Introduction
Primary plasma cell leukemia (pPCL) is a rare and
aggressive plasma cell disorder (1,2). The
prognosis of pPCL is poor. The median overall survival ~12 months
based on patients untreated with novel drugs (2). Over the past decade, the use of new
agents, such as proteasome inhibitors or immunomodulatory drugs,
followed by high-dose melphalan conditioning and autologous stem
cell transplantation (ASCT) or allogeneic hematopoietic stem cell
transplantation (allo-HSCT), has improved prognosis in younger
patients with pPCL. However, the outcome of patients with pPCL has
only slightly improved (1,3-12).
Due to the low incidence of pPCL, there are no large prospective
and randomized trials to support high-level evidence on the timing
and role of the SCT, thus making it difficult to evaluate whether
ASCT or allo-HSCT is more beneficial for pPCL patients. In the
present study, a case of pPCL that was successfully treated with
intensive chemotherapy combined with ASCT and sequential allo-HSCT
followed by maintenance treatment with ixazomib, thalidomide and
dexamethasone (IRD) was reported.
Case report
A 26-year-old man who suffered repeated nosebleeds
was admitted to the Changhai Hospital on April, 2020. A complete
blood count revealed a white blood cell count of
53.5x109/l (with 14% blasts), hemoglobin level of 68 g/l
and platelets count of 22x109/l. The serum
immunoglobulin G (IgG) level was 41.5 g/l and an IgG-kappa type-M
component was identified by serum immunofixation. The serum free
light chain kappa/lambda ratio was 193.202. Peripheral blood smear
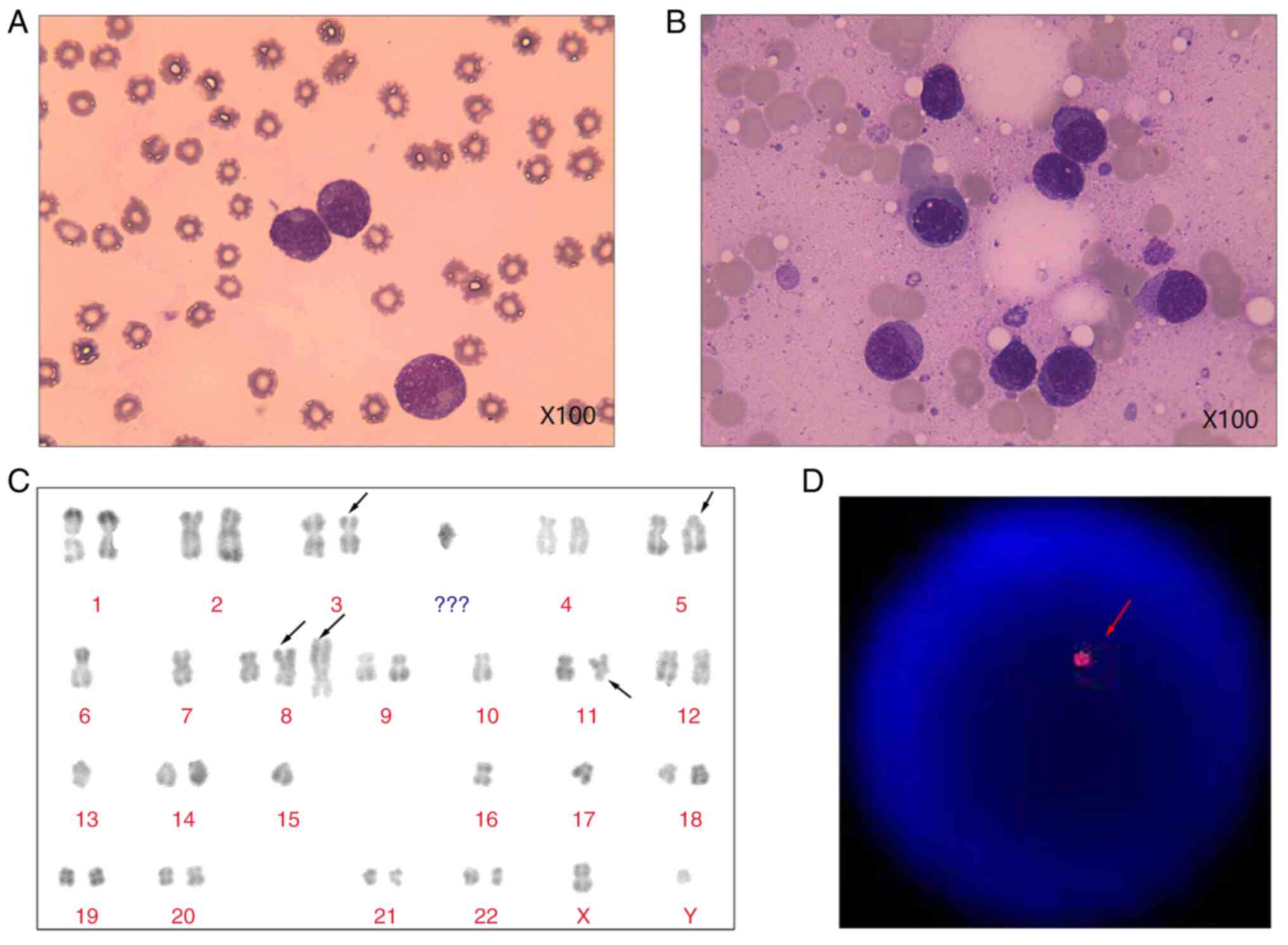
and bone marrow aspiration revealed 62 and 74% plasmacytes,
respectively (Fig. 1A and B). Immunophenotypic analysis using
multiparameter flow cytometry revealed a cluster of 60.445%
neoplastic plasma cells that were positive for CD38, CD138, CD56
and cKappa, and negative for cLambda, CD19, and CD20. The
antibodies were purchased from BD Biosciences. A total of 1 ml
freshly isolated whole BM aspirate was collected, of which 400 µl
was used to lyse erythrocytes using RBC lysis buffer (cat. no.
