Introduction
Cancer care is complex not only because of the
nature of the diagnosis but also due to the toxicity of the
treatment. Patients diagnosed with any cancer often suffer from
comorbid conditions that bring in a significant risk of drug
interactions (1). However, several
patients are frequently on supplements (herbal and non-herbal).
Patients diagnosed with cancer tend to gravitate towards
alternative medicine, primarily because of the purported nontoxic
approach (2,3). However, a clear understanding of the
merits and flaws is lacking due to a dearth of well-designed
clinical trials. The interaction of herbal medicine with several
anti-cancer therapies has also remained elusive. Elderberry
(Sambucus nigra) is a woody, deciduous shrub that grows
across the Americas, Europe, Asia and the South Pacific. Elderberry
supplements have become immensely popular in the US, particularly
after the coronavirus pandemic (COVID-19) (4,5).
Pazopanib is a multikinase inhibitor of vascular
endothelial growth factor receptors, platelet-derived growth factor
receptor and c-kit (6). Pazopanib
is approved by the US Food and Drug Administration (USFDA) for
patients with metastatic renal cell carcinoma and those with
metastatic or locally advanced unresectable soft-tissue sarcoma
(STS) who experienced progression after chemotherapy (7). Several trials in patients with
localized high-risk extremity STS have also explored the utility of
administering neoadjuvant pazopanib with preoperative radiation
therapy (RT) with mixed results (8,9). The
patient presented in the current study was consuming elderberry
supplements when she started pazopanib. The current study reports
the case of a woman diagnosed with high-risk localized
extra-skeletal myxoid chondrosarcoma treated with neoadjuvant
