Introduction
Renal cell carcinoma (RCC) consists of a
heterogeneous group of tumors originating from renal tubular
epithelial cells and is one of the 10 most frequently occurring
cancer types in the world; it accounts for 2% of all cancer
diagnoses and cancer-associated deaths worldwide (1). RCC is the most common malignant tumor
of the kidney, accounting for 85-90% of all renal cancer cases and
1-2% of all malignancies (2).
According to pathological features, mutational analysis and
syndromic correlations, RCC has been classified into several
subtypes: Clear cell carcinoma (70-90%), papillary RCC (10-15%) and
chromophobe RCC (3-5%). Classifying the subtype of RCC has clinical
significance in prognosis and therapeutic interventions (3). Despite improvements in diagnosis,
mainly improved imaging modalities and the incidental detection of
a number of tumors by imaging tests for unrelated complaints,
nearly 30% of all patients with RCC are diagnosed with a metastatic
illness (4). Contrast-enhanced
computed tomography (CECT) is the modality of choice for the
preoperative characterization and staging of renal tumors, and for
follow-up of an RCC that has been kept under active surveillance or
treated non-operatively. Native scan, arterial ‘corticomedullary’
phase, parenchymal ‘nephrographic’ phase and pelvicalyceal
‘excretory’ phase are all essential techniques, and three-plane
reconstruction is suggested in all patients (5). Tumor size and local T staging have
been shown to be independent predictors of outcome, with higher T
stages portending a poorer survival rate; for example, the 5- and
10-year disease-free survival rates after surgery for T1, T2, T3a,
T3b and T3c tumors are ~95 and 91%, 80 and 70%, 66 and 53%, 52 and
43%, and 43 and 42%, respectively (5,6). In
local T staging of RCC (Table I),
T1 and T2 are solely determined by tumor size in the absence of
invasion of surrounding structures (7). The importance of local T staging also
matters for the operational approach; for T1a tumors, partial
nephrectomy (PN) or tumor enucleation has become standard, while
for larger tumors, PN is considered (if suitable), but is not yet
conventional (8). Staging a tumor
as T3a reduces overall tumor-related survival rates by 4-6 times,
requires radical nephrectomy and doubles the chance of distant
metastases (~16% for T2 and 34% for T3) (9,10).
Preoperative detection of the invasion of the renal vein (RV) or
its segments (T3a), the infradiaphragmatic inferior vena cava (IVC)
(T3b), the supradiphragmatic IVC or the IVC wall (T3c) is crucial,
as this will impact the operation plan and require a
multidisciplinary approach that includes cardiothoracic and
hepatobiliary teams. In some cases when distant metastasis is
present, only cytoreductive surgery is performed (11).
 | Table IPrimary renal cell carcinoma staging
(T staging). |
Table I
Primary renal cell carcinoma staging
(T staging).
| T category | T criteria |
|---|
| Tx | Primary tumor
cannot be assessed |
| T0 | No evidence of
primary tumor |
| T1 | Tumor <7 cm in
greatest dimension, limited to the kidney |
|
T1a | Tumor <4 cm in
greatest dimension, limited to the kidney |
|
T1b | Tumor >4 cm but
<7 cm in greatest dimension, limited to the kidney |
| T2 | Tumor >7 cm in
greatest dimension, limited to the kidney |
|
T2a | Tumor >7 cm but
<10 cm in greatest dimension, limited to the kidney |
|
T2b | Tumor >10 cm,
limited to the kidney |
| T3 | Tumor extends into
major veins or perinephric tissues. But not into the ipsilateral
adrenal gland and not beyond Gerota's fascia |
|
T3a | Tumor extends into
the renal vein or its segmental branches, the pelvicalyceal system,
or the perirenal and/or renal sinus fat but not beyond Gerota's
fascia |
|
T3b | Tumor extends into
the vena cava below the diaphragm |
|
T3c | Tumor extends into
the vena cava above the diaphragm or invades the wall of the vena
cava |
| T4 | Tumor invades
beyond Gerota's fascia (including contiguous extension into the
ipsilateral adrenal gland) |
The current study aimed to evaluate the accuracy of
CECT for the preoperative staging of RCC by using surgical and
pathological staging as reference methods.
Patients and methods
Study population
This is a single-center prospective study conducted
between October 2019 and November 2021 at the Radiology Center of
Sulaimani Teaching Hospital in Sulaimani, Iraq. It was performed
for individuals who had a renal mass and were diagnosed with RCC
based on clinical and imaging studies. The age of the patients
ranged from 31 to 80 years, with a median age of 52 years and a
mean age of 56.1 years. Among the patients, 35 (59.32%) were male
and 24 (40.68%) were female.
Ethical approval
The study was approved by the Arab Board Scientific
Committee of the Iraqi Ministry of Health (approval no. 22-2018).
All participants gave verbal and written informed consent for the
participation in the CECT, as well the publication of CECT data and
that of surgical and pathological data.
