Introduction
Glioblastoma multiforme (GBM) is one of the most
frequent and aggressive primary tumors in the Central Nervous
System (CNS), representing more than 60% of all brain tumors in
adults (1,2).
In over 90% of cases GBM occurs de novo (primary
GBM) without evidence of a less malignant precursor and usually
grows more rapidly and has a worse prognosis than secondary GBM,
developing from lower grade astrocytoma or oligodendrogliomas.
GBM remains incurable with a poor prognosis both for
limited therapeutic alternatives and for high risk of progression
or recurrence. The median overall survival (mOS) is about 15 months
with 2- and 1-year survival rate respectively of 27 and 41%; only
GBM associated with methylated methylguanine-DNA-methyltransferase
(MGMT) gene reach a mOS about 24 months; however, less than 10% of
patients survives at 5 years (3-5).
In newly diagnosed GBM, the standard of care (SoC)
is surgical resection followed by concomitant chemoradiotherapy
(CRT) with temozolomide (6) and
subsequently adjuvant chemotherapy (5,7-10).
However, relapse rate remains poor, with most relapses occurring
around 6-9 months after primary treatment (5,11).
In case of recurrence, treatment options are scarce
and include re-surgery, re-irradiation, traditional
chemotherapeutic drugs, alone or in combination, such as
nitrosourea compounds like fotemustine or CCNU (lomustine),
temozolomide rechallenge, or antiangiogenic drugs such as
bevacizumab, all with limited efficacy (9,12).
In addition, immune-checkpoint inhibitors, such as nivolumab or
pembrolizumab, have shown poor results. There is no uniform
consensus on standard treatment and even the guidelines fail to
facilitate therapeutic decision in recurrent setting; therefore,
enrollment in clinical trials is recommended. In several countries,
Lomustine is usually used in second line after temozolomide
failure, with mOS range of 8.6-9.8 months and median
progression-free survival (mPFS) of 1.5-2.7 months (13,14).
Fotemustine is the only third generation of
nitrosourea available In Italy, and has achieved encouraging
results, though with low-certainty evidence (15).
Regorafenib is a small-molecule multi-kinase
inhibitor already approved in second-line therapy for advanced
hepatocellular carcinoma (HCC) (16,17)
and in third-line treatment both for advanced colorectal cancer
(CRC) (18-20)
and gastrointestinal stromal tumors (GISTs) (20). Since 2019, regorafenib has been
approved by Agenzia Italiana del Farmaco (AIFA)in recurrent
glioblastoma as second line therapy, following the results of a
phase 2 trial by Lombardi et al (21) (REGOMA). REGOMA is a randomised,
comparative, multicenter phase 2 trial designed to evaluate the
efficacy of regorafenib in this setting, compared to lomustine. In
this trial, 119 patients were randomized to receive either
regorafenib or lomustine. At the median follow-up of 15.4 months,
OS, primary endpoint of the study, was greatly improved in the
regorafenib group vs. SoC cohort (7.4 vs. 5.6, respectively). The
disease control rate (DCR) was 44% in the regorafenib arm and 20%
in the lomustine control arm. Because of this, regorafenib has been
approved in Italy and, while sometimes used in other countries on a
single patient basis as an off label treatment, it is conspicuously
absent from European Association of Neuro-Oncology (EANO) (9).
Although the REGOMA trial has brought new hope in
patients with GBM, it presents some critical issues related to both
the absence of a phase 3 study, necessary to confirm the results
obtained, and to the mOS of patients treated with lomustine found
to be inferior to what is known in literature in the same
population (8,6-9,3 months). This underlines the importance of
stratifying GBM based on key molecular alterations and/or specific
prognostic factors.
Moreover, elderly GBMs often present with a dismal
prognosis, with survival around 6 months, and a limited response to
treatments. Several molecular features are being investigated and
different prognostic assessment including age, performance status
(PS), disease burden, comorbidities and other factors have been
proposed to better predict outcomes and prognosis (22).
We report our findings based on a retrospective
analysis of a cohort of 56 patients >60 years treated in two
Units in South Italy, Azienda Ospedaliera Universitaria Luigi
Vanvitelli (Naples) and Ospedale Civile San Giovanni di Dio
(Frattamaggiore, Naples). It is one of the few studies following
REGOMA trial to study regorafenib in a real-life environment and
one of the few to do so with a homogeneous ITT population: all
patients were diagnosed with recurrent glioblastoma and treatment
was initiated in a second line setting. We chose to focus on
elderly patients, defined as patients >60 years, since median
age of diagnosis of glioblastoma is 64 years, with many diagnoses
being made in 70 years or older patients (23), and seeing how for these patients is
harder not only to participate in clinical trials but also to
receive SoC therapy, due to worsening clinical conditions,
increasing comorbidities, and reduced social network.
Materials and methods
Study design and participants
Ours was a bi-centric retrospective study analyzing
the role of regorafenib in recurrent glioblastoma patients >60
years. All data were collected retrospectively.
Inclusion criteria were designed to be as close as
possible to a real-life setting: histologically confirmed
Glioblastoma diagnosis, prior therapy according to Stupp protocol,
adequate bone marrow, liver, and renal function. Performance status
(PS) was measured according to the Eastern Cooperative Oncology
Group (ECOG), and only patients with PS 0-2 were considered
eligible to treatment (alas, from fully active patients to capable
of self-care but not to any work).
Exclusion criteria were all those of routine
clinical practice: previous therapy for recurrent disease, arterial
thrombotic or embolic events within six months, uncontrolled
hypertension, myocardial infarction, need for antiviral treatment
for active hepatitis B or C, contemporary use of strong cytochrome
P3A4 inhibitors or inducers. We included in our ITT population
Isocitrate Dehydrogenase (IDH) mutant Glioblastomas, mostly
secondary glioblastomas, although the newest 2021 WHO
classification of CNS tumors define Glioblastomas strictly as IDH
wild type (24): our decision was
based on the time of initial diagnosis, due to the different
classification criteria; furthermore, IDH mutant patients were
present in the REGOMA trial population (21).
Methylated methylguanine-DNA-methyltransferase
(MGMT) methylation and IDH mutational status were assessed on
archived tumor tissue in separate laboratories for each center.
MGMT methylation status was assessed by methylation array by EPIC
array Illumina 850k according to Bady et al (25) or Methylation Specific PCR (MSP/PCR)
as per Vlassenbroeck et al (26) after bisulfite modification of DNA,
while IDH mutations status was assessed by methylation array by
EPIC array Illumina 850k (25) or
immunohistochemistry (27).
Unfortunately, information regarding previous
treatment or molecular analysis is not available for all patients,
as some of them were initially treated elsewhere and, due to the
retrospective nature of the study, it was difficult to retrieve all
data.
Procedures
Regorafenib was administered as per product label:
160 mg of regorafenib (four 40 mg tablets) per day orally for three
weeks in a four-week cycle. Dose reductions were allowed in case of
toxicities on a 40 mg scale basis to a minimum of 80 mg/day (50%
dose reduction). Treatment was continued until disease progression
(according to Response Assessment in Neuro-Oncology-RANO-Criteria),
unacceptable toxicities, death, or consent withdrawal.