pazopanib given concurrently with preoperative RT who had a
potential drug-drug interaction with over-the-counter (OTC)
elderberry supplement.
Case report
A 65-year-old woman presented to urgent care of
University Hospitals Cleveland Medical Center (Cleveland, Ohio) in
February 2022 with a lump on her left thigh, which had grown slowly
over the last six months. MRI of the femur showed a 13x7.4x7.2 cm
mass in the left sartorius, abutting the adjacent neuro-vascular
bundle. The patient was seen by an orthopedic oncologist and had a
core needle biopsy, which read as unclassified pleomorphic
spindle-shaped sarcoma (intermediate grade, 18 mitoses per 10
high-power fields, and no necrosis). The chest CT scan did not show
any metastasis. The case was discussed by the multidisciplinary
tumor board. The patient was referred to radiation oncology for
preoperative radiation and to medical oncology for discussion of
neoadjuvant pazopanib. At the first visit to medical oncology, the
patient did not report any comorbidities. The patient reported
taking an elderberry supplement (quantity unspecified) since the
beginning of the COVID-19 pandemic in 2020. The clinical exam was
significant for an ~15 cm long and 8 cm wide hard, immobile mass on
the anterior part of the left thigh. The mass was not tender and
the skin over the mass could be pinched. No regional
lymphadenopathy or organomegaly was noted on the clinical exam. The
rest of the systemic exam was unremarkable. The vital signs on the
initial visit were within normal limits. Laboratory tests on the
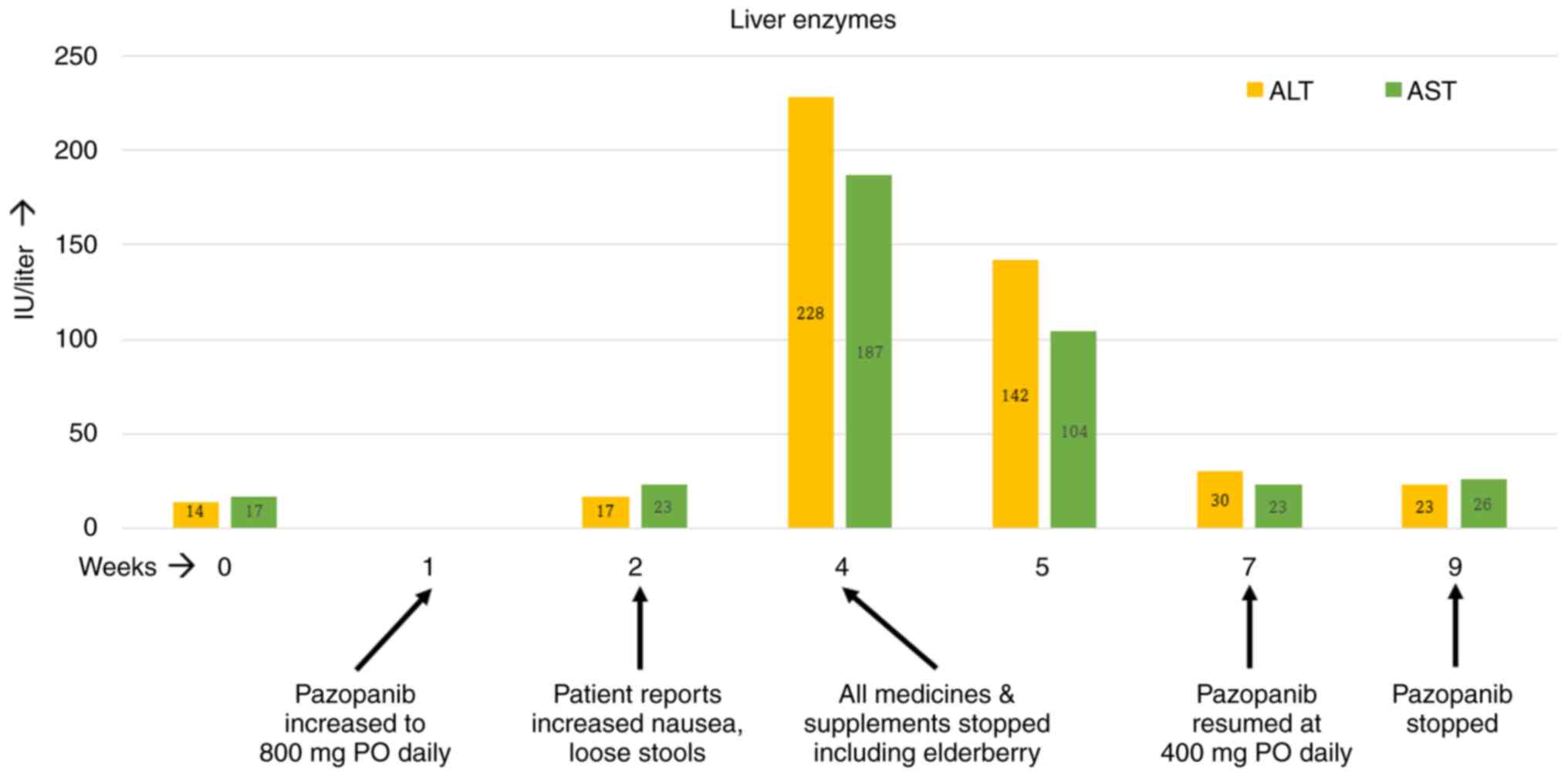
first visit were also within normal limits (Table I).
 | Table IRelevant laboratory tests at various
time-points. |
Table I
Relevant laboratory tests at various
time-points.
