Introduction
Esophageal cancer (EC) is the seventh most prevalent
type of cancer and the sixth leading cause of cancer-related
mortality worldwide (1,2). This type of cancer has two major
histological subtypes, squamous cell carcinoma (SCC) and
adenocarcinoma (AC). SCC is the primary pathological subtype in
East Asia, eastern and southern Africa, and Southern Europe, while
AC is predominant in North America and other parts of Europe
(3). Esophageal squamous cell
carcinoma (ESCC) develops from precancerous lesions, gradually
followed by the progression of hyperplasia, dysplasia and SCC
(4). Patients with locally
advanced cancer frequently develop recurrent disease after surgery
alone, and either chemotherapy or chemoradiotherapy is recommended
as an adjunct to surgery for such patients (5). The 5-year survival rate of patients
with ESCC is ~18%, which reflects late diagnosis, the
aggressiveness of the disease and a lack of effective treatment
strategies (6,7). The poor prognosis of patients with
ESCC may be due to tumor invasion and metastasis (8). In patients with advanced-stage or
metastatic ESCC, combination chemotherapy regimens extend survival;
however, the median survival rate remains <1 year (9). Therefore, therapeutic efficacy needs
to be evaluated through biomarkers to predict the prognosis and
treatment response of patients with locally advanced resectable
ESCC.
Vascular endothelial growth factor (VEGF) is a
critical and effective factor that stimulates angiogenesis and
participates in tumor invasion and metastasis (10). Serum VEGF levels are of diagnostic
and prognostic value in certain types of cancer, including EC
(11,12). Greater invasion depth and higher
histological grade are associated with a higher risk of recurrence
(13-16).
The high incidence of synchronous and metachronous metastases are
the main reasons for the poor prognosis of patients with
superficial ESCC. The recurrence of superficial ESCC following
esophagectomy normally involves regional lymph node or distant
metastases (17). Although a high
serum VEGF level is a prognostic factor for patients with locally
advanced ESCC (12), whether the
serum VEGF levels can predict recurrence is yet to be
elucidated.
In the present study, association between serum VEGF
levels and, the curative effects and prognostic values were
investigated in patients with advanced-stage ESCC following
curative esophagectomy followed by chemotherapy or concurrent
radiotherapy, particularly in patients with recurrent ESCC. The
significance of the serum VEGF levels, as a predictor of
recurrence, was also evaluated in patients with locally advanced
resectable ESCC.
Patients and methods
Patients and treatment modality
Between January, 2012 and June, 2016, a total of 173
patients with a mean age of 60.9±8.1 years, included 147 males and
26 females, with locally advanced resectable ESCC confirmed by
histopathological analysis were enrolled at Jiangsu Cancer Hospital
(Nanjing, China). These patients were diagnosed with stage III or
stage IV ESCC using to the latest TNM staging by the International
Union Against Cancer (2009). The patients were classified as having
locally advanced resectable ESCC and underwent R0 resection.
Metastasis was confirmed by imaging, including lymphatic metastasis
and distant metastasis. A total of 183 healthy controls included
142 males and 41 females were enrolled between January, 2013 and
December, 2013 at Nanjing Yian physical examination center
(Nanjing), with a mean age of 48.3±13.7 years. The present study
was approved by the Biomedical Research Ethics Committee of Jiangsu
Cancer Hospital (approval no. 2010ke-052). All of the participants
provided written informed consent. The experiments were performed
in accordance with the Declaration of Helsinki. All patients were
treated with chemotherapy, with a chemotherapy cycle once every 3-4
weeks. Among them, 57 patients were treated with concurrent
radiotherapy at four different intervals with the course of
radiotherapy being 4-6 weeks. Initially, the patients did not
receive any treatment (pre-treatment group). The patients then
underwent chemotherapy (Chemo group) or concurrent radiotherapy
(Chemo + Radio group) after R0 resection. In Chemo group, patients
received at least four cycles of chemotherapy with different
chemotherapeutic drugs. Data for the first four cycles were
analyzed. Chemotherapy regimens included the TP regimen, which was
taxane (PTX, TAX, TXT or DOC) combined with platinum, the PF
regimen which was 5-fluorouracil and its derivatives (5-FU, FT207
or CAPE) combined with platinum, and the GP regimen which was
gemcitabine (GEM) combined with platinum. The platinum was one of
DDP, LBP, CBP and NDP (18). The
patients received concurrent radiotherapy for 30-45 days at the
first course of chemotherapy, with radiotherapy schemes, such as
GTV (Gross Tumor Volume) 60-65 Gy/28-33 fractions (f), CTV
(Clinical Target Volume) 50-55 Gy/28-33 f and PTV (Planning Target
Volume) 50-66 Gy/28-33 f. A total of 5 months after the end of
chemotherapy or concurrent radiotherapy, 57 patients exhibited
recurrence at the original lesion or metastasis, including lymph
node metastasis and distal metastasis; these patients were then
classified as patients with recurrent disease (recurrent patient
group). Patients with recurrence then received further treatments,
including chemotherapy or concurrent radiotherapy. In the course of
further treatment, the chemotherapy regimens or radiotherapy
regimens were the same as those aforementioned.
Serum samples and detection of serum
VEGF levels
Blood samples were collected at the pre-treatment
stage and at four intervals during chemotherapy (at 21-28 days
after each of the 4 cycles of chemotherapy). At the beginning of
the next cycle, samples were collected and tested for VEGF levels
to monitor the previous treatment cycle. Samples from the recurrent
patient group were collected at recurrence (re-0 cycle), and at day
21-28 after the completion of the course of further treatment (re-1
and re-2 cycle). The samples were stored at -80˚C following
centrifugation for 10 min at 1,500 x g at 4˚C.
The serum levels of VEGF were measured using Luminex
FLEXMAP 3D instruments and xPONENT™ software (Luminex Corporation)
with Human cytokine/chemokine panels (cat. no. MPXHCYTO-60K-01;
MilliporeSigma). The preparation of blood samples, the setting of
detection parameters and the calculation of serum VEGF levels were
performed according to the manufacturers' protocols (19).
Statistical analysis
Serum VEGF levels are presented as the mean ± SD.
The differences between two groups were analyzed using an unpaired
Student's t-test. The comparisons of ≥3 groups were performed using
ANOVA, followed by pair-wise comparisons using the Bonferroni post
hoc test. Patient characteristics for patients with recurrent and
non-recurrent ESCC were analyzed using Pearson's χ2 test
or Fishers exact test (where the expected count in >20% of cells
was <5). The follow-up period ended on April 25, 2020, and the
overall survival (OS) was calculated. The cut-off values for the OS
and recurrence-free survival (RFS) of patients with regard to serum
VEGF levels were assessed using the receiver operating
characteristic (ROC) and the area under curve (AUC), sensitivity
and specificity were also calculated (Table SI). Multivariate analysis was
performed using the Cox proportional hazards regression model. RFS
rates were calculated from the date of surgery to the date of
recurrence using the Kaplan-Meier method and the significance of
comparisons between groups was measured using a log-rank test. When
survival curves crossed over, these data were analyzed using the
weighted, two-stage test. P<0.05 was considered to indicate a
statistically significant difference. All the statistical analyses
were performed using GraphPad Prism 5 (Dotmatics), except Pearson's
χ2 test and Fishers exact test which were performed
using SPSS Statistics 21.0 (IBM).