Outcomes
Primary endpoint was OS, while PFS, objective
response rate (ORR) and proportion of patient achieving disease
control (DC) were secondary endpoints. OS was defined as time from
treatment start to death from any cause, PFS as time from treatment
start to disease progression or death, ORR as partial (PR) or
complete response (CR) according to RANO criteria and disease
control as SD, PR or CR according to RANO criteria. OS and PFS were
estimated with Kaplan-Meier methods. Survival data were then
stratified according to age, IDH mutation and MGMT methylation
status.
Statistical analysis
Patient characteristics were reported as median with
range of values between parentheses for continuous variables and
percentages for categorical variables. Kaplan Meier estimates
helped computing survival curves, whereas survival differences were
evaluated using the log-rank test, with significance level of
P=0.05. Statistical analyses were performed using IBM SPSS
statistics v.23.0.
Results
Patients
Data were collected from 2019 to 2021 and fifty-six
patients were included in the final analysis (Tables I and II), 19 female and 37 males; median age
at start of treatment was 68 years (60-79 years). Patients showed
mainly an ECOG PS of 1, as expected due to the diagnosis and the
advanced setting.
 | Table IBaseline ITT population
characteristics (n=56). |
Table I
Baseline ITT population
characteristics (n=56).
| Variable | Value |
|---|
| Median age at
regorafenib start, years | 68 (60-79) |
| Sex, n (%) | |
|
Male | 37 (66.07) |
|
Female | 19 (33.93) |
| ECOG PS, n (%) | |
|
0 | 17 (30.36) |
|
1 | 30 (53.57) |
|
2 | 9 (16.07) |
| Surgery at time of
recurrence, n (%) | 5 (8.90) |
| IDH status, n
(%) | |
|
IDH
mutated | 3 (5.36) |
|
IDH wild
type | 32 (57.14) |
|
Unknown | 21 (37.50) |
| MGMT status, n
(%) | |
|
MGMT
methylated | 24 (42.86) |
|
MGMT
unmethylated | 20 (35.71) |
|
Unknown | 12 (21.43) |