| Parameter (normal
range) | Day 0 | Day 7 | Day 28 | Day 35 | Day 49 |
|---|
| Blood count | | | | | |
|
Hemoglobin,
(12.0-16.0) g/dl | 13.3 | 12.9 | 13.4 | 13.1 | 12.8 |
|
WBC,
(4.4-11.3) x103/Ul | 7.1 | 5.3 | 4.3 | 4.0 | 5.9 |
|
Platelets,
(150-450) x103/Ul | 322 | 276 | 257 | 210 | 308 |
| Liver enzymes | | | | | |
|
AST, (9-39)
IU/l | 17 | 23 | 187 | 104 | 23 |
|
ALT, (7-45)
IU/l | 14 | 17 | 228 | 142 | 30 |
|
LDH,
(84-246) IU/l | 178 | 245 | 278 | 200 | 161 |
| Infections | | | | | |
|
Hepatitis B
Ag | ND | ND | Neg | ND | ND |
|
Hepatitis B
core Ab | ND | ND | Neg | ND | ND |
|
Hepatitis
C | ND | ND | Neg | ND | ND |
|
HIV | ND | ND | Neg | ND | ND |
The patient started treatment with pazopanib at 400
mg per os (PO) daily for a week, and it was then increased to 800
mg PO daily. However, by the second week on the full dose of
pazopanib, the patient started noticing increased nausea and
occasional loose stools. The nausea kept getting worse over the
ensuing weeks. By week four on pazopanib, the patient's alanine
transferase (ALT) and aspartate transaminase (AST) levels had risen
by 4-5-fold. At this time, further investigation was performed with
blood tests. The patient tested negative for hepatitis B and C and
human immunodeficiency virus. Ultrasound examination of the liver
was also unremarkable. At this point, treatment with pazopanib was
stopped temporarily and all medicines and supplements taken by the
patient were reviewed. The patient was taking a calcium supplement
and multivitamins apart from the elderberry supplement, which was
permanently discontinued. Soon after stopping all medicines, the
patient felt better as her nausea and occasional loose stools
resolved within days. The patient's liver enzymes exhibited a
downward trend over the next seven days and normalized by the end
of the second week on their own. Since the patient's radiation
treatment was still ongoing, pazopanib was resumed at 400 mg PO
daily, which was administered for another two weeks. The patient
reported no GI upset this time and her liver enzymes remained
normal. As for RT, the patient received a total of 50 Gy of
external beam radiation to the left thigh mass in 25 fractions over
5 weeks. The RT started in the third week of pazopanib and ended in
the eighth week. The timeline of events is depicted in Fig. 1. Four weeks after the completion of
RT, the patient underwent surgery. The surgical specimen was from a
R0 resection and had 85% pathologic necrosis. At two years
post-surgery, the patient has no evidence of cancer recurrence.
Discussion
Elderberry has been used in folk medicine to treat
cold and flu (10). Historically,
various parts of the elderberry plant have been used for specific
ailments. A poultice made from the leaves of the elderberry plant
has been used to promote healing and relieve joint pains (11). The flower of elderberry has
anti-inflammatory and antioxidant properties (11). Hippocrates, in 400 BC, referred to
elderberry as his ‘medicine chest’, considering the broad
applications of the shrub (12).
Two small randomized studies on patients with influenza reported
that elderberry use may reduce the duration of symptoms (13-15).
The use of elderberry grew exponentially during COVID-19 despite
the lack of a demonstrated scientific benefit of the supplement
(5). Elderberry supplement is
primarily marketed as an herbal dietary supplement that can
stimulate the immune system and increase antioxidant levels in the
body. However, consuming raw elderberries, leaves and branches may
cause severe gastrointestinal (GI) toxicity (16). The clinical use of elderberry in
patients diagnosed with cancer is experimental. As with various
other herbal supplements, the interaction of elderberry with
anti-cancer therapies remains unknown to a large extent.
Several in vitro studies have explored the
interaction of various herbal products with cytochrome P450 (CYP)
enzymes in the context of non-cancer conditions, such as pregnancy.
Several products, including fennel seeds, ginger, horsetails,
raspberry leaf, cranberry and black elderberry had varying
half-maximal inhibitory concentrations (IC50s) for
CYP1A2, CYP2D6 and CYP3A4. Within the CYP family, numerous
clinically used medicines are metabolized by CYP3A4 in particular,
which is also considered a significant candidate for
pharmacokinetic interactions (17). Although several herbs have shown
in vitro inhibition of CYP3A4, grapefruit juice and St.