Inclusion and exclusion criteria
Adult patients with renal masses who had optimal
CECT, a pathology report confirming RCC and adequate local T
staging (within 3 weeks of surgery, containing native,
nephrographic and delayed phase images, and thin slices reformatted
in coronal and sagittal sections) were included in this study. The
exclusion criteria included the following: i) Patients in the
pediatric age group, as most of the renal tumors in this age group
are Wilm's tumors, and RCC is rarely encountered; ii) tumors
radiologically suspected to be RCC, but subsequently proved to be a
non-RCC tumor; iii) tumors radiologically consistent with AML,
transitional cell carcinoma or lymphoma; and iv) tumors
radiologically suspected to be RCC, but without pathological
confirmation.
Radiological evaluation
Patients with suspected RCC had their preoperative
abdominal CT scans reviewed. The study included native,
nephrographic and excretory phases with non-ionic intravenous (IV)
contrast. The contrast material routinely used for these scans was
Low Osmolar Contrast Media administered at a rate of 2-4 ml/sec
through an automated IV injector at a dose of 1-1.5 ml/kg.
The scan region extended from the diaphragm to the
symphysis pubis. CECT was performed with a thickness of 5 mm in the
axial plane, then reconstructed to a 1-2 mm thickness, and
reformatted to the coronal and sagittal planes.
Imaging data were collected, including tumor side
and size (largest tumor diameter in any plane), and perinephric fat
invasion. The latter was diagnosed when there was fat stranding in
addition to an irregular tumor edge, angular lobulation, and
nodular extension or obvious tumor invasion towards Gerota's
fascia, but without reaching it.
In the early stages, the excretory phase was
reviewed for perinephric fat stranding and the invasion of the
pelvicalyceal system (PCS) and renal sinus fat. Other parameters
that were assessed included tumor extension into the major RV or
its segmental branches, IVC extension or wall invasion in the
nephrographic and excretory phases, and the invasion of Gerota's
fascia (clear invasion by thickening and breaching, or contact
>1 cm).
Invasion of the adrenal gland and other surrounding
organs was determined by loss of the fat plane and contact (>1
cm) in all planes, loss of the fat plane between the tumor and the
organ with local change in enhancement or texture, or obvious tumor
extension into the organ. Lastly, the radiologic T stage was
recorded according to the Tumor-Node-Metastasis (TNM) staging
system (Table I) (5,6).
Operative and pathological
evaluation
Intraoperative notes were recorded, including the
operation type, perinephric fat invasion, RV or IVC tumor
extension, and surrounding organ invasion. Pathological data were
collected on tumor size, RCC type, presence of clear margins,
preservation of renal capsule or perinephric fat invasion, renal
sinus or PCS invasion, segmental or main RV extension, and
involvement of Gerota's fascia or nearby organs.
Data entry and statistical
analysis
The collected data were organized in Microsoft Excel
2016 (Microsoft Corporation) for better classification, and
statistical analysis was performed using STATA version 15
(StataCorp LP) and Microsoft Excel. Descriptive statistics (mean,
frequency, percentage) and analytical statistics (P-value) were
calculated for related data. Fisher's exact test was used for the
comparison of categorical data (as >20% of the cells contained
count numbers of <5), and unpaired the t-test was calculated for
comparison of the numerical data. The association between two
variables was measured by Pearson's correlation. P≤0.05 was
considered to indicate a statistically significant difference.
Results
In the current study, 59 patients with RCC were
included. The average age ± standard deviation of the patients was
56.24±12.70. Table II shows the
distribution of the tumors by sex, side and pole of the kidney, and
type of operation.
 | Table IIBaseline characteristics in patients
with renal cell carcinoma. |
Table II
Baseline characteristics in patients
with renal cell carcinoma.
| Base-line
characteristics | n (%) |
|---|
| Sex | |
|
Male | 35 (59.32) |
|
Female | 24 (40.68) |
| Side | |
|
Right | 32 (54.24) |
|
Left | 27 (45.76) |
| Pole of kidney | |
|
Upper | 11 (18.64) |
|
Middle | 12 (20.34) |
|
Lower | 17 (28.81) |
|
Upper and
middle | 10 (16.95) |
|
Lower and
middle | 6 (10.17) |
|
Diffuse
involvement | 3 (5.08) |
| Type of
operation | |
|
Radical
nephrectomy | 37 (62.71) |
|
Partial
nephrectomy | 19 (32.20) |
|
Enucleation
of mass | 2 (3.39) |
|
No
operation | 1 (1.69) |
The average tumor size in the radiological and
pathological staging was 6.72 and 6.47 cm, respectively; however,
the mean difference in tumor size between the radiological and
pathological findings was not statistically significant (mean
difference, 0.25 cm; 95% CI, -1.22 to 1.72; t=0.34; P=0.734).
