations in the head and neck. Head
Neck Pathol. 14:121–131. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chamberlain F, Cojocaru E, Scaranti M,
Noujaim J, Constantinou A, Thway K, Fisher C, Messiou C, Strauss
DC, Miah A, et al: Adult soft tissue myoepithelial carcinoma:
Treatment outcomes and efficacy of chemotherapy. Med Oncol.
37(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gleason BC and Fletcher CD: Myoepithelial
carcinoma of soft tissue in children: An aggressive neoplasm
analyzed in a series of 29 cases. Am J Surg Pathol. 31:1813–1824.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rekhi B, Sable M and Jambhekar NA:
Histopathological, immunohistochemical and molecular spectrum of
myoepithelial tumours of soft tissues. Virchows Arch. 461:687–697.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Giridhar P, Gupta P, Mallick S, Upadhyay
AD and Rath GK: Impact of adjuvant therapy on survival in patients
with myoepithelial carcinoma: A systematic review and individual
patient data analysis of 691 patients. Radiother Oncol.
140:125–130. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kabarriti R, Quinn TJ, Ewart MR, Mehta KJ,
Lomita C, Geller DS, Kalnicki S and Fox JL: Neoadjuvant radiation
therapy for the management of myoepithelial carcinoma of the upper
extremity. Int J Cancer. 142:854–862. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miccio JA, Oladeru OT, Yang J, Xue Y, Hoda
ST, Ryu S, Stessin AM and Parker RI: Myoepithelial carcinoma: The
role of radiation therapy. A case report and analysis of data from
the surveillance, epidemiology, and end results (SEER) registry. J
Pediatr Hematol Oncol. 38:274–278. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hata M, Tokuuye K, Shioyama Y, Nomoto S,
Inadome Y, Fukumitsu N, Nakayama H, Sugahara S, Ohara K, Noguchi M,
et al: Malignant myoepithelioma in the maxillary sinus: Case report
and review of the literature. Anticancer Res. 29:497–501.
2009.PubMed/NCBI
|
|
11
|
Noronha V, Cooper DL, Higgins SA, Murren
JR and Kluger HM: Metastatic myoepithelial carcinoma of the vulva
treated with carboplatin and paclitaxel. Lancet Oncol. 7:270–271.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rastrelli M, Passuello N, Cecchin D, Basso
U, Tosi AL and Rossi CR: Metastatic malignant soft tissue
myoepithelioma: A case report showing complete response after
locoregional and systemic therapy. J Surg Case Rep.
2013(rjt109)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bisogno G, Tagarelli A, Schiavetti A,
Scarzello G, Ferrari A, Cecchetto G and Alaggio R: Myoepithelial
carcinoma treatment in children: A report from the TREP project.
Pediatr Blood Cancer. 61:643–646. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mourtzoukou D, Zaidi S, Jones RL, Fisher C
and Thway K: Soft tissue myoepithelial carcinoma metastatic to the
cecum: Highlighting an unusual metastatic pattern and the need for
diagnostic awareness. Rare Tumors. 8(6086)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hoggard TM, Henderson-Jackson E, Bui MM,
Caracciolo J, Teer JK, Yoder S, Binitie O, Gonzalez RJ, Brohl AS
and Reed DR: Myoepithelial carcinoma with RB1 mutation: Remarkable
chemosensitivity to carcinoma of unknown origin therapy. BMC
Cancer. 17(250)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shenoy N: Aggressive myoepithelial
carcinoma with EWSR1-POU5F1 fusion highly responsive to Ewing
sarcoma combination chemotherapy. Cancer. 126:5198–5201.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tian Z, Yang Y, Yang Y, Zhang F, Li P,
Wang J, Yang J, Zhang P, Yao W and Wang X: High cumulative
doxorubicin dose for advanced soft tissue sarcoma. BMC Cancer.
20(1139)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Antonescu CR, Zhang L, Chang NE, Pawel BR,
Travis W, Katabi N, Edelman M, Rosenberg AE, Nielsen GP, Dal Cin P
and Fletcher CD: EWSR1-POU5F1 fusion in soft tissue myoepithelial
tumors. A molecular analysis of sixty-six cases, including soft
tissue, bone, and visceral lesions, showing common involvement of
the EWSR1 gene. Genes Chromosomes Cancer. 49:1114–1124.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang SC, Chen HW, Zhang L, Sung YS,
Agaram NP, Davis M, Edelman M, Fletcher CD and Antonescu CR: Novel
FUS-KLF17 and EWSR1-KLF17 fusions in myoepithelial tumors. Genes
Chromosomes Cancer. 54:267–275. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Flucke U, Mentzel T, Verdijk MA, Slootweg
PJ, Creytens DH, Suurmeijer AJ and Tops BB: EWSR1-ATF1 chimeric
transcript in a myoepithelial tumor of soft tissue: A case report.
Hum Pathol. 43:764–768. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Agaram NP, Chen HW, Zhang L, Sung YS,
Panicek D, Healey JH, Nielsen GP, Fletcher CD and Antonescu CR:
EWSR1-PBX3: A novel gene fusion in myoepithelial tumors. Genes
Chromosomes Cancer. 54:63–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bodis S, Kroiss S, Tchinda J, Fritz C,
Wagner U and Bode PK: Myoepithelial carcinoma of soft tissue with
an EWSR1-KLF15 gene fusion in an infant. Pediatr Dev Pathol.
24:371–377. 2021.PubMed/NCBI View Article : Google Scholar
|