John's Wort have proven clinically relevant in vivo
interactions with CYP3A4(18).
Pazopanib is metabolized in the liver primarily through the CYP3A4
enzyme with a minor contribution from CYP1A2 and CYP2C8(19). Strong inhibitors of CYP3A4 are to
be avoided for concurrent use with pazopanib. If it is necessary to
administer potent CYP3A4 inhibitors, then the dose of pazopanib
should be reduced to 400 mg daily (from the recommended dose of 800
mg PO daily). The USFDA label for pazopanib strictly prohibits the
concurrent use of grapefruit juice with pazopanib.
Two studies have reported the in vitro
activity of black elderberry against CYP3A4 (18,20)
However, Schrøder-Aasen et al (18) only tested Echinacea
purpurea, which was part of a commercial product called
Sambucus Force (marketed as Elderberry Defense). The authors
demonstrated the significant inhibition of CYP3A4 by E.
purpurea and drew an inference regarding the activity of S.
nigra without performing any dedicated experiments. Langhammer
and Nilsen (20) conducted
dedicated experiments with S. nigra and reported 100%
inhibition of CYP3A4 at high concentrations. Since high
concentrations of the herb (S. nigra) were needed to reach
the IC50, the authors concluded that S. nigra
does not inhibit CYP3A4 in vitro. However, Schrøder-Aasen
et al (18) did acknowledge
that S. nigra may have inhibitory effects on CYP3A4 at high
concentrations. Despite the increasing popularity of
elderberry-based products, no other studies have evaluated the
metabolic activity of elderberry on CYP3A4, neither in vitro
nor in vivo.
STS are a rare group of cancers of mesenchymal
origin. Although sarcomas can arise anywhere in the body, the
extremities and retroperitoneum are the most common sites of origin
(21). Intending to preserve the
limb, surgery with perioperative (pre- or post-operative RT) is the
treatment of choice in patients with localized sarcoma (22). In the present case, neoadjuvant
pazopanib was added to radiation to improve the local control rates
based on the results of the PASAART-1 and -2 trials (8). The GI toxicity experienced by the
patient soon after starting pazopanib and the resolution of
symptoms after stopping elderberry hint towards a possible
drug-drug interaction. The patient was able to resume pazopanib
without the return of adverse effects when elderberry was no longer
consumed. It should be acknowledged that the patient restarted
pazopanib at a lower dose. In the phase 1 study of pazopanib, there
was a linear growth in the maximum concentration of the drug with
increasing doses (the doses were tested from 50 to 2,000 mg).
However, there was no linear relationship observed between the dose
and the toxicity of pazopanib. Only one patient developed a grade 3
rise in AST levels and was on a dose of 300 mg PO twice a day.
Hence, it is unlikely that starting at half the dose of pazopanib
prevented the resurgence of the hepatic and GI toxicity (23). Although it may seem that elderberry
is a weak inhibitor of CYP3A4, it may lead to significant drug
interactions if consumed in high quantities (18).
Since the patient of the present study was receiving
treatment with curative intent in the neoadjuvant setting, a
detailed workup (including liver biopsy, CYP3A4 activity, pazopanib
levels, etc.) for proving the drug-drug interaction was deemed as
time-consuming and not in the best interest of the patient.