Overall, the pathological measurements revealed that
71.19% (n=42) of the tumors were smaller, 11.89% (n=7) were of the
same size and 16.94% (n=10) were larger than the radiological
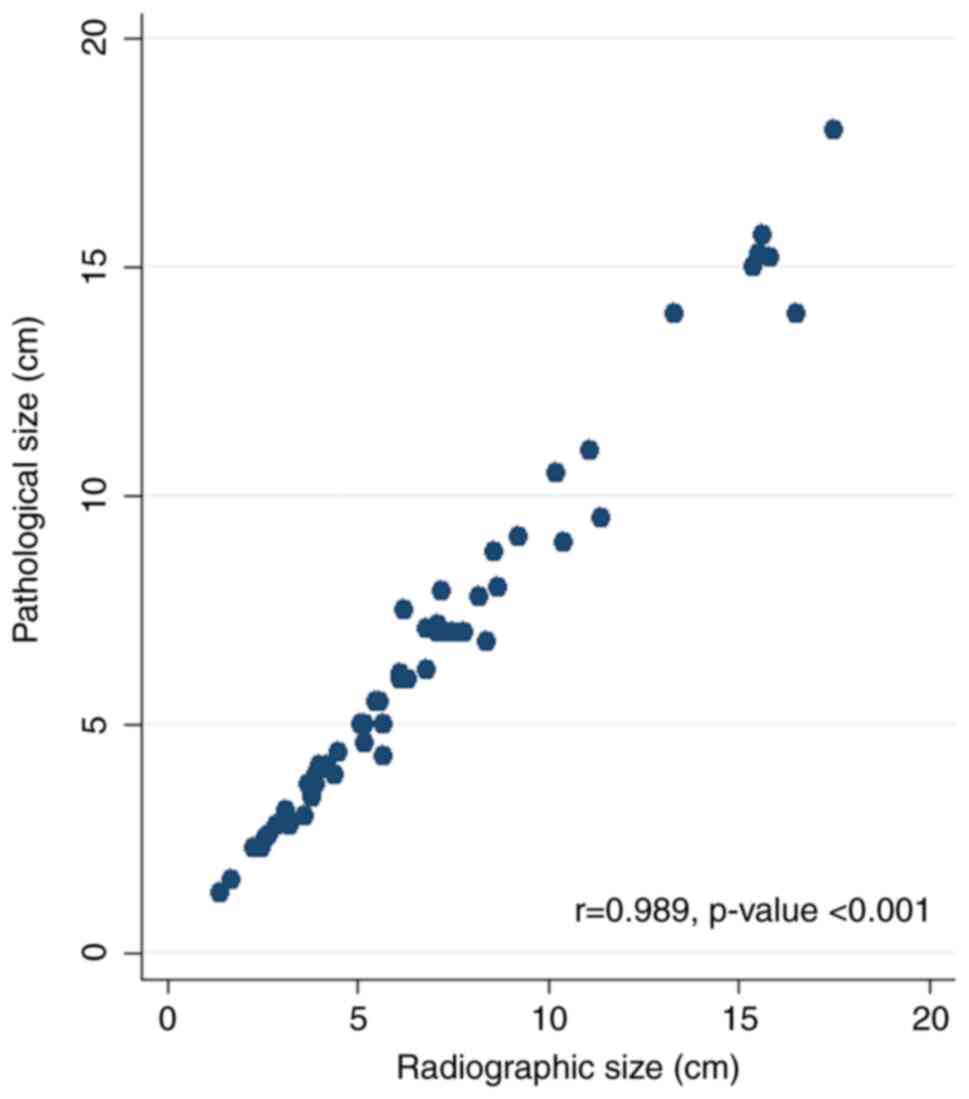
measurements. The scatter plot shows a positive linear correlation
between the pathological and radiological tumor sizes (r=0.989;
P<0.001; Fig. 1).