| Corticosteroid use,
n (%) | |
|
Yes | 48 (85.71) |
|
No | 8 (14.29) |
| Third line
treatment following PD, n (%) | |
|
Yes | 19 (33.93) |
|
No | 37 (66.07) |
 | Table IIAEs during regorafenib treatment. |
Table II
AEs during regorafenib treatment.
| AEs | Grade 1 | Grade 2 | Grade 3 |
|---|
| Hand foot skin
reaction, n | 3 | 1 | 2 |
| Rash/desquamation,
n | 1 | 2 | 0 |
| Piastrinopenia,
n | 0 | 5 | 2 |
| Neutropenia, n | 2 | 0 | 0 |
| Hypertension,
n | 3 | 3 | 0 |
| Fatigue, n | 9 | 10 | 2 |
| Voice changes,
n | 1 | 5 | 1 |
| Vomiting, n | 1 | 3 | 0 |
| Hepatic AEs, n | 1 | 2 | 0 |
| Aspartate
aminotransferase elevation, n | 1 | 3 | 2 |
| Hyperbilirubinemia,
n | 4 | 0 | 0 |
| Proteinuria, n | 2 | 3 | 1 |
| Fever, n | 4 | 0 | 0 |
| Cardiac, n | 1 | 2 | 0 |
| Diarrhea, n | 4 | 1 | 0 |
| Total, n (%) | 37(43) | 40(46) | 10(11) |
IDH and MGMT data were available for most patients.
IDH mutations were identified only in 3 out of 35 patient whose
mutational status was known, whereas MGMT was found methylated in
24 patients and unmethylated in 20 patients; for the remaining 12,
MGMT methylation status was unknown.
Survival outcomes
Longest treatment period with regorafenib was for 8
cycles. At cut-off date (25/03/2022), none of the enrolled patients
were still treated with regorafenib and only three patients were
not reported dead (two alive, still in treatment; one lost at
follow up). 19 patients were treated at regorafenib progression
with a third line therapy, 17 with fotemustine and 2 with
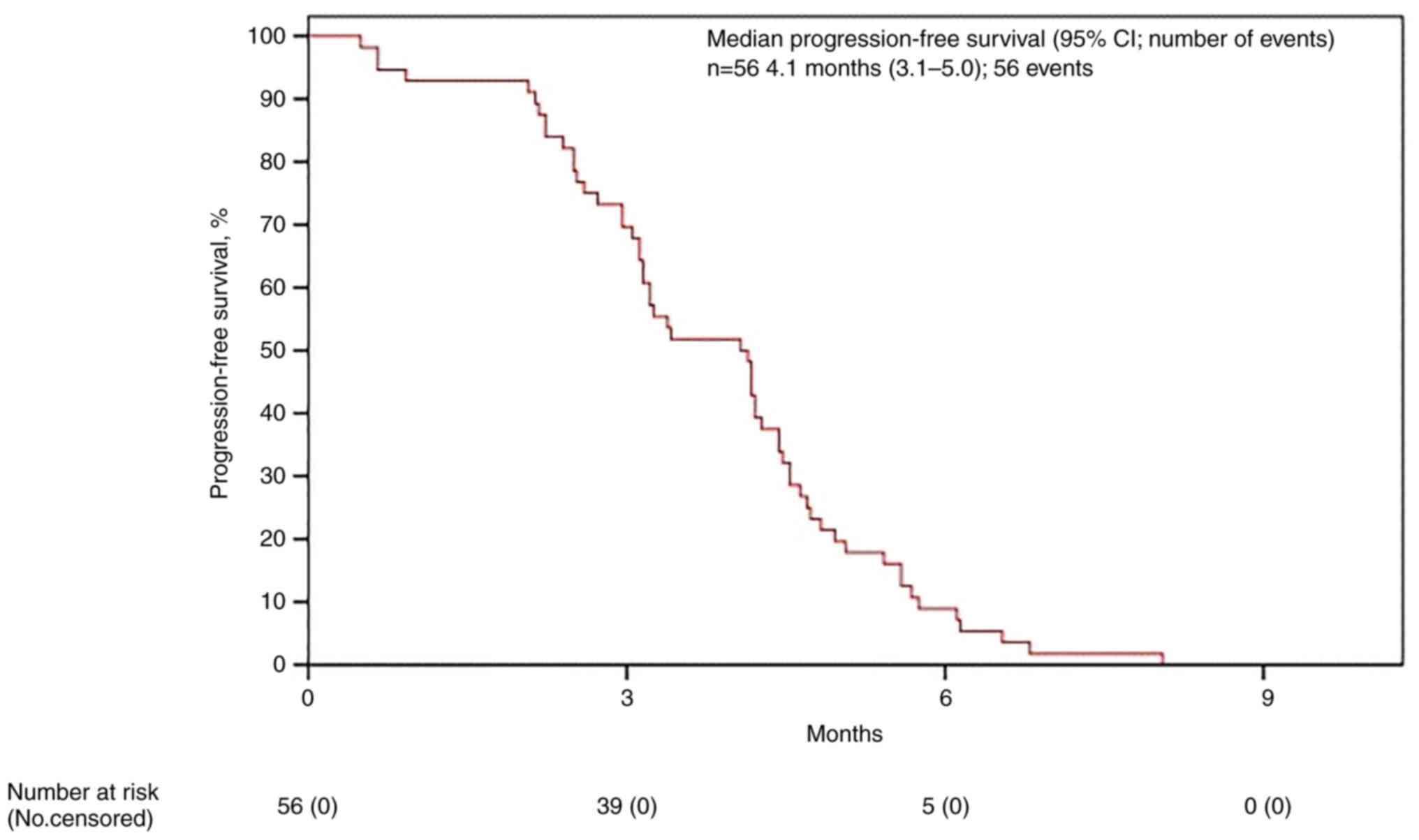
lomustine. mPFS was estimated as 4.0 months (95% IC 3.1-5.0)
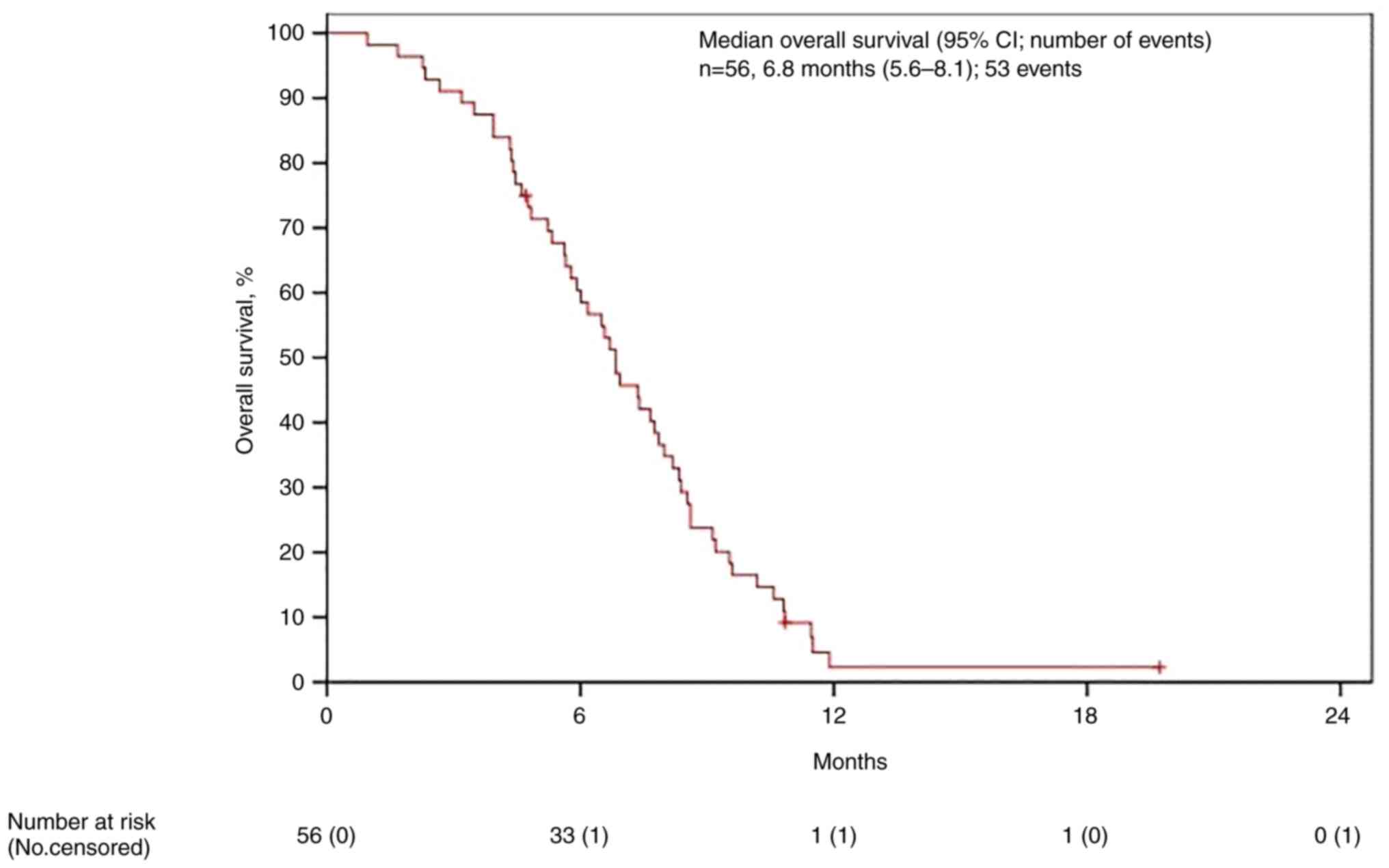
(Fig. 1) and mOS as 6.8 (95% IC
5.6-8.0) (Fig. 2). Data were than
stratified for MGMT status and for age.
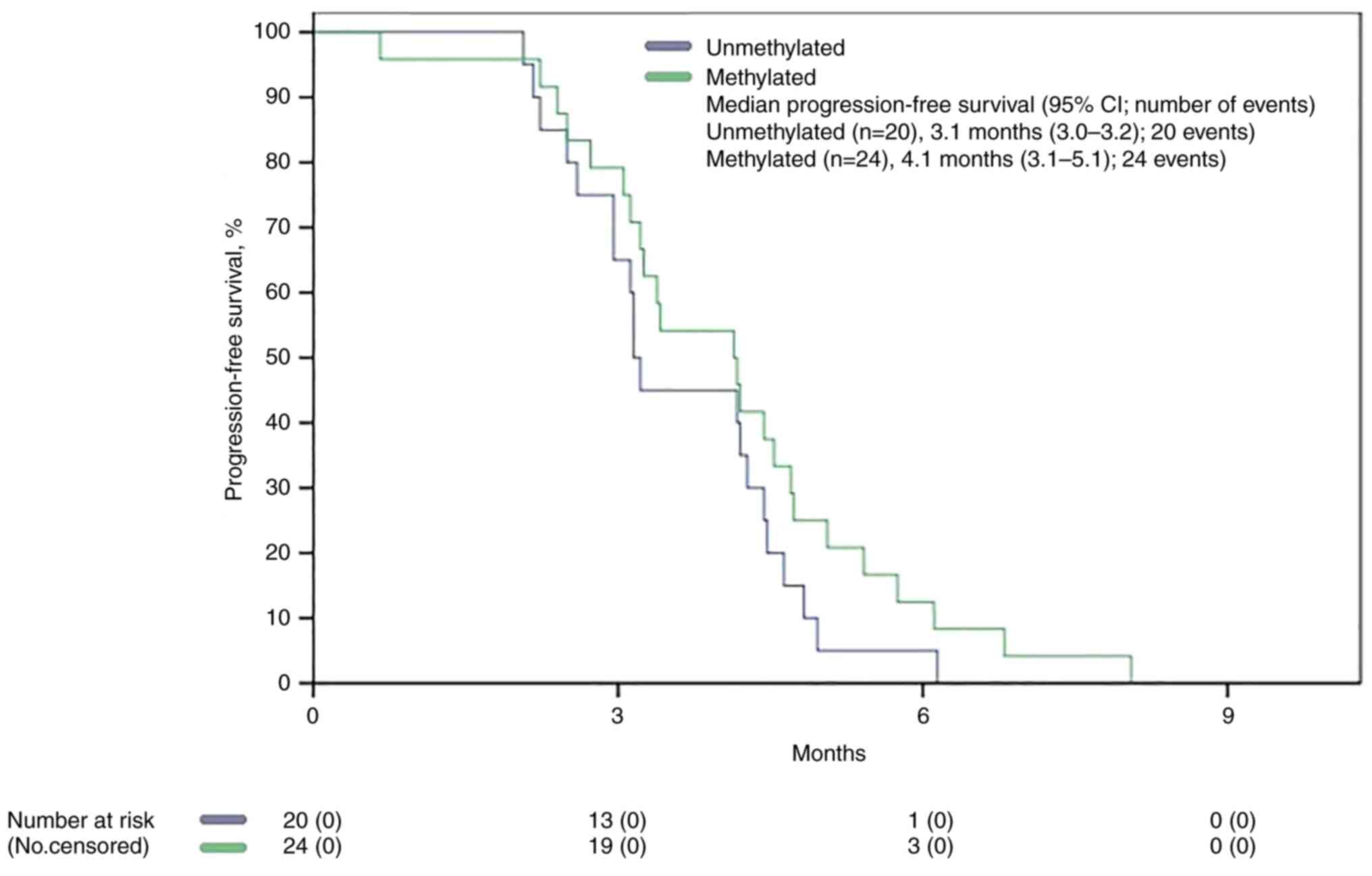
No significant difference was found between the two
populations based on MGMT status in mPFS (3.1 months vs. 4.1
months, P 0.170) (Fig. 3), whereas