Results
Serum VEGF levels are increased in
patients with locally advanced resectable ESCC and are maintained
at high levels in patients with recurrent disease following
therapy
Compared with the healthy controls, patients with
stage III and stage IV ESCC had significantly higher serum VEGF
levels (P<0.001), with the mean serum VEGF level in the patients
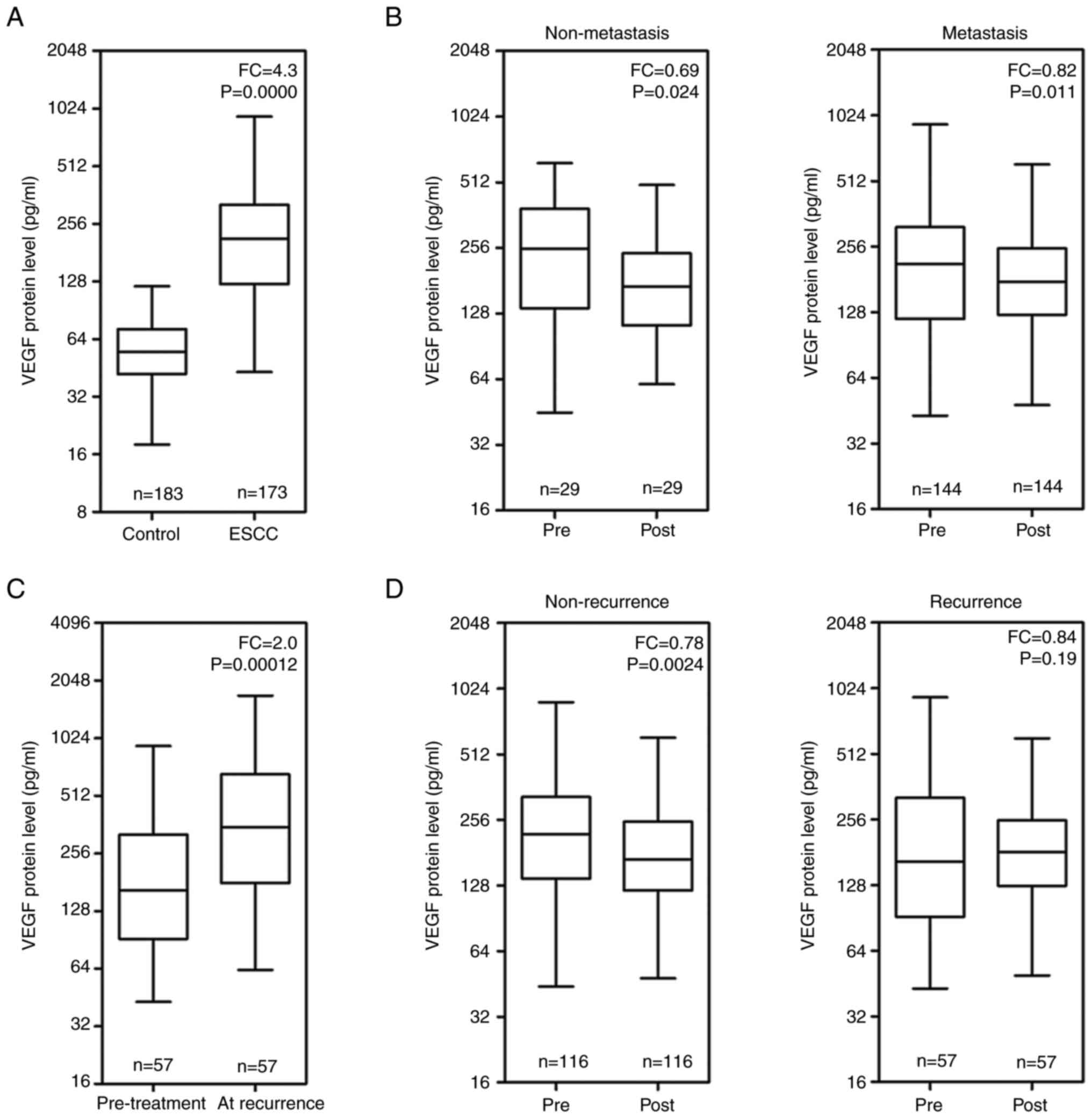
being 4.3-fold that of the controls (Fig. 1A). Compared with the pre-treatment
stage, the serum VEGF levels significantly decreased in both
non-metastatic and metastatic patients with locally advanced
resectable ESCC following treatment (P<0.05; Fig. 1B). The serum VEGF levels in 57
patients with locally advanced resectable ESCC with recurrent
disease were significantly higher at the time of recurrence
compared with the levels in the same patients at the pre-treatment
stage (P<0.001; Fig. 1C). The
serum VEGF level was significantly lower in patients with no
disease recurrence following treatment compared with the levels at
the pre-treatment stage (P<0.01). However, the difference in the
serum VEGF levels between the pre-treatment and post-treatment
stage was not significant in patients with recurrence (P>0.05;
Fig. 1D).
The clinical characteristics of patients at the
pre-treatment stage were presented in Table SII. The serum VEGF levels in the
patients with recurrent disease were significantly higher compared
with those in patients with no recurrence (P<0.001). Serum VEGF
levels in patients with lymph node metastasis were significantly
higher compared with those in patients with distal metastasis
(P<0.05). The clinical characteristics of patients with
recurrence or no recurrence were presented in Table SIII.
Serum VEGF levels changed in patients
with locally advanced resectable ESCC following chemotherapy or
concurrent radiotherapy
Serum VEGF levels markedly decreased at the 1st
treatment cycle compared with pre-treatment, then increased
gradually after the 1st treatment cycle in patients treated with
concurrent radiotherapy and after the 3rd treatment cycle in
patients treated with chemotherapy alone (Fig. 2A). Compared with patients with
metastatic locally advanced resectable ESCC, patients with
non-metastatic locally advanced resectable ESCC exhibited a greater
degree of decline in serum VEGF levels following treatment with the
four treatment cycles (Fig. 2B).
Serum VEGF levels tended to increase in patients with a survival
time ≤60 months after the 3rd treatment cycle (Fig. 2C). Serum VEGF levels in patients
with recurrent disease markedly increased, in a gradual manner, at
the 3rd and 4th treatment cycles compared with those in patients
with no recurrence (Fig. 2D).
Serum VEGF levels in patients with recurrent disease treated with
concurrent radiotherapy were notably lower at the 1st and 2nd
treatment cycles, and then notably higher at the 4th treatment
cycle compared with those in patients with recurrent disease
treated with chemotherapy alone (Fig.
2E). Following recurrence, patients continued to receive
further treatments, including chemotherapy or concurrent
radiotherapy. However, the serum VEGF levels in the patients with
recurrent disease demonstrated no significant differences after two
cycles of further treatments (P>0.05; Fig. 2F).