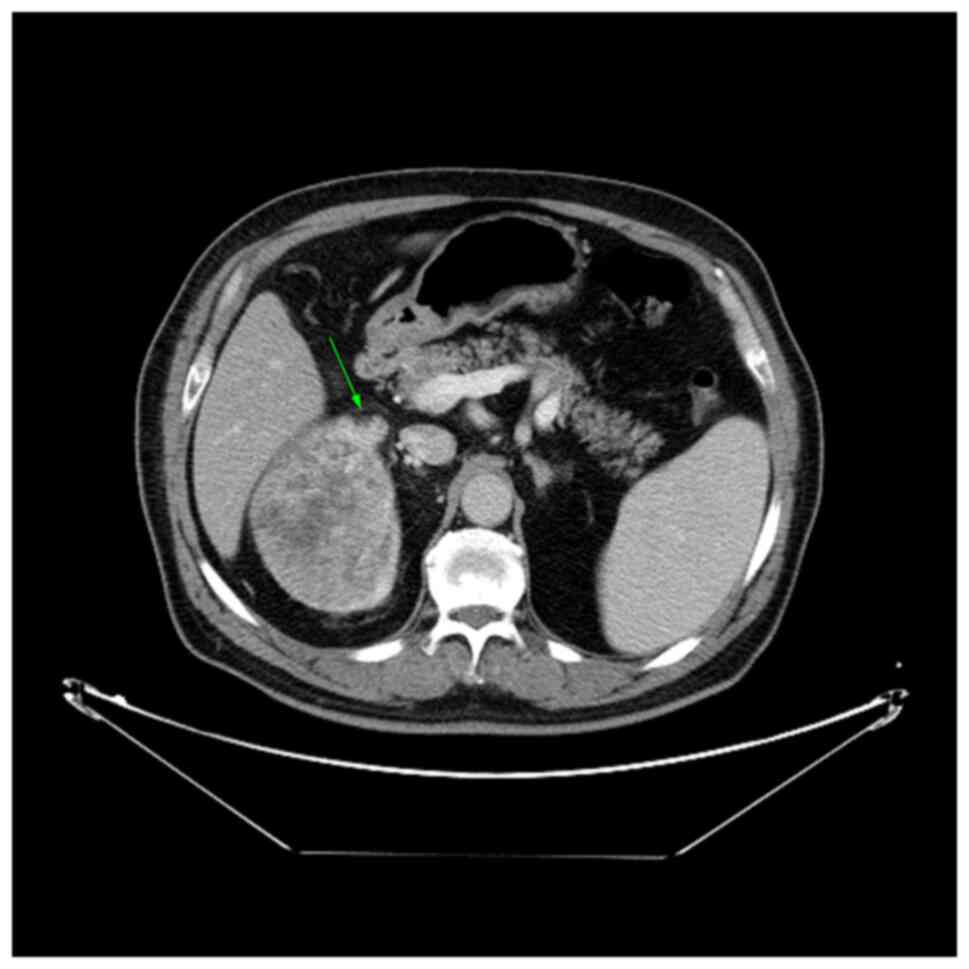
Fig. 2 demonstrates
one of the criteria for perinephric fat invasion, namely, a nodular
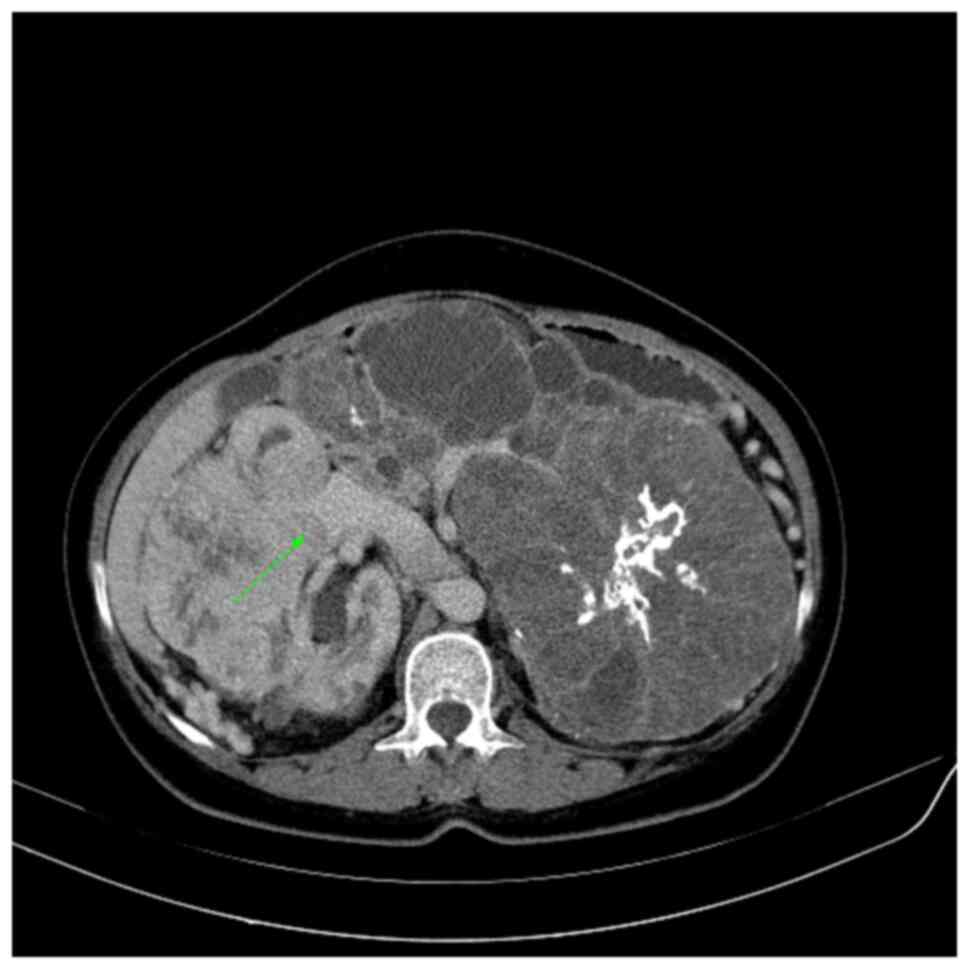
extension from the tumor to the perinephric fat (T3a). Fig. 3 shows an enhanced filling defect in
the right main renal vein in the arterial and nephrographic phases
without extension to the IVC.