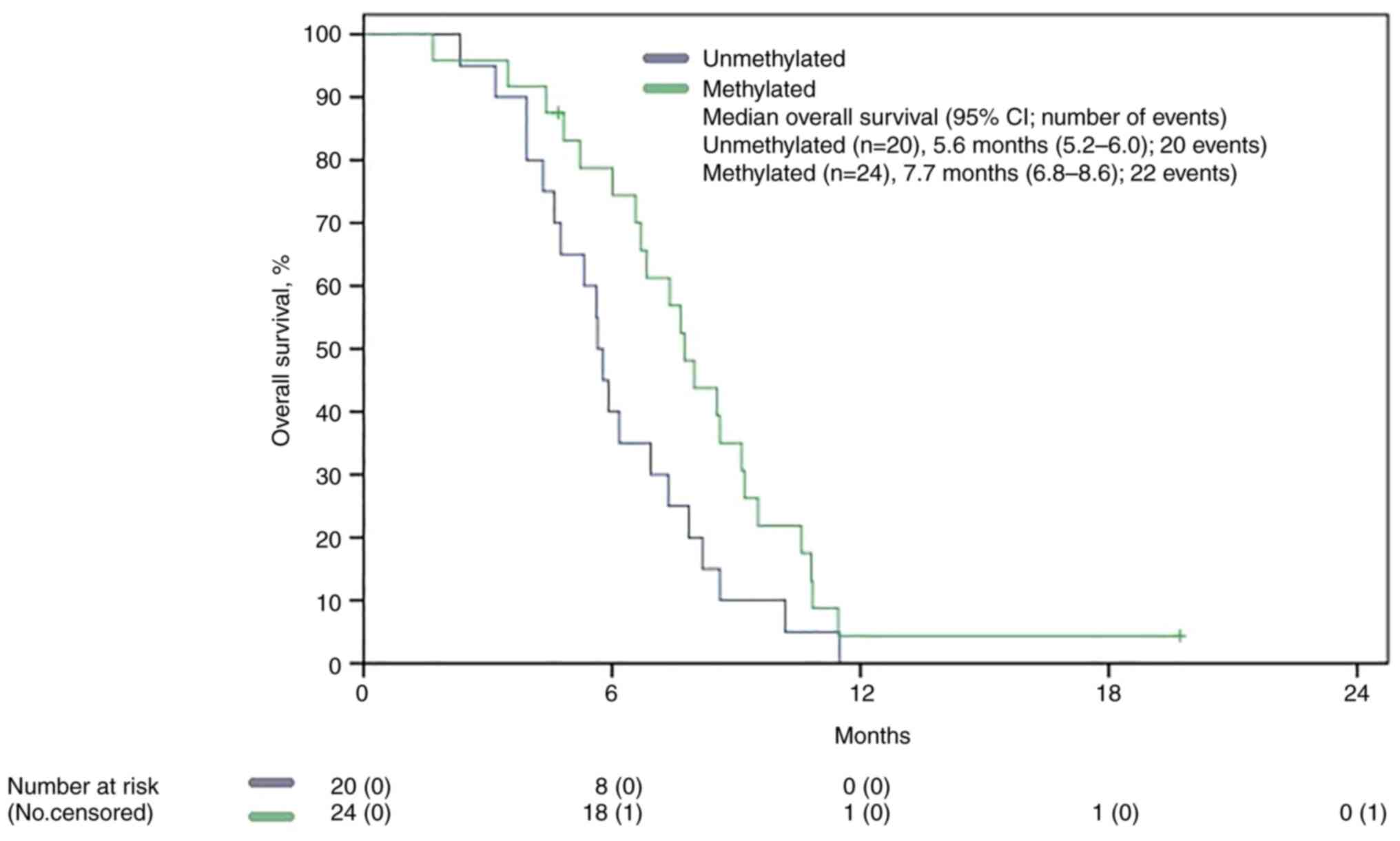
mOS in MGMT methylated patient was statistically significant
superior to that of the unmethylated group, 7.7 months (5,29-6,01)
vs. 5.6 months (5,29-6,01), P 0.048 (Fig. 4.)
Age did not appear to be a significant influence on
PFS. We stratified ITT population according to age twice: one time
using 65 years as cut-off, a second time using 70 years. Patients
aged >65 years were 39, whereas patients with >70 years were
18. Neither study showed any significance difference of mPFS
between the two populations (P 0.074 using 65 years as cut-off, P
0.332 using 70 years).
Other clinical measures
Adverse events (AEs) were recorded for all almost
patients and only 25% of the ITT (14 patients) did not report any
toxicities. Eighty-seven AEs were reported totally: of these, the
majority were grade 2 (46%) and grade 1 (43%) according to Common
Terminology Criteria for Adverse Events (CTCAE) scale, with only 10
grade 3 events (11%). No patient reported grade 4 AEs. The only
grade 3 reported AEs were hand-foot skin reactions (HFSR),
piastrinopenia, fatigue, voice changes, proteinuria, and
aminotransferase elevation. Most reported toxicities (>5%) have
been fatigue (24%), piastrinopenia (8%), voice changes (8%), HFSR
(7%), hypertension (7%), proteinuria (7%), aspartate
aminotransferase (AST) elevation (7%) and diarrhea (6%). (Table III).
 | Table IIIBest response during regorafenib
treatment. |
Table III
Best response during regorafenib
treatment.
| Outcome | No. (%) |
|---|
| Partial
response | 5 (8.9) |
| Complete
response | 0 (0.0) |
| Stable disease | 22 (39.3) |
| Progressive
disease | 29 (51.8) |
Nearly half of the ITT population (44.6%) needed
dose reduction, due to AEs or clinical condition, to improve
tolerability and allow for treatment continuation. Out of the 25
patients who needed reductions, dose was lowered to 80 mg per day
for three of them.