Furthermore, the transaminitis and GI toxicity resolved within two
weeks, which would have made further testing futile. However, based
on the timeline and sequence of events, there may have been a
significant drug-drug interaction. The present report aims to
highlight the importance of understanding the interaction (both
beneficial and detrimental) between herbal supplements and
cytotoxic medicines. In vivo mouse experiments have shown
the activity of elderberry in breast, bladder and ovarian cancer
(24). It is thought that
elderberry flower exerts its medicinal effect due to the abundance
of polyphenolic compounds contained in it, primarily flavonols,
phenolic acid and anthocyanins. In preclinical experiments, rutin,
the most common polyphenol in elderberry flowers, affected the
viability of neuroblastoma, leukemia and breast cancer cells
(24). However, the lack of data
demonstrating clinical activity limits the interpretability of the
preclinical results. The oncology community is only beginning to
realize the importance of integrative oncology. There is an
inevitable shift in our perception and acceptance of traditional
medicine, including herbal medicine. However, the supplement market
is not strictly regulated. The USFDA regulates dietary supplements
under the Federal Food, Drug and Cosmetic Act as amended by the
Dietary Supplement Health and Education Act from 1994. Under the
provisions of this act, dietary supplements do not need any prior
approval and the safety of the product is determined primarily
through post-market surveillance (25). Considering the complexity of cancer
care and the rise in consumption of herbal supplements, it becomes
prudent to conduct clinical trials in a controlled setting to
determine clinical interactions between allopathic and traditional
medicines.
In conclusion, the present study signifies the
importance of understanding the potential drug interactions between
herbal products and cytotoxic drugs in cancer patients. This report
also emphasizes the importance of diligently reconciling the
medicine list, including OTC herbal supplements, which often go
underreported. More studies are needed to understand the in
vitro and in vivo activity of herbal supplements and
their potential impact on cancer care.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
NA and AM wrote the manuscript and researched the
references. NA conceived the study and prepared the tables and
figures. AM provided expert opinions. Both authors have read and
approved the final version of the manuscript. Both authors have
confirmed the authenticity of the raw data.
Ethics approval and consent to
participate
The case report was exempted from review by the
Institutional Review Board at the Case Western Reserve University
School of Medicine (Cleveland, USA).
Patient consent for publication
The patient provided consent for the publication of
her data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Riechelmann RP, Tannock IF, Wang L, Saad
ED, Taback NA and Krzyzanowska MK: Potential drug interactions and
duplicate prescriptions among cancer patients. J Natl Cancer Inst.
99:592–600. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kessel KA, Lettner S, Kessel C, Bier H,
Biedermann T, Friess H, Herrschbach P, Gschwend JE, Meyer B,
Peschel C, et al: Use of complementary and alternative medicine
(CAM) as part of the oncological treatment: Survey about patients'
attitude towards CAM in a university-based oncology center in
Germany. PLoS One. 11(e0165801)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Buckner CA, Lafrenie RM, Dénommée JA,
Caswell JM and Want DA: Complementary and alternative medicine use
in patients before and after a cancer diagnosis. Curr Oncol.
25:e275–e281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Choudhury NR: Elderberry supplements
market outlook (2023 to 2033). https://www.futuremarketinsights.com/reports/elderberry-supplements-market.
Accessed November 19, 2023.
|
|
5
|
Adams KK, Baker WL and Sobieraj DM: Myth
busters: Dietary supplements and COVID-19. Ann Pharmacother.
54:820–826. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schutz FA, Choueiri TK and Sternberg CN:
Pazopanib: Clinical development of a potent anti-angiogenic drug.
Crit Rev Oncol Hematol. 77:163–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee ATJ, Jones RL and Huang PH: Pazopanib
in advanced soft tissue sarcomas. Signal Transduct Target Ther.
4(16)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van Meekeren M, Bovee J, van Coevorden F,
van Houdt W, Schrage Y, Koenen AM, Miah AB, Zaidi S, Hayes AJ,
Thway K, et al: A phase II study on the neo-adjuvant combination of
pazopanib and radiotherapy in patients with high-risk, localized
soft tissue sarcoma. Acta Oncol. 60:1557–1564. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weiss AR, Chen YL, Scharschmidt TJ, Xue W,
Gao Z, Black JO, Choy E, Davis JL, Fanburg-Smith JC, Kao SC, et al:
Outcomes after preoperative chemoradiation with or without
pazopanib in non-rhabdomyosarcoma soft tissue sarcoma: A report
from Children's oncology group and NRG oncology. J Clin Oncol.