CECT accurately detected perinephric fat invasion in
12 out of 15 cases and excluded invasion in 42 out of 44
pathologically negative cases. CECT was able to detect PCS and
sinus fat invasion in 15 out of 17 pathologically proven patients,
while excluding invasion in 40 out of 42 negative cases (Table III).
 | Table IIISensitivity, specificity and
predictive values of detecting invasion of perinephric fat, PCS and
sinus fat using contrast-enhanced computed tomography. |
Table III
Sensitivity, specificity and
predictive values of detecting invasion of perinephric fat, PCS and
sinus fat using contrast-enhanced computed tomography.
| Parameter | Perinephric fat
invasion | PCS or sinus fat
extension |
|---|
| Sensitivity, % | 80.00 | 88.23 |
| Specificity, % | 95.45 | 95.23 |
| PPV, % | 85.71 | 88.23 |
| NPV, % | 93.33 | 95.23 |
Regarding renal vein invasion, CECT detected 8 out
of 10 pathologically proven patients and excluded 46 out of 49
negative cases. CECT detected both cases of IVC invasion and
excluded 56 out of 57 pathologically negative cases. Furthermore,
four out of five cases of Gerota's fascia invasion were diagnosed
radiologically, and 52 out of 54 patients were accurately excluded.
Adrenal invasion was diagnosed in 1 out of 2 cases, while all 57
pathologically negative cases were excluded using CECT (Table IV).
 | Table IVSensitivity, specificity and
predictive values of detecting invasion of the renal vein, IVC,
Gerota's fascia and adrenal gland using contrast-enhanced computed
tomography. |
Table IV
Sensitivity, specificity and
predictive values of detecting invasion of the renal vein, IVC,
Gerota's fascia and adrenal gland using contrast-enhanced computed
tomography.
| Parameter | Renal vein
invasion | IVC extension | Gerota's fascia
invasion | Adrenal gland
direct invasion |
|---|
| Sensitivity, % | 80.00 | 100.00 | 80.00 | 50.00 |
| Specificity, % | 93.87 | 98.25 | 96.30 | 100.00 |
| PPV, % | 72.72 | 66.70 | 66.67 | 100.00 |
| NPV, % | 95.83 | 100.00 | 98.11 | 98.30 |
Table V clarifies
the important parameters by which radiologists decide RCC local
staging. In this table, a comparison between the radiological
suspicion of invasion and pathological true invasion has been
performed. These points are important to radiologists to calculate
the sensitivity and specificity of CT scans for detecting invasion
of these structures.
 | Table VAssociation of radiological detection
of types of invasion with pathological assessment. |
Table V
Association of radiological detection
of types of invasion with pathological assessment.
| Type of
invasion | Radiological, n
(%) | Pathological, n
(%) | P-value |
|---|
| Renal vein
invasion | | | 0.002 |
|
Segmental
branch | 8 (13.56) | 7 (11.86) | |
|
Main renal
vein | 3 (5.08) | 3 (5.08) | |
|
No
invasion | 48 (81.36) | 49 (83.05) | |
| IVC extension | | | 0.002 |
|
Infradiaphragmatic
invasion | 2 (3.39) | 1 (1.69) | |
|
IVC wall
invasion | 1 (1.69) | 1 (1.69) | |
|
No
extension | 56 (94.92) | 57 (96.61) | |
| Surrounding organ
invasion other than adrenal gland | | | <0.0001 |
|
Liver | 2 (3.39) | 1 (1.69) | |
|
Colon | 1 (1.69) | 1 (1.69) | |
|
Abdominal
muscle | 1 (1.69) | 1 (1.69) | |
|
Diaphragm | 1 (1.69) | 1 (1.69) | |
|
Psoas | 1 (1.69) | 0 (0.00) | |
|
Tail of
pancreas | 1 (1.69) | 0 (0.00) | |
|
No | 52 (88.14) | 55 (93.22) | |
Table VI provides
an overall view of the present study results, summarizing the
sensitivity, specificity, predictive values and accuracy of
contrast-enhanced computed tomography for local T staging. This
data enables other specialists who work on RCC (e.g., urologists,
oncologists and nephrologists) to understand the accuracy of CT
scans in the local staging of RCC.
 | Table VISensitivity, specificity, predictive
values and accuracy of contrast-enhanced computed tomography for
local T staging. |
Table VI
Sensitivity, specificity, predictive
values and accuracy of contrast-enhanced computed tomography for
local T staging.