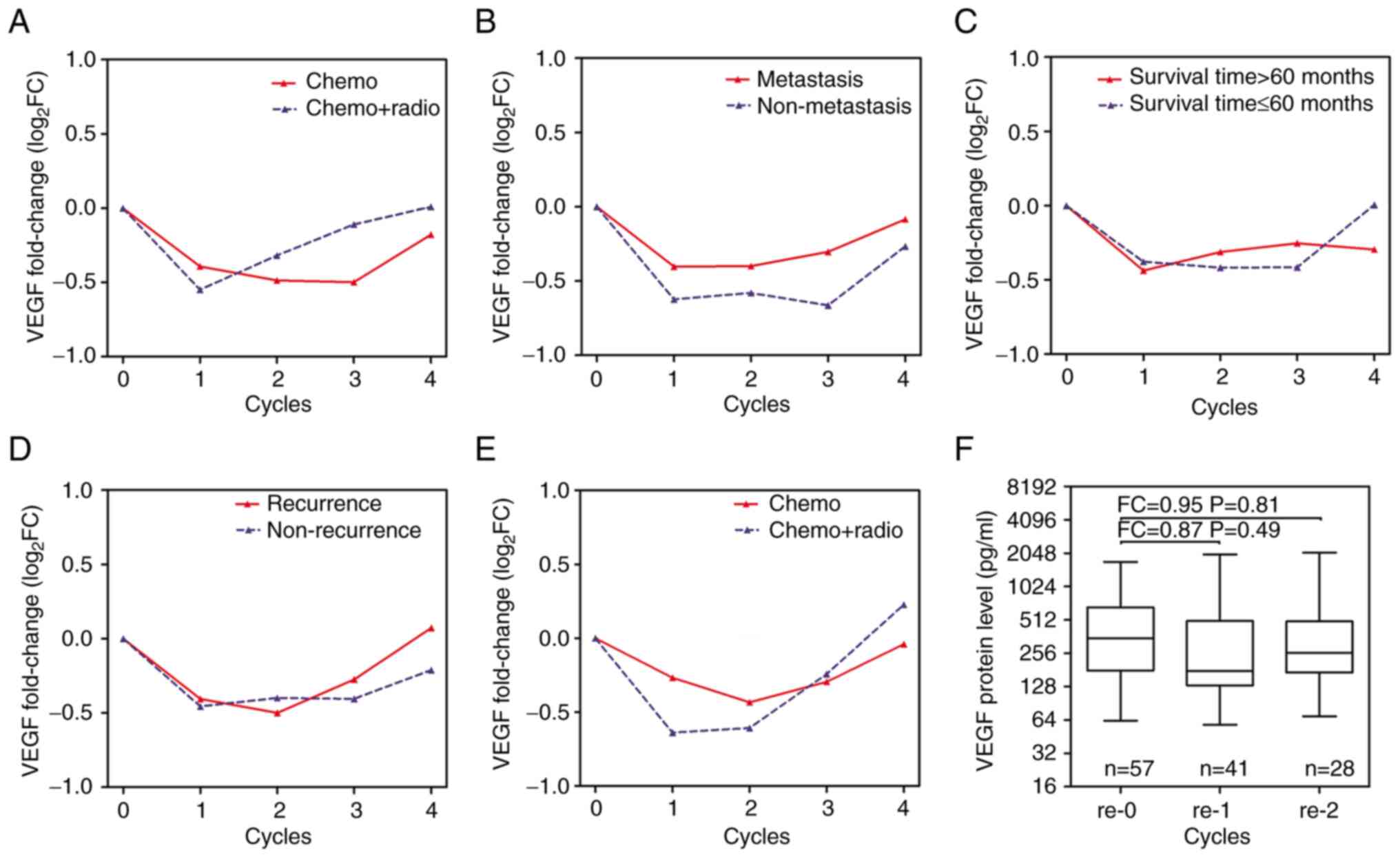 | Figure 2Changes in the serum VEGF level in
patients with locally advanced resectable ESCC following treatment.
Mean changes in serum VEGF levels for (A) treatment modality, (B)
metastasis, (C) survival time and (D) recurrence relative to the
pre-treatment stage in patients with locally advanced resectable
ESCC. (E) Mean changes in serum VEGF levels for treatment modality
relative to the pre-treatment stage in 57 patients with recurrent
disease. (F) Median values of VEGF levels in 57 patients with
recurrent disease following further treatment. The levels were
assessed at recurrence (re-0 cycle) and at the 1st (re-1 cycle),
2nd cycle (re-2 cycle) following further treatments in 57 patients
with recurrent disease. Chemo, patients received at least four
cycles of chemotherapy; Chemo + Radio, patients received concurrent
radiotherapy at the 1st cycle of chemotherapy; FC, fold change
which is the ratio of the mean of VEGF levels at each cycle to the
mean value of VEGF levels at 0 cycle or re-0 cycle; VEGF, vascular
endothelial growth factor; log2FC, log value of the fold
change; chemo, chemotherapy; radio, radiotherapy. |
Serum VEGF levels remain high in
patients with recurrent disease at the fourth treatment cycle
No significant difference was demonstrated in serum
VEGF levels between the patients who underwent chemotherapy and
concurrent radiotherapy, for patients with either non-metastatic or
metastatic disease (P>0.05; Fig.
3A). Similarly, no significant difference was demonstrated in
serum VEGF levels between the patients who underwent chemotherapy
and concurrent radiotherapy, for those with no recurrence and those
with recurrent disease (P>0.05; Fig. 3B). Although serum VEGF levels
fluctuated during the four treatment cycles, the median values of
the serum VEGF levels following chemotherapy were always lower than
those in the non-metastatic patients at the pre-treatment stage
(P>0.05; Fig. 3C). Patients
with metastatic disease who received chemotherapy or concurrent
radiotherapy, median serum VEGF levels at the 1st treatment were
significantly decreased compared with those at pre-treatment
(P<0.05; Fig. 3D). Serum VEGF
levels significantly decreased in patients with no recurrence
treated with chemotherapy alone, at the 1st, 2nd and 3rd cycles,
compared with the levels at the pre-treatment stage (P<0.01;
Fig. 3E). A significant decrease
in serum VEGF levels was demonstrated in patients with no
recurrence treated with concurrent radiotherapy, at the 1st
treatment cycle, compared with the levels at the pre-treatment
stage (P<0.05). Although no significant differences were
demonstrated in the serum VEGF levels at the 2nd, 3rd and 4th
treatment cycles, the median values of the serum VEGF levels
trended to decrease gradually in patients with no recurrence that
underwent concurrent radiotherapy (Fig. 3E). Serum VEGF levels did not
demonstrate a significant change in patients with recurrence during
either treatment compared with the levels at the pre-treatment
stage (P>0.05; Fig. 3F).