R1010, Beijing Solarbio Science & Technology Co., Ltd.). After
washing once, the mononuclear cells were obtained and stained with
monoclonal antibodies for 15 min at room temperature. Following
mononuclear cells were washed, collected, and analyzed following
the manufacturer's instructions using a FACSAria II instrument (BD
Biosciences). The karyotype of the patient was 41-44, XY, -X, del
(3) (p21), -4, inv (5) (p11q13), -6, -7, add (8) (p21), +add (8) (p21), -9, -10, -11, -12, del (11) (q23), del (13) (q132q22), -13, -15, -16, -17, +mar
[CP4]/46, XY [7] (Fig. 1C).
Fluorescence in situ hybridization analysis using
locus-specific identifier probes was performed with Isis Software
(MetaSystems). Hybridized chromosome slides were analysed using an
epifluorescence microscope Axio imager A2 (Carl Zeiss AG). The
result showed the typical TP53 deletions. (Fig. 1D). The image of positron emission
tomography-computed tomography (PET-CT) revealed hypermetabolic
activity in the bone marrow (data not shown). Therefore, the
patient was diagnosed as having IgG-kappa pPCL.
The patient received four cycles of the VTD-PACE
regimen (bortezomib, thalidomide, dexamethasone and 4 days of
continuous infusions of cisplatin, doxorubicin, cyclophosphamide
and etoposide) and were well-tolerated. After the end of
chemotherapy sessions, previous laboratory-assessed abnormalities
were obviously subsided, and consisted with the very good partial
response revealed by the positivity of serum immunofixation. Next,
high-dose melphalan (100 mg/m2 for two consecutive days)
was administered along with ASCT, and in so doing, stringent
complete remission (sCR) was achieved. For the cure of pPCL, the
patient underwent matched, unrelated allo-HSCT, along with
myeloablative conditioning with the FAB regimen (fludarabine,
cytarabine and busulfan) at the time of six months after ASCT. The
numbers of infused mononuclear cells and CD34+ cells
were 6.83x108/kg and 3.10x106/kg,
respectively. A post-transplant cyclophosphamide-based regimen was
administered to prevent acute graft-vs.-host disease (aGVHD).
Neutrophil and platelet were engrafted at 14 days after allo-HSCT.
The patient developed grade I skin aGVHD 35 days after allo-HSCT
and responded well to steroid treatment. IRD regimen was used
(ixazomib, thalidomide and dexamethasone) as a maintenance therapy,
which was planned to last 1 year.
The follow-up ended on November 1, 2022 (19 months
after allo-HSCT) and the patient remained in sCR. The present study
was performed in accordance with the ethical standards formulated
of the Helsinki Declaration, and informed consent was obtained from
the patient and his family.
Discussion
pPCL is a rare, aggressive plasma cell disorder, for
which there are no established therapeutic regimens. Although the
availability of novel agents and increasing use of SCT strategies
have resulted in improved outcomes, long-term survival remains poor
(2,4-6,11).
Jurczyszyn et al (7) reported that pPCL patients who
underwent upfront ASCT (n=55) had a superior median overall
survival (OS) than those (n=98) who did not receive ASCT (35 months
vs. 13 months, P<0.001). Another retrospective study suggested
that allo-HSCT was more beneficial for PCL patients. The results
demonstrated that the median progression-free survival (PFS) was 6
months in the ASCT group (n=9) compared with 18 months in the
allo-HSCT group (n=7), and the median OS was 19 months and 40
months, respectively (10).