| Stages | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | P-value |
|---|
| T1a | 90.00 | 97.44 | 94.74 | 95.00 | 94.92 | <0.001 |
| T1b | 75.00 | 91.49 | 69.23 | 93.48 | 88.14 | <0.001 |
| T2a | 60.00 | 98.15 | 75.00 | 96.36 | 94.92 | 0.002 |
| T2b | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.017 |
| T3a | 69.23 | 95.65 | 81.82 | 91.67 | 89.83 | <0.001 |
| T3b | 100.00 | 98.28 | 50.00 | 100.00 | 98.31 | 0.034 |
| T3c | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 0.017 |
| T4 | 83.33 | 96.23 | 71.43 | 98.08 | 94.92 | <0.001 |
Discussion
When used properly, a CT scan is considered a highly
accurate measure (100% sensitivity and 95% specificity) for
detecting renal masses. According to previous studies, CT scans can
detect and stage renal masses with up to 91% accuracy, making them
the imaging modality of choice (12,13).
The current study found that the mean pathological diameter of the
tumors was less than the radiological mean diameter (6.47 vs. 6.72
cm), with a mean difference of 0.25 cm. This size discrepancy, in
which radiological size is larger, is well known from previous
studies. Choi et al (14)
reported a 0.17-cm discrepancy. Chen et al (15) observed a 0.22-cm discrepancy.
Meanwhile, Nazim et al (12) found a 0.38-cm discrepancy. The
reduction in tumor size observed on pathological examination has
been attributed to a decrease in the blood volume in a highly
vascular renal tumor following ligation or blockage of the renal
artery (16). The reduction in
tumor size may also be due to the use of 10% buffered formalin for
pathological specimen fixation. Pathological size is an important
indicator of the prognosis of patients. However, radiological size
estimation is an essential component for selecting the appropriate
treatment in RCC (12).
The involvement of perinephric fat tissue is a
critical element in therapeutic planning. In fact, perirenal fat
tissue infiltration alters the surgical technique from conservative
to radical nephrectomy (13). One
of the most challenging aspects of staging renal tumors is
detecting perinephric fat invasion, which causes tumors of any size
to be classified as T3a. Catalano et al (13) demonstrated perinephric fat invasion
with a sensitivity and specificity of ~96 and 93%, respectively.
El-Hefnawy et al (17)
observed a specificity of 80%, while Sokhi et al (10) reported a sensitivity and
specificity of 83 and 76%, respectively. Liu et al (18) showed a sensitivity and specificity
of 32 and 86%, respectively. The current study found a sensitivity
and specificity of 80 and 95%, respectively, which was somewhat
higher than previous studies. These values depend on the criteria
for perinephric fat invasion, which varied among the studies. In
the present study, perinephric fat stranding was considered as
perinephric fat invasion in addition to other features, such as
perinephric nodules, an irregular tumor edge and angular
lobulation.
Using two phases (non-contrast and nephrographic),
Sokhi et al (10) found
renal sinus fat invasion sensitivity and specificity to be 71-88
and 71-79%, respectively. In the current study, PCS and renal sinus
fat invasion were detected by CECT with higher sensitivity and
specificity (88 and 95%, respectively) when compared with prior
studies. The higher sensitivity and specificity of the present
study can be attributed to the use of additional excretory phases,
which are effective in differentiating PCS compression or invasion.
Perinephric and renal sinus fat invasion are the most difficult to
diagnose with CT imaging, as perinephric fat stranding unrelated to
tumor invasion, a large tumor size, a previously unhealthy kidney,
and the presence of microscopic and radiologically undetectable
invasion all complicate interpretation. Sinus fat invasion is also
difficult to differentiate from compression (10,19).
Accurate preoperative assessment of invasion and the
extent of tumor thrombi in the RV and IVC is essential for a
surgeon to determine the right surgical strategy for thrombectomy
and to reduce the risk of perioperative tumoral embolism (20). RV can be radiologically assessed
for invasion when there is a hypodense filling defect that is
continuous with the tumor or an extended RV with an intraluminal
lesion, and the result becomes more specific when it is enhanced
(10,21). Tumors can be classified as stage
pT3a due to renal vein infiltration, renal sinus invasion or
extracapsular extension, which can be small and thus difficult to
detect on CT (2). In the study by
Sokhi et al (10), a renal
vein invasion with a specificity of 91-93%, but a sensitivity of
59-69%, was reported. Bradley et al (22) discovered renal vein involvement
with a sensitivity and specificity of 84 and 98%, respectively. The
sensitivity and specificity of the current study were 80 and 94%,
respectively. Karlo et al (23) noted that the tumor edge touching
the sinus fat was an accurate CT sign of branch RV invasion. Sokhi
et al (10) also
demonstrated that the presence of suspected sinus fat invasion,
numerous perinephric septa, stranding or vascularity, and thickened
perirenal fascia, especially in the case of a necrotic and
irregular tumor edge, should alert the radiologist to perform a
more proper examination of the renal veins.