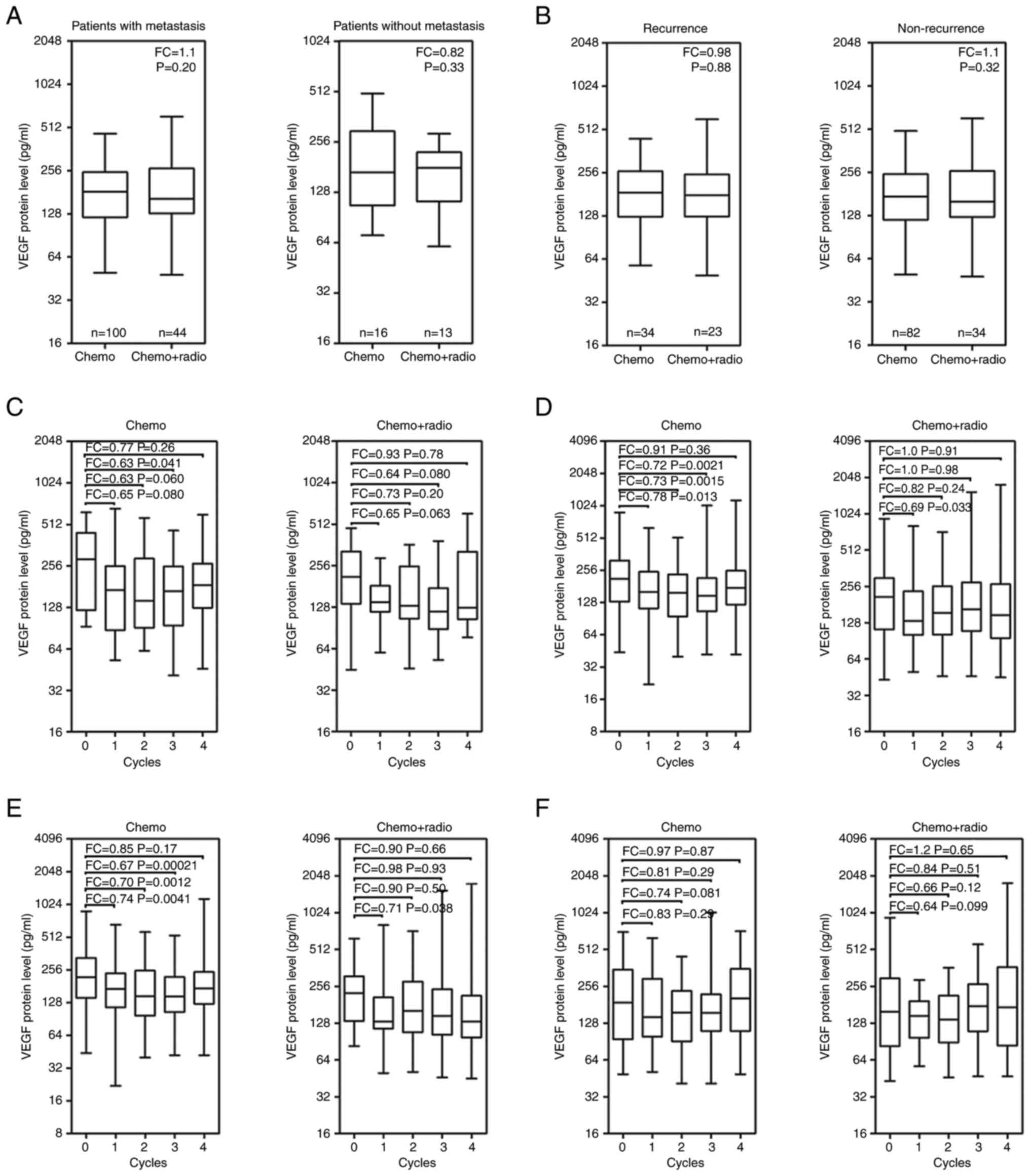 | Figure 3Boxplots of serum VEGF levels in
patients with locally advanced resectable ESCC following the
treatments. Median VEGF levels in (A) metastatic and non-metastatic
patients, and (B) patients with recurrence and no recurrence
treated with Chemo and Chemo + Radio, respectively. Median VEGF
levels from pre-treatment (0 cycles) to the 1st, 2nd, 3rd and 4th
cycles of treatment in (C) non-metastatic patients, (D) metastatic
patients, (E) non-recurrent patients and (F) recurrent patients.
Chemo, patients received at least four times of chemotherapy; Chemo
+ Radio, patients received concurrent radiotherapy at the 1st cycle
of chemotherapy; FC, fold change; VEGF, vascular endothelial growth
factor. In C-F, FC was the ratio of the mean of VEGF levels at each
cycle to the mean value of VEGF levels at 0 cycle. |
Prognostic value of the serum VEGF
level in patients with locally advanced resectable ESCC
Patient characteristics, including age, sex, stage,
metastasis, treatment modality, treatment evaluation and serum VEGF
levels were analyzed with regard OS using univariate analysis. The
OS of patients with ESCC differed significantly between patients
with serum VEGF levels <137 and ≥137 pg/ml at the pre-treatment
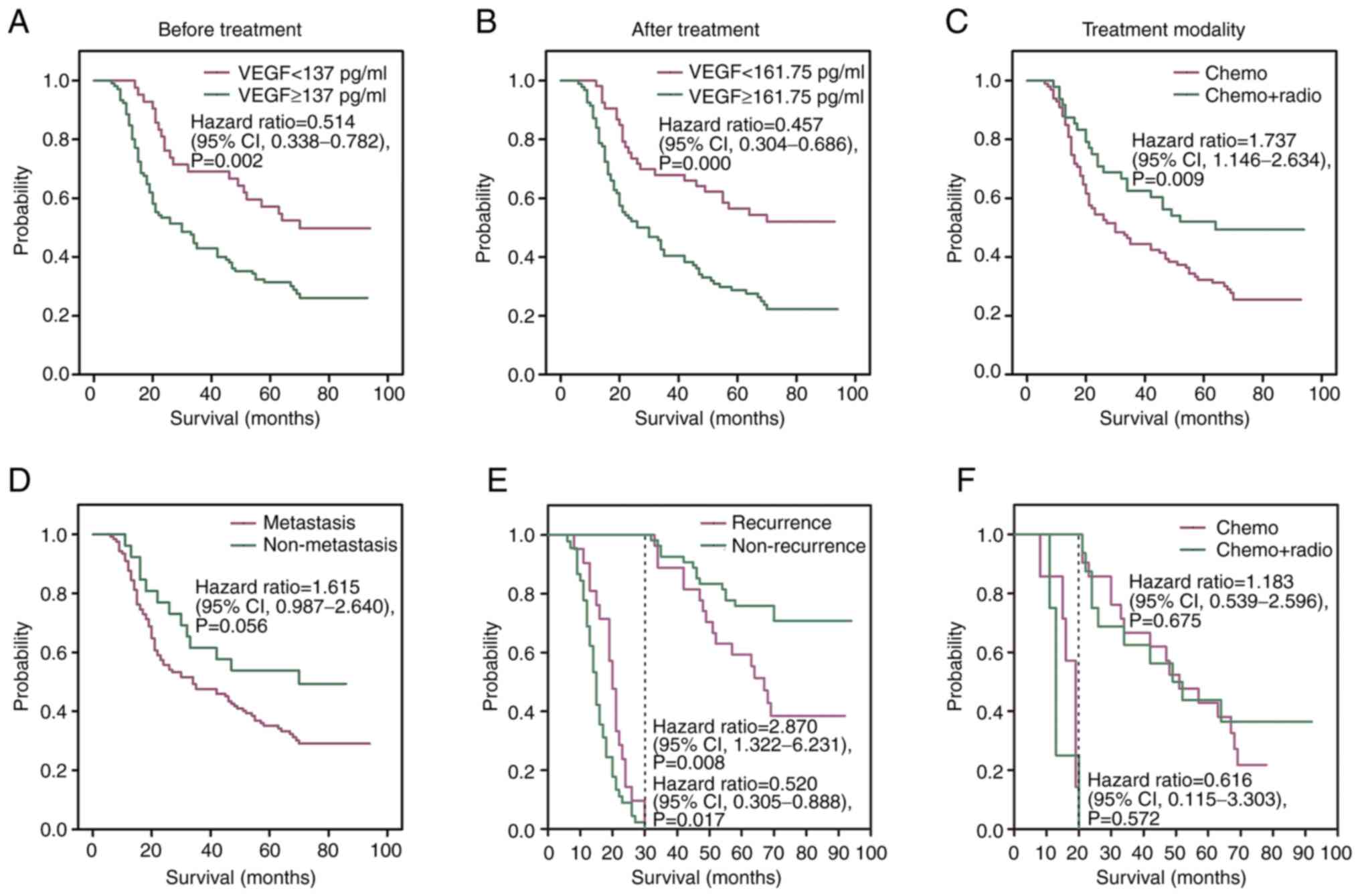
stage (P=0.002; Fig. 4A). The
median OS rates were 70 and 25 months in patients with locally
advanced resectable ESCC with serum VEGF levels <161.75 and
≥161.75 pg/ml following treatment, respectively (P=0.0002; Fig. 4B). Furthermore, patients treated
with concurrent radiotherapy had a significantly longer OS
(51.4±27.8 months), compared with the OS of patients treated with
chemotherapy (40.9±27.9 months) (P=0.009; Fig. 4C). Although metastasis was
associated with a poor prognosis, no significant differences were
demonstrated in the OS of patients with non-metastatic and