Out of the 56 patients, none was reported as having
a CR, 5 patients (8.9%) developed PR and 22 patients (39.3%) showed
stable disease (SD) as their best response. DCR was then 48.2% with
an ORR of 8.9%. 29 patients (51.8%) developed progressive disease
at their first MRI imaging (Table
IV).
 | Table IVPatient data. |
Table IV
Patient data.
| Patient | Year of birth | Age at diagnosis,
years | Sex | ECOG PS | IDH1/2 | MGMT | Surgery | Date of
surgery | Type of
surgery | No. of adjuvant TMZ
cycles | Date of
recurrence | Second surgery | Second
radiotherapy | CCS | Regorafenib
start | Dose reduction | Best response to
regorafenib | Recurrence date
after regorafenib | Therapy at
recurrence | Date of death |
|---|
| P001 | 1945 | 71 | F | 2 | Unknown | Methylated | Yes | NA | Partial | 11 | May-19 | No | No | No | Dec-19 | Yes | PD | Feb-20 | No | Mar-20 |
| P002 | 1950 | 69 | M | 1 | Unmutated | Methylated | Yes | May-20 | Complete | 6 | Apr-21 | No | No | No | Apr-21 | Yes | SD | Aug-21 | Fotemustine | Oct-21 |
| P003 | 1948 | 70 | M | 1 | Unknown | Methylated | Yes | Jan-19 | Complete | 6 | Jan-20 | Yes | Yes | Yes | Feb-20 | Yes | SD | Jun-20 | Fotemustine | Dec-20 |
| P004 | 1980 | 63 | M | 0 | Unmutated | Methylated | Yes | Apr-20 | Complete | 12 | Nov-21 | No | No | No | Dec-21 | No | PD | Dec-21 | No | Jan-22 |
| P005 | 1951 | 68 | M | 1 | Unmutated | Unmethylated | Yes | Nov-19 | Complete | 6 | Dec-20 | No | No | No | Dec-20 | No | PD | Mar-21 | No | May-21 |
| P006 | 1953 | 66 | M | 1 | Unmutated | Unmethylated | Yes | Jun-20 | Complete | 6 | Aug-21 | No | No | No | Aug-21 | No | SD | Jan-22 | No | Feb-22 |
| P007 | 1954 | 64 | M | 1 | Unmutated | Methylated | No | | | 6 | Apr-20 | No | No | No | Apr-20 | Yes | PD | Aug-20 | Fotemustine | Nov-20 |
| P008 | 1952 | 66 | F | 2 | Unknown | Unknown | No | | | 1 | Jan-20 | No | No | No | Feb-20 | No | PD | Jul-20 | No | Sept-20 |
| P009 | 1950 | 67 | M | 1 | Unknown | Methylated | Yes | Sept-18 | Partial | 6 | Aug-19 | No | No | No | Sept-19 | Yes | SD | Feb-20 | No | Mar-20 |
| P010 | 1957 | 61 | M | 1 | Unmutated | Unmethylated | No | | | 4 | May-19 | No | No | No | May-19 | Yes | PD | Aug-19 | No | Nov-19 |
| P011 | 1956 | 62 | M | 2 | Unmutated | Unknown | Yes | Jun-19 | Partial | NA | Oct-21 | No | No | No | Nov-21 | No | PD | Dec-21 | No | Dec-21 |
| P012 | 1950 | 69 | M | 1 | Unmutated | Methylated | Yes | Nov-19 | Partial | 4 | Aug-20 | No | No | No | Sept-20 | Yes | PD | Dec-20 | No | Jan-21 |
| P013 | 1952 | 66 | M | 1 | Mutated | Unmethylated | Yes | May-19 | Partial | 6 | Mar-20 | No | No | No | Mar-20 | No | SD | Aug-20 | No | Sept-20 |
| P014 | 1962 | 65 | M | 0 | Unknown | Unmethylated | Yes | Aug-18 | Partial | 2 | Jul-19 | No | No | No | Aug-19 | No | PD | Oct-19 | No | Nov-19 |
| P015 | 1958 | 59 | M | 2 | Unmutated | Unmethylated | Yes | Mar-18 | Complete | 16 | Oct-19 | No | No | No | Nov-19 | Yes | PD | Feb-20 | No | Feb-20 |
| P016 | 1947 | 70 | M | 1 | Unknown | Unknown | Yes | Jul-2018 | Partial | 9 | Feb-21 | No | No | No | Apr-21 | Yes | SD | Sept-21 | Lomustina | Alive |
| P017 | 1958 | 60 | M | 1 | Unmutated | Unmethylated | Yes | Jul-19 | Partial | 3 | Feb-20 | Yes | Yes | Yes | Mar-20 | Yes | SD | Aug-20 | Fotemustine | Nov-20 |
| P018 | 1951 | 68 | F | 0 | Unmutated | Methylated | Yes | Feb-20 | Complete | 6 | Apr-21 | Yes | Yes | Yes | Apr-21 | No | PD | Aug-21 | No | Oct-21 |
| P019 | 1959 | 60 | F | 1 | Unknown | Unmethylated | Yes | Aug-19 | Partial | 4 | Jun-20 | No | No | No | Jul-20 | Yes | PD | Oct-20 | No | Nov-20 |
| P020 | 1948 | 70 | F | 1 | Unknown | Unknown | Yes | Na | Partial | NA | Jan-20 | No | No | No | Jan-20 | No | PD | May-20 | No | Sept-20 |
| P021 | 1947 | 72 | M | 0 | Unmutated | Unmethylated | Yes | May-20 | Partial | 3 | Feb-21 | No | No | No | Mar-21 | No | PD | May-21 | No | Jun-21 |
| P022 | 1947 | 71 | M | 2 | Unmutated | Methylated | Yes | Dec-18 | Partial | 6 | Sept-19 | No | No | No | Oct-19 | No | SD | Mar-20 | Fotemustine | Jul-20 |
| P023 | 1957 | 66 | F | 1 | Unknown | Unknown | Yes | Na | Partial | NA | Nov-20 | No | No | No | Nov-20 | No | SD | Jun-21 | No | Aug-21 |
| P024 | 1949 | 70 | M | 1 | Unknown | Methylated | Yes | Jul-20 | Complete | 3 | Mar-21 | No | No | No | Mar-21 | Yes | SD | Aug-21 | Fotemustine | Feb-22 |
| P025 | 1948 | 70 | M | 2 | Unmutated | Methylated | Yes | Nov-19 | Complete | 6 | Sept-20 | No | No | No | Sept-20 | No | PR | Mar-21 | No | Apr-21 |
| P026 | 1951 | 68 | M | 1 | Unmutated | Unmethylated | Yes | Jul-20 | Partial | 6 | Aug-21 | No | No | No | Aug-21 | Yes | PD | Oct-21 | No | Jan-22 |
| P027 | 1946 | 72 | F | 1 | Unmutated | Methylated | Yes | Sept-19 | Complete | 6 | Aug-20 | No | No | No | Sept-20 | No | SD | Feb-21 | Fotemustine | May-21 |
| P028 | 1957 | 64 | M | 1 | Unknown | Unknown | Yes | Na | Partial | NA | May-19 | No | No | No | May-19 | No | PD | Aug-19 | No | Oct-19 |
| P029 | 1952 | 67 | F | 0 | Unknown | Unknown | Yes | Jan-20 | Complete | 6 | Mar-21 | No | No | No | Mar-21 | No | SD | Oct-21 | No | Jan-22 |
| P030 | 1955 | 64 | F | 0 | Unmutated | Unmethylated | Yes | Feb-20 | Complete | 5 | Jan-21 | No | No | No | Feb-21 | Yes | SD | Jul-21 | Fotemustine | Dec-21 |
| P031 | 1951 | 66 | M | 0 | Unmutated | Unmethylated | Yes | Oct-18 | Complete | 6 | Sept-19 | No | No | No | Sept-19 | Yes | SD | Mar-20 | Fotemustine | Jun-20 |