IVC extension, like RV tumor extension, is described
when there is an enhancing filling defect within the IVC or a
non-enhancing lesion continuous with the renal tumor. Whether the
extension is infra- or supra-diaphragmatic does not appear to
affect the prognosis, but the invasion of the IVC wall greatly
reduces survival rate (20,24).
For IVC extension and invasion, magnetic resonance imaging (MRI)
demonstrated great accuracy, up to 100% (25). However, MRI is only used as a
problem-solving tool in indeterminate cases. Türkvatan et al
(20) reported IVC invasion with
100% accuracy. Nazim et al (12) showed a sensitivity and specificity
of 100 and 97%, respectively. The sensitivity and specificity for
detecting IVC invasion in the current study were 100 and 98%,
respectively.
The T3a stage is the most difficult to define
precisely. Reznek (25) calculated
the sensitivity and specificity for T3a stage diagnosis to be 46
and 98%, respectively. In an investigation by El-Hefnawy et
al (17), T3a sensitivity and
specificity were found to be 51 and 80%, respectively. The overall
sensitivity and specificity for T3 in the current study were 80 and
97%, respectively, while the sensitivity and specificity for T3a
were 69.2 and 95.6%, respectively.
Gerota's fascia invasion and expansion are difficult
to distinguish. The studies by Reznek (25) and Tsili and Argyropoulou (26) discussed the difficulty of Gerota's
fascia infiltration without mentioning the accuracy of CT or MRI
(25,26). Bradley et al (22) showed that thickening of Gerota's
fascia had a sensitivity and specificity of 52 and 90%,
respectively. The sensitivity and specificity of the present study
were 80 and 96%, respectively.
The absence of barrier planes between renal cancer
and the surrounding structures raises the possibility of
neighboring organ invasion (stage T4) (17). Direct expansion of RCC beyond
Gerota's fascia and into adjacent organs is difficult to identify
without a proven localized change in attenuation within the
diseased organ (20). Larger
tumors touching adjacent organs make it challenging to determine
whether invasion is evident radiologically (22). The study by Reznek (25) claimed that organ invasion should be
suggested only when there is enlargement or alteration in density,
but did not discuss CECT accuracy; however, the study did report an
MRI accuracy for organ invasion of ~97% (25). In the current study, radiological
invasion of surrounding organs employing the selected criteria
provided a sensitivity of 100%, a positive predictive value of 57%
and a specificity of 95%.
According to the study by Liu et al (18), the overall accuracy of T staging is
75%. El-Hefnawy et al (17)
reported an overall T staging accuracy of 65%. Türkvatan et
al (20) demonstrated an
accuracy of 89% in a study of 57 cases. In an investigation by Kim
et al (27), an accuracy of
about 87% in 144 cases was reported. The overall local T staging
accuracy of the current study was estimated to be at least 80%.
These differences might be attributed to the number of patients
included in the study, as well as the imaging characteristics
employed for local staging.
Despite the advantages of the current study, it also
has crucial limitations, as it was a single-center study and it had
a small sample size.
In conclusion, CECT is accurate in the local T
staging of RCC, with a high sensitivity and specificity regarding
the assessment of tumor size, extension to nearby structures and
venous invasion.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The research was registered in the Research Registry
(registration number: researchregistry7516) and is available at the
following link: https://www.researchregistry.com/browse-the-registry#home/?view_2_search=7516&view_2_page=1.
Authors' contributions
SHT, FHK, and RJR performed the radiological
assessments and confirm the authenticity of all the raw data. FHK
contributed to manuscript drafting. LAA pathologically examined the
specimens, was a major contributor to the study conception and
revised the manuscript. SMF and AMS were major contributors to the
study conception, and the revision and final revision of the
manuscript. FHF and DHR contributed to the conception and the
design of the study. IA and RB analyzed and interpreted the data.
SSF, BAA and SHM collected patient data and thoroughly revised the
content of the manuscript. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Arab Board Scientific
Committee of the Iraqi Ministry of Health (approval no. 22-2018).
Written informed consent was obtained from all the patients.
Patient consent for publication
The representative patient in Figs. 2 and 3 provided consent for the publication of
diagnostic images and data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3(17009)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Warren AY and Harrison D: WHO/ISUP
classification, grading and pathological staging of renal cell
carcinoma: Standards and controversies. World J Urol. 36:1913–1926.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ljungberg B, Campbell SC, Cho HY, Jacqmin
D, Lee JE, Weikert S and Kiemeney LA: The epidemiology of renal
cell carcinoma. Eur Urol. 60:615–621. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ng CS, Wood CG, Silverman PM, Tannir NM,
Tamboli P and Sandler CM: Renal cell carcinoma: Diagnosis, staging,
and surveillance. AJR Am J Roentgenol. 191:1220–1232.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Frederick LG, Page DL, Fleming ID, Fritz
AG, Balch CM, Haller DG and Morrow M: AJCC Ccancer Staging Manual.