metastatic disease (P=0.056; Fig.
4D). The two-stage test demonstrated the OS between patients
with recurrence and patients without recurrence, with 30 months as
a node. Compared with patients with recurrence, patients without
recurrence had a significantly longer OS among patients with who
survived >30 months (P=0.008; Fig.
4E). Among patients who survived <30 months, patients with
recurrent had a significantly greater survival time compared with
patients without-recurrence (P=0.017; Fig. 4E). The two-stage test also
demonstrated the OS between patients with recurrence treated with
concurrent radiotherapy and chemotherapy, with 20 months as a node.
However, post-operative concurrent radiotherapy for patients with
recurrence did not significantly prolong OS compared with
chemotherapy only among patients with survival <20 months or
survival ≥20 months, (P=0.572 and P=0.675, respectively; Fig. 4F).
Serum VEGF level is a predictor of
recurrence in patients with locally advanced resectable ESCC
Age, sex, stage, metastasis, treatment modality,
treatment evaluation and serum VEGF level, were indicated to be
significant univariate factors, which predicted post-operative
recurrence. The serum VEGF level following treatment was the only
independent predictor of recurrence in patients with locally
advanced resectable ESCC following treatment, as demonstrated by
multivariate Cox logistic regression analysis (Table I). No significant difference in the
recurrence-free survival rates was demonstrated between patients
with high serum VEGF levels (≥102 pg/ml) and low serum VEGF levels
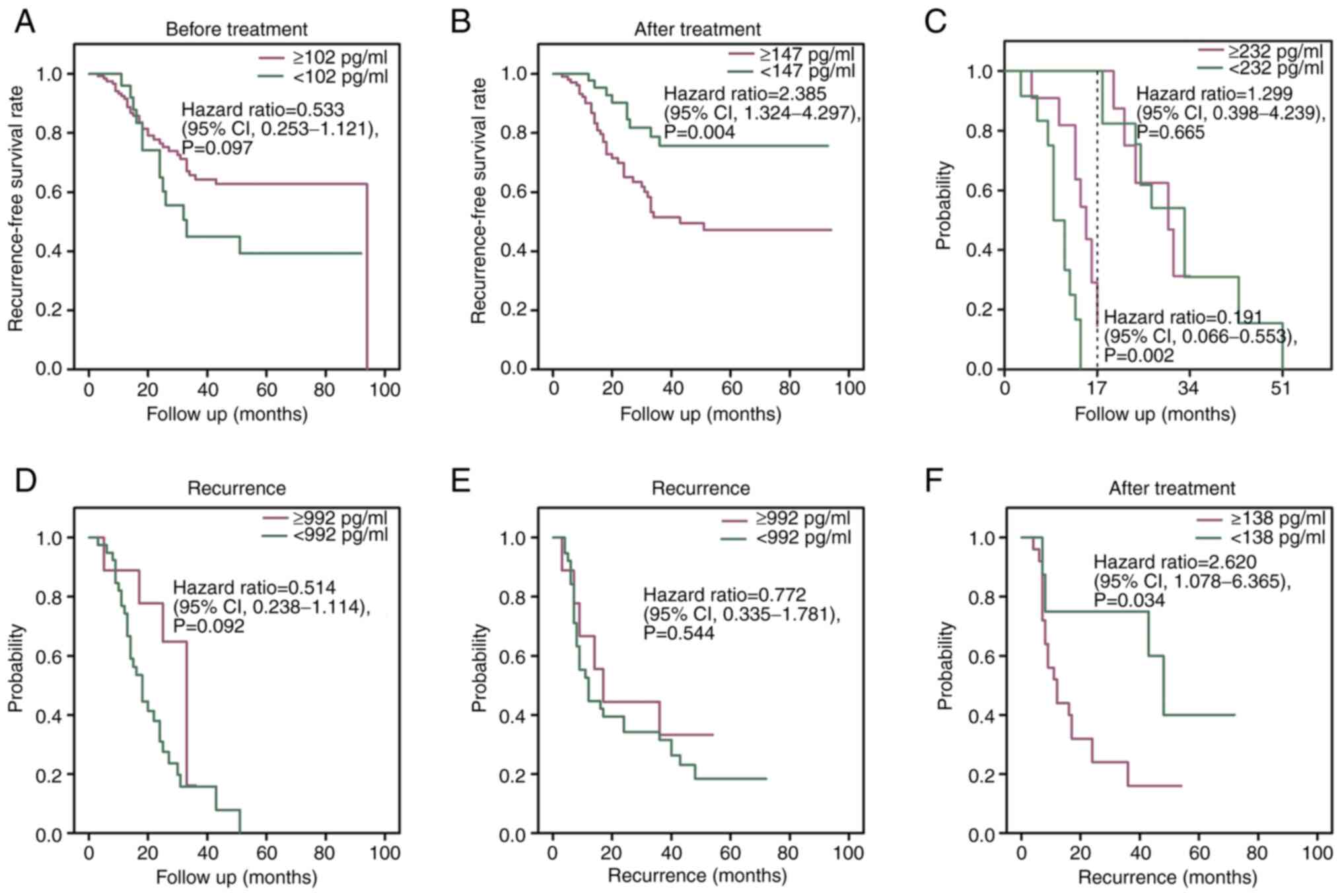
(<102 pg/ml) at the pre-treatment stage (P=0.097; Fig. 5A). However, patients with low serum
VEGF levels (<147 pg/ml) had significantly higher RFS rates
compared with those with high serum VEGF levels (≥147 pg/ml)
following treatment (P=0.004; Fig.