| P032 | 1951 | 67 | F | 1 | Unknown | Unmethylated | Yes | Jan-19 | Complete | 6 | Dec-19 | No | No | No | Dec-19 | Yes | PD | Apr-20 | No | May-20 |
| P033 | 1949 | 69 | M | 0 | Unknown | Methylated | Yes | Mar-19 | Complete | 5 | Jan-20 | No | No | No | Feb-20 | No | PD | Jun-20 | No | Oct-20 |
| P034 | 1951 | 68 | M | 0 | Unmutated | Methylated | Yes | Jun-20 | Complete | 3 | Feb-21 | No | No | No | Feb-21 | No | PD | May-21 | Fotemustine | Oct-21 |
| P035 | 1962 | 75 | F | 1 | Unknown | Unknown | Yes | Na | Partial | NA | Dec-19 | No | No | No | Dec-19 | No | PD | Dec-19 | No | Feb-20 |
| P036 | 1978 | 59 | M | 1 | Mutated | methylated | Yes | Oct-18 | Partial | 0 | Jul-20 | No | No | No | Jul-20 | Yes | SD | Mar-21 | Fotemustina | Alive |
| P037 | 1953 | 65 | F | 0 | Unmutated | Methylated | Yes | Mar-19 | Complete | 6 | Jan-20 | No | No | No | Feb-20 | Yes | PD | May-20 | Fotemustine | Oct-20 |
| P038 | 1957 | 62 | M | 0 | Unknown | Methylated | No | | | 3 | Oct-20 | No | No | No | Nov-20 | No | PR | Jun-21 | Fotemustine | Sept-21 |
| P039 | 1947 | 72 | F | 0 | Unknown | Methylated | Yes | Aug-20 | Complete | 6 | Oct-21 | Yes | Yes | Yes | Oct-21 | No | SD | Feb-22 | No | Alive |
| P040 | 1943 | 76 | F | 0 | Unmutated | Unmethylated | Yes | May-20 | Complete | 6 | Jun-21 | No | No | No | Jun-21 | Yes | SD | Oct-21 | No | Jan-22 |
| P041 | 1948 | 71 | M | 2 | Unmutated | Unknown | Yes | Oct-20 | Partial | 0 | Mar-21 | No | No | No | Apr-21 | Yes | PD | May-21 | No | Jul-21 |
| P042 | 1949 | 70 | M | 0 | Unmutated | Unmethylated | Yes | Jan-20 | Partial | 4 | Nov-20 | No | No | No | Nov-20 | Yes | PD | Feb-21 | No | Apr-21 |
| P043 | 1948 | 70 | M | 1 | Unmutated | Unmethylated | Yes | Feb-19 | Complete | 6 | Jan-20 | No | No | No | Feb-20 | Yes | PD | May-20 | Fotemustine | Sept-20 |
| P044 | 1947 | 72 | M | 0 | Unmutated | Unmethylated | Yes | Jan-20 | Complete | 5 | Nov-20 | No | No | No | Nov-20 | No | SD | Apr-21 | No | May-21 |
| P045 | 1953 | 66 | M | 1 | Unmutated | Methylated | Yes | Jul-20 | Complete | 5 | Apr-21 | No | No | No | May-21 | No | PD | Aug-21 | No | Dec-21 |
| P046 | 1940 | 78 | M | 0 | Unknown | Methylated | Yes | Dec-19 | Complete | 6 | Dec-20 | No | No | No | Dec-20 | No | SD | May-21 | Fotemustine | Sept-21 |
| P047 | 1951 | 65 | F | 1 | Unknown | Unknown | Yes | Na | Partial | NA | Dec-20 | No | No | No | Dec-20 | No | PD | Mar-21 | No | Apr-21 |
| P048 | 1957 | 62 | M | 2 | Mutated | Unmethylated | Yes | Nov-19 | Complete | 6 | Nov-20 | No | No | No | Nov-20 | Yes | SD | Jun-21 | Fotemustine | Nov-21 |
| P049 | 1954 | 64 | M | 0 | Unmutated | Methylated | Yes | Feb-19 | Complete | 6 | Jan-20 | No | No | No | Feb-20 | No | PR | Jul-20 | Fotemustine | Jan-21 |
| P050 | 1948 | 70 | M | 1 | Unmutated | Methylated | Yes | Oct-18 | Complete | 3 | Jun-19 | No | No | No | May-19 | No | PD | Aug-19 | No | Oct-19 |
| P051 | 1944 | 75 | M | 1 | Unmutated | Unmethylated | No | | | 2 | Apr-20 | No | No | No | May-20 | Yes | PD | Jul-20 | No | Sept-20 |
| P052 | 1959 | 59 | F | 1 | Unmutated | Methylated | Yes | Nov-18 | Complete | 6 | Nov-19 | Yes | Yes | Yes | Dec-19 | No | PR | Jun-20 | No | Sept-20 |
| P053 | 1953 | 66 | F | 2 | Unknown | Unmethylated | No | | | 2 | Jan-21 | No | No | No | Jan-21 | No | SD | Jun-21 | No | Aug-21 |
| P054 | 1966 | 62 | F | 1 | Unmutated | Unknown | Yes | Jun-19 | Partial | 9 | Aug-20 | No | No | No | Nov-20 | Yes | SD | Jun-21 | Lomustina | Nov-21 |
| P055 | 1955 | 63 | F | 1 | Unmutated | Methylated | Yes | Jul-19 | Complete | 3 | Feb-20 | No | No | No | Feb-20 | No | PR | Aug-20 | No | Oct-20 |
| P056 | 1955 | 64 | M | 1 | Unknown | Unknown | Yes | Na | Partial | NA | Feb-20 | No | No | No | Feb-20 | No | PD | Aug-20 | No | Sept-20 |
Discussion
To our knowledge, this was one of the few analyses
following REGOMA to study regorafenibas treatment at first
recurrence in glioblastoma patients in a real-life setting and
possibly the only one to focus on an older population. Median age
of patients treated with regorafenib in REGOMA was 54.8 years
(46.8-61.3), whereas in our project median age reached 68 years.
(60-79). Although appearing small, our sample size is comparable to
other publications in the same setting due to the relative
incidence and prognosis of glioblastoma. Thus, we believe our
results to be representative of a real life population.
Our results were mostly comparable to those of
REGOMA, with a DCR of 48% (vs. 44%), although our patients only
showed SD or PR with no CR.CR were instead reported in REGOMA trial
in 2% of patients. It must be noted that neither a subsequent
prospective study by Lombardi et al (28) published in 2021 nor a 2019
bicentric retrospective analysis by Tzaridis et al (29) showed evidence of CR; indeed, a 2019
retrospective analysis by Kebir et al (30) on six high grade astrocytomas showed
a DCR of 0%.
mOS was slightly lower in our findings, 6.8 months
vs. 7.4 months, easily due to an older and more comorbid population
than that of the REGOMA trial and, of course, to the
characteristics of a real-life retrospective setting, that allows
for less stringent inclusion criteria. Anyhow, results were largely
superimposable, confirming the benefit of regorafenib on OS on the
ITT.