However, results from the Center for International Blood and Marrow
Transplant Research (CIBMTR) exhibited superior outcomes with
upfront ASCT over allo-HSCT for patients with pPCL (3-year OS: 64
vs. 39%; 3-year relapse: 61 vs. 38%). Although relapse rates were
lower with allo-HSCT, treatment-related mortality was significantly
higher, ultimately resulting in the lack of a survival benefit
(4). In addition, other results
from the CIBMTR comparing ASCT and allo-HSCT showed that the
survival outcomes were comparable (11).
Due to a lack of multicenter clinical trial-based
evidence, it is not clear whether ASCT or allo-HSCT should be
pursued (Table I) (3-12).
Thus, developing complex treatment algorithms that combine novel
agents, SCT and post-transplantation remission strategies should be
considered. A recent study confirmed the safety and efficacy of
sequential ASCT-allo-HSCT in relapsed Hodgkin lymphoma (13). Similarly, in multiple myeloma
patients, sequential ASCT-allo-HSCT demonstrated PFS and OS at 5
years of 41 and 80%, respectively, with a non-relapse mortality of
12% (14). In patients with pPCL,
who have a higher risk of relapse after ASCT than patients with
Hodgkin lymphoma or multiple myeloma, allo-HSCT could be used as a
potential consolidation strategy after ASCT to improve clinical
outcomes. Thus, to improve the survival of the young individual
with pPCL in the present report, after initial induction of
VTD-PACE treatment, sequential ASCT-allo-HSCT followed by
maintenance therapy with IRD were administered. The patient
maintained sCR for more than 19 months after allo-HSCT.
 | Table IPublished data on patients with pPCL
who underwent ASCT or Allo-HSCT. |
Table I
Published data on patients with pPCL
who underwent ASCT or Allo-HSCT.
| Year | Number of patients
underwent SCT | Treatment | Survival | (Refs.) |
|---|
| 2011 | 23 | ASCT (n=17);
Allo-HSCT (n=2); tandem auto/allo- HSCT (n=4) | Total transplanted
patients: median OS was 38.1 months | (3) |
| 2012 | 147 | ASCT (n=97) | 3 years PFS was 34%;
3 years OS was 64%; 3 years relapse was 61%; 3 years NRM was
5% | (4) |
| | | Allo-HSCT (n=50) | 3 years PFS was 20%;
3 years OS was 39%; 3 years relapse was 38%; 3 years NRM was
41% | |
| 2016 | 26 | Allo-HSCT (n=1) | NA | (5) |
| | | ASCT (n=2) | NA | |
| 2018 | 23 | ASCT | Median PFS was 5.5
months; median OS was 18.1 months | (6) |
| 2018 | 55 | ASCT | Median OS was 35
months | (7) |
| 2018 | 19 | ASCT (n=13);
Allo-HSCT (n=1); Tandem auto/allo- HSCT (n=4); tandem auto/auto-
HSCT (n=1) | Total transplanted
patients: median OS was 35.5 months | (8) |
| 2019 | 28 | ASCT | Median PFS was 25
months; median OS was 36 months | (9) |
| 2020 | 16 | ASCT (n=9) | Median PFS was 6
months; median OS was 19 months | (10) |
| | | Allo-HSCT (n=7) | Median PFS was 18
months; median OS was 40 months | |
| 2020 | 348 | ASCT (n=277) | 4 years PFS was 17%;
4 years OS was 28%; 4 years relapse was 76%; 4 years NRM was
7% | (11) |
| | | Allo-HSCT (n=
71) | 4 years PFS was 19%;
4 years OS was 31%; 4 years relapse was 69%; 4 years NRM was
12% | |
In conclusion, the present case report demonstrated
the early use of novel agents and an upfront sequential ASCT and
allo-HSCT approach with encouraging results in pPCL, which could
achieve a deeper response and improve prognosis. Further
mechanistic studies are required to investigate this
phenomenon.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (NSFC; grant no. 81771779) and Initial
Scientific Research Fund of Young scholars from Changhai Hospital
in Shanghai (grant no. 2020QNA03).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WF, AH and YL collected, verified and interpreted
the patient information and drafted the manuscript. ML and GT
performed laboratory analysis. JY and XN designed the research,
interpreted the data and critically reviewed and revised the
manuscript. WF and XN confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the guidelines of the Declaration of Helsinki, and granted an
exemption from requiring ethics approval by the Ethics Committee of
Changhai Hospital (Shanghai, China).
Patient consent for publication
Informed consent regarding the publication of
clinical data was obtained from the patient and his family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nandakumar B, Kumar SK, Dispenzieri A,
Buadi FK, Dingli D, Lacy MQ, Hayman SR, Kapoor P, Leung N, Fonder
A, et al: Clinical characteristics and outcomes of patients with
primary plasma cell leukemia in the era of novel agent therapy.