5B). The two-stage test demonstrated that the time from the
post-operative stage to recurrence when compared between serum VEGF
levels <232 and ≥232 pg/ml in 57 patients with recurrent disease
following chemotherapy or concurrent radiotherapy, had 17 months as
a node. Among patients who survived ≥17 months, serum VEGF levels
(232 pg/ml) demonstrated no significant correlation with the time
from the post-operative stage to recurrence (P=0.665; Fig. 5C). However, patients who survived
<17 months, with serum VEGF levels <232 pg/ml had a
significantly shorter time period from the post-operative stage to
recurrence than those with serum VEGF ≥232 pg/ml (P=0.002; Fig. 5C). Furthermore, serum VEGF levels
(992 pg/ml) at recurrence in 57 recurrent patients were not
associated with the time from the post-operative stage to
recurrence (P=0.092; Fig. 5D). The
survival from recurrence to the end of follow-up was also
calculated. Patients with recurrent disease with high serum VEGF
levels (≥992 pg/ml) and low serum VEGF levels (<992 pg/ml) at
recurrence had similar survival times (P=0.544; Fig. 5E).
 | Table IUnivariate and multivariate analyses
to predict postoperative recurrence. |
Table I
Univariate and multivariate analyses
to predict postoperative recurrence.
| | Univariate |
Multivariate3 |
|---|
| Group | Total, n | Recurrence, n
(%) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Gender | | | 0.699 | | |
|
Male | 147 | 51 (34.6) | | | |
|
Female | 26 | 6 (23.1) | | | |
| Age, years | | | 0.205 | | |
|
≤60 | 88 | 35 (39.8) | | | |
|
60-70 | 62 | 19 (30.6) | | | |
|
≥70 | 23 | 3 (13.0) | | | |
| TNM staging | | | 0.308 | | |
|
III | 62 | 23 (37.1) | | | |
|
Ⅳ | 111 | 34 (30.6) | | | |
| Metastasis | | | 0.741 | | |
|
Yes | 144 | 48 (33.3) | | | |
|
No | 29 | 9 (31.0) | | | |
| Metastasis
mode | | | 0.1354 | | |
|
Lymph node
metastasis | 84 | 29 (34.5) | | | |
|
Distal
metastasis | 13 | 4 (30.8) | | | |
|
Both | 47 | 15 (31.9) | | | |
|
Treatment1 | | | 0.290 | | |
|
Chemo | 116 | 34 (29.3) | | | |
|
Chemo +
Radio | 57 | 23 (40.4) | | | |
|
Evaluation2 | | | 0.968 | | |
|
Improved | 127 | 44 (34.6) | | | |
|
No
healed | 9 | 5 (55.6) | | | |
|
Missing | 37 | | | | |
| Serum VEGF | | | <0.001 | 1.747
(0.780-3.909) | 0.175 |
|
≥147 | 112 | 42 (37.5) | | | |
|
<147 | 61 | 15 (24.6) | | | |
All patients with recurrent disease received further
treatments, including chemotherapy or concurrent radiotherapy. A
low serum VEGF level following further treatment was beneficial for
the survival of patients with recurrent disease. Patients with
recurrent disease with low serum VEGF levels (<138 pg/ml)
following further treatment had a significantly longer survival
time compared with those with high serum VEGF levels (≥138 pg/ml)
(P=0.034; Fig. 5F). The changes in
serum VEGF levels before and after treatment in individual patients
with recurrent disease were examined. Compared with VEGF levels
before treatment, the VEGF levels of 27 patients increased and 30
patients decreased after treatment. There were no differences in
the concentrations of serum VEGF, RFS and OS between high degree
(>100% increase) group and low degree (≤100% increase) group in
patients who had higher VEGF levels after treatment than before
treatment (P>0.05; Table II).
However, compared with the low degree (≤50% decrease) group, serum
VEGF levels in the high degree (>50% decrease) group were
significantly lower in patients who had lower VEGF levels after
treatment than before treatment (P<0.05; Table II).
 | Table IIThe individual changes in serum VEGF
levels before and after treatment in patients with recurrent
disease. |
Table II
The individual changes in serum VEGF
levels before and after treatment in patients with recurrent
disease.
| VEGF levels after
treatment | Degree | Total (n=57) | Serum VEGF, mean ±
SD pg/ml | P-value | Recurrence-free
survival, mean ± SD months | P-value | Survival, mean ± SD
months | P-value |
|---|
| Up | High
(>100%) | 10 | 242.05±80.41 | 0.462 | 18.89±6.60 | 0.337 | 51.67±23.37 | 0.712 |
| | Low (≤100%) | 17 | 206.28±137.39 | | 23.14±11.81 | | 47.71±25.48 | |
| Down | High (>50%) | 9 | 120.58±52.27 | 0.01 | 19.17±11.89 | 0.942 | 33.17±23.37 | 0.618 |
| | Low (≤50%) | 21 | 210.00±101.24 | 9 | 18.79±10.69 | | 38.89±24.42 | |
Discussion
The majority of patients with EC are diagnosed at an
advanced stage and the prognosis of metastatic patients is
extremely poor, with a median OS of 4-6 months, due to the
aggressiveness of the disease (20,21).
Although surgical resection is a potential mainstay for curable EC,
locoregional recurrence occurs in 23.8-58.0% of cases (22-26).
In the present study, 32.9% (57/173) of the patients experienced
recurrence, which suggested that the cohort of patients with
advanced-stage disease in the present study was representative.
However, the serum VEGF levels remained elevated in patients with
recurrence following treatment compared with the levels at the
pre-treatment stage, which tended to increase at the 4th treatment
cycle; this indicated that the serum VEGF level may be a potential
biomarker for recurrence in patients with locally advanced
resectable ESCC.
Extensive local infiltration and regional lymph node
metastasis render the complete removal of tumors difficult, which
is one of the possible reasons for the failure of ESCC treatment
(27). In the present study, the
serum VEGF levels in 57 patients with recurrent ESCC were not only
higher at recurrence compared with the pre-treatment stage, but
also remained elevated following further treatment, which indicated
that a high serum VEGF level was associated with recurrence in
patients with locally advanced resectable ESCC. Moreover, the
patients with serum VEGF levels ≥147 pg/ml had a significantly
higher risk of developing recurrence following treatment. The mean
serum VEGF levels in the patients with recurrence were ~2-fold
higher than those in the patients with no recurrence at the
pre-treatment stage (488.65 vs. 256.45 pg/ml), while the serum VEGF
levels in the patients with no recurrence significantly decreased
following treatment. These results indicated that the serum VEGF
level was a predictor of recurrence in patients with locally
advanced resectable ESCC, particularly in patients with high serum
VEGF levels following chemotherapy or concurrent radiotherapy.