6th Edition. Springer, New York, NY, 2002.
|
|
7
|
Tsui KH, Shvarts O, Smith RB, Figlin RA,
deKernion JB and Belldegrun A: Prognostic indicators for renal cell
carcinoma: A multivariate analysis of 643 patients using the
revised 1997 TNM staging criteria. J Urol. 163:1090–1095, 1295.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang N, Wu Y, Wang J, Xu J, Na R and Wang
X: The effect of discrepancy between radiologic size and pathologic
tumor size in renal cell cancer. Springerplus.
5(899)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chandrasekar T, Klaassen Z, Goldberg H,
Kulkarni GS, Hamilton RJ and Fleshner NE: Metastatic renal cell
carcinoma: Patterns and predictors of metastases-a contemporary
population-based series. Urol Oncol Semin Orig Investig.
35:661.e7–661.e14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sokhi HK, Mok WY and Patel U: Stage T3a
renal cell carcinoma: Staging accuracy of CT for sinus fat,
perinephric fat or renal vein invasion. Br J Radiol.
88(20140504)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rendon RA, Kapoor A, Breau R, Leveridge M,
Feifer A, Black PC and So A: Surgical management of renal cell
carcinoma: Canadian kidney cancer forum consensus. Can Urol Assoc
J. 8:E398–E412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nazim SM, Ather MH, Hafeez K and Salam B:
Accuracy of multidetector CT scans in staging of renal carcinoma.
Int J Surg. 9:86–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Catalano C, Fraioli F, Laghi A, Napoli A,
Pediconi F, Danti M, Nardis P and Passariello R: High-resolution
multidetector CT in the preoperative evaluation of patients with
renal cell carcinoma. AJR Am J Roentgenol. 180:1271–1277.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choi SM, Choi DK, Kim TH, Jeong BC, Seo
SI, Jeon SS, Lee HM, Choi HY and Jeon HG: A comparison of
radiologic tumor volume and pathologic tumor volume in renal cell
carcinoma (RCC). PLoS One. 10(e0122019)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen W, Wang L, Yang Q, Liu B and Sun Y:
Comparison of radiographic and pathologic sizes of renal tumors.
Int Braz J Urol. 39:189–194. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Herr HW: Radiographic vs surgical size of
renal tumours after partial nephrectomy. BJU Int. 85:19–21.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
El-Hefnawy AS, Mosbah A, El-Diasty T,
Hassan M and Shaaban AA: Accuracy of multi-detector computed
tomography (MDCT) in staging of renal cell carcinoma (RCC):
Analysis of risk factors for mis-staging and its impact on surgical
intervention. World J Urol. 31:887–891. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Y, Song T, Huang Z, Zhang S and Li Y:
The accuracy of multidetector computed tomography for preoperative
staging of renal cell carcinoma. Int Braz J Urol. 38:627–636.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zagoria RJ, Bechtold RE and Dyer RB:
Staging of renal adenocarcinoma: Role of various imaging
procedures. AJR Am J Roentgenol. 164:363–370. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Türkvatan A, Akdur PO, Altinel M, Olçer T,
Turhan N, Cumhur T, Akinci S and Ozkul F: Preoperative staging of
renal cell carcinoma with multidetector CT. Diagn Interv Radiol.
15:22–30. 2009.PubMed/NCBI
|
|
21
|
Bonsib SM: Renal veins and venous
extension in clear cell renal cell carcinoma. Mod Pathol. 20:44–53.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bradley AJ, MacDonald L, Whiteside S,
Johnson RJ and Ramani VA: Accuracy of preoperative CT T staging of
renal cell carcinoma: Which features predict advanced stage? Clin
Radiol. 70:822–829. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Karlo CA, Donati OF, Marigliano C, Tickoo
SK, Hricak H, Russo P and Akin O: Role of CT in the assessment of
muscular venous branch invasion in patients with renal cell
carcinoma. AJR Am J Roentgenol. 201:847–852. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hatcher PA, Anderson EE, Paulson DF,
Carson CC and Robertson JE: Surgical management and prognosis of
renal cell carcinoma invading the vena cava. J Urol. 145:20–24.
1991.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Reznek RH: CT/MRI in staging renal cell
carcinoma. Cancer Imaging. 4 (Spec No A):S25–S32. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tsili AC and Argyropoulou MI: Advances of
multidetector computed tomography in the characterization and
staging of renal cell carcinoma. World J Radiol. 7:110–127.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim CK, Choi D, Park BK, Kang TW and Kim
JH: Diagnostic performance of multidetector-row CT for predicting
the preoperative staging of renal cell carcinoma. J Korean Soc
Radiol. 60:109–116. 2009.
|