Mayo Clin Proc. 96:677–687. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gonsalves WI, Rajkumar SV, Go RS,
Dispenzieri A, Gupta V, Singh PP, Buadi FK, Lacy MQ, Kapoor P,
Dingli D, et al: Trends in survival of patients with primary plasma
cell leukemia: A population-based analysis. Blood. 124:907–912.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pagano L, Valentini CG, De Stefano V,
Venditti A, Visani G, Petrucci MT, Candoni A, Specchia G, Visco C,
Pogliani EM, et al: Primary plasma cell leukemia: A retrospective
multicenter study of 73 patients. Ann Oncol. 22:1628–1635.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mahindra A, Kalaycio ME, Vela-Ojeda J,
Vesole DH, Zhang MJ, Li P, Berenson JR, Bird JM, Dispenzieri A,
Gajewski JL, et al: Hematopoietic cell transplantation for primary
plasma cell leukemia: Results from the Center for International
Blood and Marrow Transplant Research. Leukemia. 26:1091–1097.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Royer B, Minvielle S, Diouf M, Roussel M,
Karlin L, Hulin C, Arnulf B, Macro M, Cailleres S, Brion A, et al:
Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction
followed by stem cell transplantation for primary plasma cell
leukemia: A prospective phase II study of the intergroupe
francophone du myelome. J Clin Oncol. 34:2125–2132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gowda L, Shah M, Badar I, Bashir Q, Shah
N, Patel K, Kanagal-Shamanna R, Mehta R, Weber DM, Lee HC, et al:
Primary plasma cell leukemia: Autologous stem cell transplant in an
era of novel induction drugs. Bone Marrow Transplantation.
54:1089–1093. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jurczyszyn A, Radocha J, Davila J, Fiala
MA, Gozzetti A, Grzasko N, Robak P, Hus I, Waszczuk-Gajda A,
Guzicka-Kazimierczak R, et al: Prognostic indicators in primary
plasma cell leukaemia: A multicentre retrospective study of 117
patients. Br J Haematol. 180:831–839. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ganzel C, Rouvio O, Avivi I, Magen H,
Jarchowsky O, Herzog K, Cohen Y, Tadmor T, Horwitz NA, Leiba M, et
al: Primary plasma cell leukemia in the era of novel agents for
myeloma-a multicenter retrospective analysis of outcome. Leuk Res.
68:9–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mina R, Joseph NS, Kaufman JL, Gupta VA,
Heffner LT, Hofmeister CC, Boise LH, Dhodapkar MV, Gleason C, Nooka
AK, et al: Survival outcomes of patients with primary plasma cell
leukemia (pPCL) treated with novel agents. Cancer. 125:416–423.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lemieux C, Johnston LJ, Lowsky R, Muffly
LS, Craig JK, Shiraz P, Rezvani A, Frank MJ, Weng WK, Meyer E, et
al: Outcomes with autologous or allogeneic stem cell
transplantation in patients with plasma cell leukemia in the era of
novel agents. Biol Blood Marrow Transplant. 26:e328–e332.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dhakal B, Patel S, Girnius S, Bachegowda
L, Fraser R, Davila O, Kanate AS, Assal A, Hanbali A, Bashey A, et
al: Hematopoietic cell transplantation utilization and outcomes for
primary plasma cell leukemia in the current era. Leukemia.
34:3338–3347. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Katodritou E, Terpos E, Delimpasi S,
Kotsopoulou M, Michalis E, Vadikolia C, Kyrtsonis MC, Symeonidis A,
Giannakoulas N, Vadikolia C, et al: Real-world data on prognosis
and outcome of primary plasma cell leukemia in the era of novel
agents: A multicenter national study by the Greek Myeloma Study
Group. Blood Cancer J. 8(31)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bento L, Boumendil A, Finel H, Khvedelidze
I, Blaise D, Fegueux N, Castagna L, Forcade E, Chevallier P,
Mordini N, et al: Tandem autologous-reduced intensity allogeneic
stem cell transplantation in high-risk relapsed Hodgkin lymphoma: A
retrospective study of the Lymphoma Working Party-EBMT. Bone Marrow
Transplant. 56:655–663. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
LeBlanc R, Ahmad I, Terra R, Boudreault
JS, Ogez D, Lamore K, Delisle JS, Bambace N, Bernard L, Cohen S, et
al: Outcomes in newly diagnosed young or high-risk myeloma patients
receiving tandem autologous/allogeneic transplant followed by
bortezomib maintenance: A phase II study. Bone Marrow Transplant.
57:252–260. 2022.PubMed/NCBI View Article : Google Scholar
|