Similarly, a previous study reported that, multivariate analysis
indicated that the VEGF score was a significant parameter of the
peritoneal recurrence of gastric cancer (28). VEGF has also been reported to be an
independent predictor of tumor recurrence following orthotopic
liver transplantation in hepatocellular carcinoma (29). Another study reported that compared
with VEGF expression determined using immunohistochemistry (IHC),
the pre-operative serum VEGF level was a useful predictor of
post-operative recurrence in non-metastatic clear cell renal cell
carcinoma, with tumors from only 26 patients (31.3%) demonstrating
overexpression of VEGF using IHC (30). In the present study, the serum VEGF
level at the pre-treatment stage was less effective predictor of
recurrence compared with the serum VEGF level in patients with
locally advanced resectable ESCC following chemotherapy or
concurrent radiotherapy.
Low serum VEGF levels in patients with locally
advanced resectable ESCC contribute to a longer median OS compared
with high serum VEGF levels. However, no marked difference was
demonstrated in the median OS between the patients with recurrence
and those with no recurrence, which suggested that recurrence does
not determine OS in patients with locally advanced resectable ESCC.
Although the serum VEGF level at recurrence in patients with
recurrence was not associated with survival from the time of
recurrence to the end of follow-up, patients with low serum VEGF
levels following further treatment had a longer survival from time
of recurrence to the end of follow-up. These results indicated that
the serum VEGF level also acted as a prognostic factor for patients
with locally advanced resectable ESCC who exhibited recurrence. The
serum VEGF level was associated with survival not only in patients
with locally advanced resectable ESCC following treatment, but also
in patients with recurrence following further treatments. A low
serum VEGF level was correlated with longer survival time in
patients with no recurrence and in those with recurrence following
treatment. It has been previously reported that following surgery,
certain patients develop locoregional recurrence and distant
metastasis (31-33).
VEGF is a prognostic factor for patients with locally advanced ESCC
treated with concurrent chemoradiotherapy. Low VEGF levels
following treatment and decreasing levels of VEGF during concurrent
chemoradiotherapy have been previously reported to be significantly
associated with improved clinical outcomes in patients with
advanced-stage ESCC (12).
Biologically, ESCC shares numerous characteristics with head and
neck SCC (34). An association has
also been previously reported between VEGF and the OS and
metastasis-free survival of patients with head and neck SCC treated
with radio-chemotherapy or radiotherapy (35). Furthermore, the serum VEGF level is
a potential target for chemotherapeutic strategies in patients with
oral carcinoma (36). The data in
the present study suggested that serum VEGF levels may have
predictive and prognostic potential in patients with locally
advanced resectable ESCC treated with chemotherapy or concurrent
radiotherapy.
The present study has certain limitations which
should be noted. First, post-operative serum VEGF levels in
patients with locally advanced resectable ESCC were used as
measurements and, serum VEGF levels may be affected by R0
resection. Lai et al reported a significant decrease in
serum VEGF levels from pre- to post-surgery in non-small cell lung
cancer (37). Therefore, the VEGF
levels in patients with locally advanced resectable ESCC may have
decreased after R0 resection. Second, the standard deviation of
serum VEGF levels was relatively large, particularly following
chemotherapy or concurrent radiotherapy, while serum VEGF levels in
certain patients were always low before and during treatments,
which suggested that the individual differences in VEGF were
relatively large.
In conclusion, the present study demonstrated that
patients with ESCC with a tendency for recurrence have high serum
VEGF levels during chemotherapy or concurrent radiotherapy and high
serum VEGF levels at recurrence compared with the pre-treatment
stage. The serum VEGF levels no longer decrease in patients with
recurrence following further treatment. The serum VEGF levels
following chemotherapy or concurrent radiotherapy were associated
with the OS and RFS of patients with locally advanced resectable
ESCC. The serum VEGF levels in patients with recurrent ESCC were
not influenced by the time duration between the post-operative
stage to recurrence; however, low serum VEGF levels were associated
with longer survival time following further treatment. Serum VEGF
levels are critical for the prognosis and prediction of recurrence
in patients with locally advanced resectable ESCC.
Supplementary Material
The cut-off values of serum VEGF
levels for the overall survival and recurrence-free survival.
Relation of pre-treatment serum VEGF
to clinicopathological characteristics of 173 patients with locally
advanced resectable esophageal squamous cell carcinoma.
Patient characteristics in relation to
recurrent and non-recurrent patients with locally advanced
resectable esophageal squamous cell carcinoma.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The 333 Project of
The Science and Technology Department of Jiangsu Province (grant
no. BRA2020389).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and RM designed the experiments. HC, ZW and JZ
performed the experiments. HC managed the project. CJ and MD
collated and investigated data. CJ, MD and XX analyzed the data. HX
determined the serum VEGF concentration using the ELISA and Luminex
methods and wrote the manuscript. RM reviewed the manuscript. HX,
HC, JZ, CJ, ZW, JW, MD, XX and RM confirm the authenticity of all
of the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed under approval of
Biomedical Research Ethics Committee of Jiangsu Cancer Hospital
(approval no. 2010ke-052). Written informed consent was obtained
from all individual participants included in the study.
Patient consent for publication
Written permission for publication was obtained from
all patients who participated in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang FL and Yu SJ: Esophageal cancer:
Risk factors, genetic association, and treatment. Asian J Surg.
41:210–215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Y, Li Y and Chen X: NOTCH and
esophageal squamous cell carcinoma. Adv Exp Med Biol. 1287:59–68.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lordick F, Mariette C, Haustermans K,
Obermannová R and Arnold D: ESMO Guidelines Committee. Oesophageal
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 27:v50–v57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kang X, Chen K, Li Y, Li J, D'Amico TA and
Chen X: Personalized targeted therapy for esophageal squamous cell
carcinoma. World J Gastroenterol. 21:7648–7658. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Y, Xiong Z, Beasley A, D'Amico T and
Chen X: Personalized and targeted therapy of esophageal squamous
cell carcinoma: An update. Ann N Y Acad Sci. 1381:66–73.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cools-Lartigue J, Spicer J and Ferri LE:
Current status of management of malignant disease: Current
management of esophageal cancer. J Gastrointest Surg. 19:964–972.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Smyth EC, Lagergren J, Fitzgerald RC,
Lordick F, Shah MA, Lagergren P and Cunningham D: Oesophageal
cancer. Nat Rev Dis Primers. 3(17048)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chang SH, Kanasaki K, Gocheva V, Blum G,
Harper J, Moses MA, Shih SC, Nagy JA, Joyce J, Bogyo M, et al:
VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine
protease inhibitor balance in venules, causing basement membrane
degradation and mother vessel formation. Cancer Res. 69:4537–4544.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang C, Wang J, Chen Z, Gao Y and He J:
Immunohistochemical prognostic markers of esophageal squamous cell
carcinoma: A systematic review. Chin J Cancer.