41:4842–4848. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
National Center for Complementary and
Integrative Health: Elderberry. National Institutes of Health,
2020. https://www.nccih.nih.gov/health/elderberry. Accessed
November 19, 2023.
|
|
11
|
Ulbricht C, Basch E, Cheung L, Goldberg H,
Hammerness P, Isaac R, Khalsa KP, Romm A, Rychlik I, Varghese M, et
al: An evidence-based systematic review of elderberry and
elderflower (Sambucus nigra) by the natural standard research
collaboration. J Diet Suppl. 11:80–120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pembleton M: Can elderberry treat the flu?
The New York Times. NYT Parenting, 2020. https://www.nytimes.com/2020/04/17/parenting/elderberry-benefits-dangers.html.
Accessed September 23, 2023.
|
|
13
|
Zakay-Rones Z, Varsano N, Zlotnik M, Manor
O, Regev L, Schlesinger M and Mumcuoglu M: Inhibition of several
strains of influenza virus in vitro and reduction of symptoms by an
elderberry extract (Sambucus nigra L.) during an outbreak of
influenza B Panama. J Altern Complement Med. 1:361–369.
1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zakay-Rones Z, Thom E, Wollan T and
Wadstein J: Randomized study of the efficacy and safety of oral
elderberry extract in the treatment of influenza A and B virus
infections. J Int Med Res. 32:132–140. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tiralongo E, Wee SS and Lea RA: Elderberry
supplementation reduces cold duration and symptoms in
air-travellers: A randomized, double-blind placebo-controlled
clinical trial. Nutrients. 8(182)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Centers for Disease Control (CDC).
Poisoning from elderberry juice-California. MMWR Morb Mortal Wkly
Rep. 33:173–174. 1984.PubMed/NCBI
|
|
17
|
Zhou SF, Xue CC, Yu XQ, Li C and Wang G:
Clinically important drug interactions potentially involving
mechanism-based inhibition of cytochrome P450 3A4 and the role of
therapeutic drug monitoring. Ther Drug Monit. 29:687–710.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schrøder-Aasen T, Molden G and Nilsen OG:
In vitro inhibition of CYP3A4 by the multiherbal commercial product
Sambucus force and its main constituents Echinacea purpurea and
Sambucus nigra. Phytother Res. 26:1606–1613. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Thorn CF, Sharma MR, Altman RB and Klein
TE: PharmGKB summary: Pazopanib pathway, pharmacokinetics.
Pharmacogenet Genomics. 27:307–312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Langhammer AJ and Nilsen OG: In vitro
inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs
commonly used in pregnancy. Phytother Res. 28:603–610.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Mangla A and Yadav U: Leiomyosarcoma.
StatPearls Publishing, Treasure Island, FL, 2023.
|
|
22
|
Mangla A: Should neoadjuvant treatment be
adopted more widely for patients with extremity soft tissue sarcoma
in low-income countries? JCO Glob Oncol. 9(e2300110)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hurwitz HI, Dowlati A, Saini S, Savage S,
Suttle AB, Gibson DM, Hodge JP, Merkle EM and Pandite L: Phase I
trial of pazopanib in patients with advanced cancer. Clin Cancer
Res. 15:4220–4227. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kolesarova A, Baldovska S, Kohut L and
Sirotkin AV: Black elder and its constituents: Molecular mechanisms
of action associated with female reproduction. Pharmaceuticals
(Basel). 15(239)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thakkar S, Anklam E, Xu A, Ulberth F, Li
J, Li B, Hugas M, Sarma N, Crerar S, Swift S, et al: Regulatory
landscape of dietary supplements and herbal medicines from a global
perspective. Regul Toxicol Pharmacol. 114(104647)2020.PubMed/NCBI View Article : Google Scholar
|