At the same time, mPFS was then doubled in our
study, 4.0 months vs. 2.0 months. Kebir et al (30) also showed a higher mPFS (3.5
months) compared to REGOMA. As in that case, our discordant results
may be due to the nature of the study: being a bicentric
retrospective analysis, MRI time schedule was easily dependent on
investigators choices and variable on a case-to-case basis, thus
determining a formally higher result.
As reported above, mOS was found statistically
superior in patients with methylated MGMT promoter (mOS 7.7 months
vs. 5.6 months), with no difference in mPFS (mPFS 3.1 months vs.
4.1 months). MGMT promoter methylation has been since long
identified as a predictive factor of increased survival from
alkylating agents (9,31). Unfortunately, no benefit has yet
been identified in patients treated with regorafenib (9,28).
While our data need to be evaluated in other studies, especially
prospective trials with larger populations, one must consider the
possibility of the two subgroups being unbalanced for confounder
factors, determining such a result. Indeed, while the proportion of
patient in the two subgroups that completed at least 6 cycles of
temozolomide is quite similar (50% in the unmethylated group vs.
62.5% in the methylated group), there was a higher percentage of
patient that underwent radical surgery at diagnosis in the latter
group (77% vs. 41%).
Age did not appear to be a prognostic factor, with
no difference in mPFS in the two subgroup analysis performed. This
is consistent with previous results from Lombardi et al
(28) real-life trial and may help
considering older patients with good performance status for
treatment with regorafenib, irrespective of age. Furthermore, if we
compare our results to those of Lombardi regarding ORR, they are
largely superimposable. DCR reached 48% in our study compared to
46% in the latter, whereas ORR resulted 9 to 7.4% (28).
Our study may help in referring older patients with
good PS to treatment with regorafenib, irrespective of age,
especially since our population, although significantly older
(median age of 68 years vs.55 years of Lombardi trial or 54.8 years
of REGOMA trial), presented similar survival and control rates.
Survival was also analyzed according to IDH status,
but our results cannot be generalized due to the strong unbalance
between the two groups, with only 3 patients reporting a mutation
in IDH (data similar to that of REGOMA trial with only 2 patients
in the regorafenib arm and 0 in the lomustine arm). Survival was
prolonged in the IDH mutant population, with a mPFS of 6.1 months
and mOS of 11.5 months vs. respectively 3.1 and 6.1 months in the
IDH wild type group (mOS p 0.041; mPFS p 0.009). Mutations in IDH
have been generally reported in so-called secondary glioblastomas
(32) and are an important
prognostic factors associated with longer OS, although, as
specified before, the new WHO system does not allow for IDH mutant
glioblastoma but classifies them into astrocytomas (grade 2 to 4)
or oligodendrogliomas (grade 2 or 3) (24).
Regorafenib was overall well tolerated in our
population, with mainly grade 1 and 2 AEs. Only 25% of the ITT (14
patients) did not report any toxicities. 56% of the patients in
Regorafenib arm developed at least one grade 3-4 AE in the REGOMA
trial and 90% ported at least one all-grade drug-related toxicity
in a subsequent study by Lombardi et al (28): with this in mind, one must consider
the possibility of low accuracy in toxicity reports for this study.
Even adjusting for this eventuality, safety profile was comparable
to other trials involving regorafenib, both in glioblastoma and in
other setting, thus advocating for its use in older but medically
fit patients as a real therapeutic alternative.
The only grade 3 reported AEs were HFSR,
piastrinopenia, fatigue, voice changes, proteinuria, and
aminotransferase elevation. HFSR accounted for 7% of overall AEs
(11% of the ITT, 6 patients) with only 2 patients showing grade 3
AEs. Incidence is thus lower than what was reported in REGOMA trial
(grade 1-2, 22%, grade 3, 10%) or other trials in GBM (29), which was even lower than data from
CORRECT (33) and RESORCE
(34) trials (overall HFS rate 47%
in the CORRECT trial and 53% in the RESORCE trial). All grade
thrombocytopenia was reported by 13% of the cohort, with 5 grade 2
AEs and 2 grade 3 AEs, a result slightly more favorable than that
of REGOMA trial, with 20% of grade 1-2 and 2% (1 patient) grade 3
thrombocytopenia and similar to the subsequent analysis by Lombardi
et al (28).
AST elevation and hyperbilirubinemia were also among
the most common AEs, with 11% manifesting AST elevation and 7%
increase in bilirubinemia, similar to what was expected based on
prior studies (21,34).
As already exposed, one of our main limitations is
the retrospective nature of our study, determining a higher risk of
incomplete data, information and recall bias, and the small
population. However, our results are fairly superimposable to those
of the available literature. This helps generating a framework in
which regorafenib is a valid approach even in elderly patients due
to both survival rates and toxicity profile being similar to those
of a younger population. In any case, more phase 3 trials are
needed to unravel the question of whether regorafenib is definitely
superior to lomustine and define the best strategy at recurrence.
An observational prospective study (REGOMA-Oss, [NCT04810182]) is
already ongoing and will analyze the role of regorafenib in
recurrent GBM in real world patients.
Unfortunately, predictive biomarkers of response to
regorafenib are not yet available. In a recent study by Santangelo
et al (35) based on
patients from REGOMA trial, a group of 5 RNA biomarkers (HIF1a and
CDKN1A mRNA, miR-93-5p, miR-3607-3p and miR301a-3p) identified a
favorable subgroup of patients. These findings, given the
relatively small population and the study design, must be validated
in larger and in prospective trials (35).
Nevertheless, new studies are already exploring
other strategies for regorafenib. GBM AGILE trial an international,
seamless Phase II/III response adaptive randomization platform
trial designed to evaluate multiple therapies, with regorafenib
being used both at first diagnosis after concomitant CRT with
temozolomide or at first recurrence. A phase II trial is evaluating
regorafenib use in bevacizumab refractory high-grade gliomas (not
only GBM but also gliosarcoma, small cell glioblastoma etc. can be
included) [NCT04051606], while another phase II basket trial is
investigating the association of regorafenib and nivolumab in
several tumor types [NCT04704154].
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MF, FC and RA conceived the study. VF developed the
methodology. VF, MP, SF, MCat, MCar, AB, IDG, AA SISF, TS, DS, MB,
MCo, RP performed data analysis. MF, MP, VF,TS, DS, MB, MCo, RP, FC
and RA performed data acquisition, analysis and interpretation.
MCar, AV, IDG, AA, SISF, TS, DS, MB, MCo and RP provided resources.
MF, MP, VF, IDG, AA, SISF and RA curated data. MF, MP, SF and MCat
wrote the original draft. MF, TS, DS, MB, MCo, RP and FC reviewed
and edited the manuscript. FC and RA supervised the study. FC and
RA were involved in project administration. MF and RA confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study did not require ethical approval. Ethical
review and approval were waived for this study due to the
retrospective nature. The requirement for patient consent to
participate was waived due to the retrospective nature of the
study.