36(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen YH, Lu HI, Lo CM, Wang YM, Chou SY,
Hsiao CC, Huang CC, Shih LH, Chen SW and Li SH: The crucial role of
blood VEGF kinetics in patients with locally advanced esophageal
squamous cell carcinoma receiving curative concurrent
chemoradiotherapy. BMC Cancer. 18(837)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang S, Chen X, Fan J and Lu L: Prognostic
significance of lymphovascular invasion for thoracic esophageal
squamous cell carcinoma. Ann Surg Oncol. 23:4101–4109.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Araki K, Ohno S, Egashira A, Saeki H,
Kawaguchi H and Sugimachi K: Pathologic features of superficial
esophageal squamous cell carcinoma with lymph node and distal
metastasis. Cancer. 94:570–575. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Song Z, Wang J, Lin B and Zhang Y:
Analysis of the tumor length and other prognosis factors in pT1-2
node-negative esophageal squamous cell carcinoma in a Chinese
population. World J Surg Oncol. 10(273)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang Q, Luo K, Chen C, Wang G, Jin J,
Kong M, Li B, Liu Q, Li J, Rong T, et al: Identification and
validation of lymphovascular invasion as a prognostic and staging
factor in node-negative esophageal squamous cell carcinoma. J
Thorac Oncol. 11:583–592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xue LY, Qin XM, Liu Y, Liang J, Lin H, Xue
XM, Zou SM, Zhang MY, Zhang BH, Hui ZG, et al: Clinicopathological
parameters predicting recurrence of pT1N0 esophageal squamous cell
carcinoma. World J Gastroenterol. 24:5154–5166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ye Z, Zhao H, Zhou W, Ye T, Geng C, Li X,
Yuan L, Du M, Xu H and Wang Q: Lower serum matrix
metalloproteinase-9 in metastatic patients with esophageal squamous
cell carcinoma after concurrent radiotherapy was significant for
prognosis. Onco Targets Ther. 13:12857–12866. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ma R, Xu H, Wu J, Sharma A, Bai S, Dun B,
Jing C, Cao H, Wang Z, She JX and Feng J: Identification of serum
proteins and multivariate models for diagnosis and therapeutic
monitoring of lung cancer. Oncotarget. 8:18901–18913.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Njei B, McCarty TR and Birk JW: Trends in
esophageal cancer survival in United States adults from 1973 to
2009: A SEER database analysis. J Gastroenterol Hepatol.
31:1141–1146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li B, Liu Y, Peng J, Sun C and Rang W:
Trends of esophageal cancer incidence and mortality and its
influencing factors in China. Risk Manag Healthc Policy.
14:4809–4821. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Miyata H, Yamasaki M, Kurokawa Y,
Takiguchi S, Nakajima K, Fujiwara Y, Konishi K, Mori M and Doki Y:
Survival factors in patients with recurrence after curative
resection of esophageal squamous cell carcinomas. Ann Surg Oncol.
18:3353–3361. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu J, Tao H, Song D and Chen C: Recurrence
risk model for esophageal cancer after radical surgery. Chin J
Cancer Res. 25:549–555. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Oppedijk V, van der Gaast A, van Lanschot
JJ, van Hagen P, van Os R, van Rij CM, van der Sangen MJ, Beukema
JC, Rütten H, Spruit PH, et al: Patterns of recurrence after
surgery alone versus preoperative Chemoradiotherapy and surgery in
the CROSS trials. J Clin Oncol. 32:385–391. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ and
Fu XL: Patterns of failure after radical surgery among patients
with thoracic esophageal squamous cell carcinoma: Implications for
the clinical target volume design of postoperative radiotherapy.
PLoS One. 9(e97225)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo XF, Mao T, Gu ZT, Ji CY, Fang WT and
Chen WH: Clinical study on postoperative recurrence in patients
with pN0 esophageal squamous cell carcinoma. J Cardiothorac Surg.
9(150)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang LS, Chow KC, Chi KH, Liu CC, Li WY,
Chiu JH and Huang MH: Prognosis of esophageal squamous cell
carcinoma: Analysis of clinicopathological and biological factors.
Am J Gastroenterol. 94:1933–1940. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aoyagi K, Kouhuji K, Yano S, Miyagi M,
Imaizumi T, Takeda J and Shirouzu K: VEGF significance in
peritoneal recurrence from gastric cancer. Gastric Cancer.
8:155–163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang X, Wu Z, Peng Y, Li D, Jiang Y, Pan
F, Li Y, Lai Y, Cui Z and Zhang K: Correlationship between Ki67,
VEGF, and p53 and hepatocellular carcinoma recurrence in liver
transplant patients. Biomed Res Int. 2021(6651397)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fujita N, Okegawa T, Terado Y, Tambo M,
Higashihara E and Nutahara K: Serum level and immunohistochemical
expression of vascular endothelial growth factor for the prediction
of postoperative recurrence in renal cell carcinoma. BMC Res Notes.
7(369)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Y, Wang L, Yang Q, Li J, He M, Yao J,
Qi Z, Li B and Qiao X: Patterns of recurrence in patients with
stage pT3N0M0 thoracic esophageal squamous cell carcinoma after
two-field esophagectomy. Zhonghua Zhong Liu Za Zhi. 38:48–54.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
Li CL, Zhang FL, Wang YD, Han C, Sun GG,
Liu Q, Cheng YJ, Jing SW and Yang CR: Characteristics of recurrence
after radical esophagectomy with two-field lymph node dissection
for thoracic esophageal cancer. Oncol Lett. 5:355–359.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shen WB, Gao HM, Zhu SC, Li YM, Li SG and
Xu JR: Analysis of postoperative failure in patients with stage
pT3N0M0 thoracic esophageal
squamous cell carcinoma and consideration of postoperative
radiotherapy. World J Surg Oncol. 15(192)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
The Cancer Genome Atlas Research, Analysis
Working Group: Asan University; BC Cancer Agency; Brigham and
Women's Hospital; Broad Institute; Brown University; Case Western
Reserve University; Dana-Farber Cancer Institute; Duke University
et al. Integrated genomic characterization of oesophageal
carcinoma. Nature. 541:169–175. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Butkiewicz D, Gdowicz-Kłosok A, Krześniak
M, Rutkowski T, Krzywon A, Cortez AJ, Domińczyk I and Składowski K:
Association of genetic variants in ANGPT/TEK and VEGF/VEGFR with
progression and survival in head and neck squamous cell carcinoma
treated with radiotherapy or radiochemotherapy. Cancers (Basel).
12(1506)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aggarwal S, Devaraja K, Sharma SC and Das
SN: Expression of vascular endothelial growth factor (VEGF) in
patients with oral squamous cell carcinoma and its clinical
significance. Clin Chim Acta. 436:35–40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lai Y, Wang X, Zeng T, Xing S, Dai S, Wang
J, Chen S, Li X, Xie Y, Zhu Y and Liu W: Serum VEGF levels in the
early diagnosis and severity assessment of non-small cell lung
cancer. J Cancer. 9:1538–1547. 2018.PubMed/NCBI View Article : Google Scholar
|