Patient consent for publication
The requirement for patient consent for publication
was waived due to the retrospective nature of the study.
Competing interests
MF: Advisory boards for MSD and Merck Serono. All
other authors declare that they have no competing interests.
References
|
1
|
Ostrom QT, Francis SS and Barnholtz-Sloan
JS: Epidemiology of brain and other CNS tumors. Curr Neurol
Neurosci Rep. 21(68)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hanif F, Muzaffar K, Perveen K, Malhi SM
and Simjee SU: Glioblastoma multiforme: A review of its
epidemiology and pathogenesis through clinical presentation and
treatment. Asian Pac J Cancer Prev. 18(3)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2012-2016. Neuro Oncol.
21:v1–v100. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ostrom QT, Cote DJ, Ascha M, Kruchko C and
Barnholtz-Sloan JS: Adult Glioma incidence and survival by race or
ethnicity in the United States from 2000 to 2014. JAMA Oncol.
4:1254–1262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Molinaro AM, Hervey-Jumper S, Morshed RA,
Young J, Han SJ, Chunduru P, Zhang Y, Phillips JJ, Shai A,
Lafontaine M, et al: Association of maximal extent of resection of
Contrast-enhanced and non-contrast-enhanced tumor with survival
within molecular subgroups of patients with newly diagnosed
glioblastoma. JAMA Oncol. 6:495–503. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Buckner JC, Shaw EG, Pugh SL, Chakravarti
A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, et
al: Radiation plus Procarbazine, CCNU, and vincristine in low-grade
glioma. N Engl J Med. 374:1344–1355. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weller M, van den Bent M, Preusser M, Le
Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven
L, et al: EANO guidelines on the diagnosis and treatment of diffuse
gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Overview|Brain tumours (primary) and brain
metastases in over 16s|Guidance|NICE.
|
|
11
|
McBain C, Lawrie TA, Rogozińska E,
Kernohan A, Robinson T and Jefferies S: Treatment options for
progression or recurrence of glioblastoma: A network meta-analysis.
Cochrane Database Syst Rev. 5(CD013579)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lombardi G, Idbaih A, le Rhun E, Preusser
M, Zagonel V and French P: A new landscape for systemic
pharmacotherapy of recurrent glioblastoma? Cancers (Basel). 12:1–4.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Batchelor TT, Mulholland P, Neyns B,
Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S,
Ashby LS, et al: Phase III randomized trial comparing the efficacy
of cediranib as monotherapy, and in combination with lomustine,
versus lomustine alone in patients with recurrent glioblastoma. J
Clin Oncol. 31:3212–3218. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wick W, Gorlia T, Bendszus M, Taphoorn M,
Sahm F, Harting I, Brandes AA, Taal W, Domont J, Idbaih A, et al:
Lomustine and bevacizumab in progressive glioblastoma. N Engl J
Med. 377:1954–1963. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Addeo R, Lamberti G, Simonetti G, Iodice
P, Marinelli A, Montella L, Cappabianca S, Gaviani P, Caraglia M,
Prete SD and Silvani A: Biweekly fotemustine schedule for recurrent
glioblastoma in the elderly: Activity and toxicity assessment of a
multicenter study. CNS Oncol. 8(CNS32)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Røed Skårderud M, Polk A, Kjeldgaard
Vistisen K, Larsen FO and Nielsen DL: Efficacy and safety of
regorafenib in the treatment of metastatic colorectal cancer: A
systematic review. Cancer Treat Rev. 62:61–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Martini G, Troiani T, Cardone C, Vitiello
P, Sforza V, Ciardiello D, Napolitano S, Della Corte CM, Morgillo
F, Raucci A, et al: Present and future of metastatic colorectal
cancer treatment: A review of new candidate targets. World J
Gastroenterol. 23:4675–4688. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
de La Fouchardière C: Regorafenib in the
treatment of metastatic colorectal cancer. Future Oncol.
14:2239–2246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lombardi G, de Salvo GL, Brandes AA, Eoli
M, Rudà R, Faedi M, Lolli I, Pace A, Daniele B, Pasqualetti F, et
al: Regorafenib compared with lomustine in patients with relapsed
glioblastoma (REGOMA): A multicentre, open-label, randomised,
controlled, phase 2 trial. Lancet Oncol. 20:110–119.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bruno F, Pellerino A, Palmiero R, Bertero
L, Mantovani C, Garbossa D, Soffietti R and Rudà R: Glioblastoma in
the elderly: Review of molecular and therapeutic aspects.
Biomedicines. 10(664)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mason M, Laperriere N, Wick W, Reardon DA,
Malmstrom A, Hovey E, Weller M and Perry JR: Glioblastoma in the
elderly: Making sense of the evidence. Neurooncol Pract. 3:77–86.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bady P, Sciuscio D, Diserens AC, Bloch J,
van den Bent MJ, Marosi C, Dietrich PY, Weller M, Mariani L,
Heppner FL, et al: MGMT methylation analysis of glioblastoma on the
Infinium methylation BeadChip identifies two distinct CpG regions
associated with gene silencing and outcome, yielding a prediction
model for comparisons across datasets, tumor grades, and
CIMP-status. Acta Neuropathol. 124:547–560. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vlassenbroeck I, Califice S, Diserens AC,
Migliavacca E, Straub J, Di Stefano I, Moreau F, Hamou MF, Renard
I, Delorenzi M, et al: Validation of real-time methylation-specific
PCR to determine O6-methylguanine-DNA methyltransferase gene
promoter methylation in glioma. J Mol Diagn. 10:332–337.
2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Capper D, Weissert S, Balss J, Habel A,
Meyer J, Jäger D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H,
et al: Characterization of R132H mutation-specific IDH1 antibody
binding in brain tumors. Brain Pathol. 20:245–254. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lombardi G, Caccese M, Padovan M, Cerretti
G, Pintacuda G, Manara R, Di Sarra F and Zagonel V: Regorafenib in
recurrent glioblastoma patients: A large and monocentric real-life
study. Cancers (Basel). 13(4731)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tzaridis T, Gepfner-Tuma I, Hirsch S,
Skardelly M, Bender B, Paulsen F, Schaub C, Weller J, Schäfer N,
Herrlinger U and Tabatabai G: Regorafenib in advanced high-grade
glioma: A retrospective bicentric analysis. Neuro Oncol.
21:954–955. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kebir S, Rauschenbach L, Radbruch A,
Lazaridis L, Schmidt T, Stoppek AK, Pierscianek D, Stuschke M,
Forsting M, Sure U, et al: Regorafenib in patients with recurrent
high-grade astrocytoma. J Cancer Res Clin Oncol. 145:1037–1042.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hegi ME, Diserens A-C, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ohgaki H and Kleihues P: The definition of
primary and secondary glioblastoma. Clin Cancer Res. 19:764–772.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Grothey A, van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Santangelo A, Rossato M, Lombardi G,
Benfatto S, Lavezzari D, De Salvo GL, Indraccolo S, Dechecchi MC,
Prandini P, Gambari R, et al: A molecular signature associated with
prolonged survival in Glioblastoma patients treated with
Regorafenib. Neuro Oncol. 23:264–276. 2021.PubMed/NCBI View Article : Google Scholar
|