1. Introduction
Tumors remain an important threat to lives and
health of individuals with the burden of cancer incidence and
mortality rapidly increasing throughout the world. With advances in
medical technology, oncologists are increasingly developing
additional strategies for oncology treatments, such as neoadjuvant
chemoradiotherapy (CRT), concurrent CRT (CCRT), immunotherapy and
targeted therapy; however, radiotherapy (RT) continues to play a
vital role in the response to the disease spectrum of most cancers
(1).
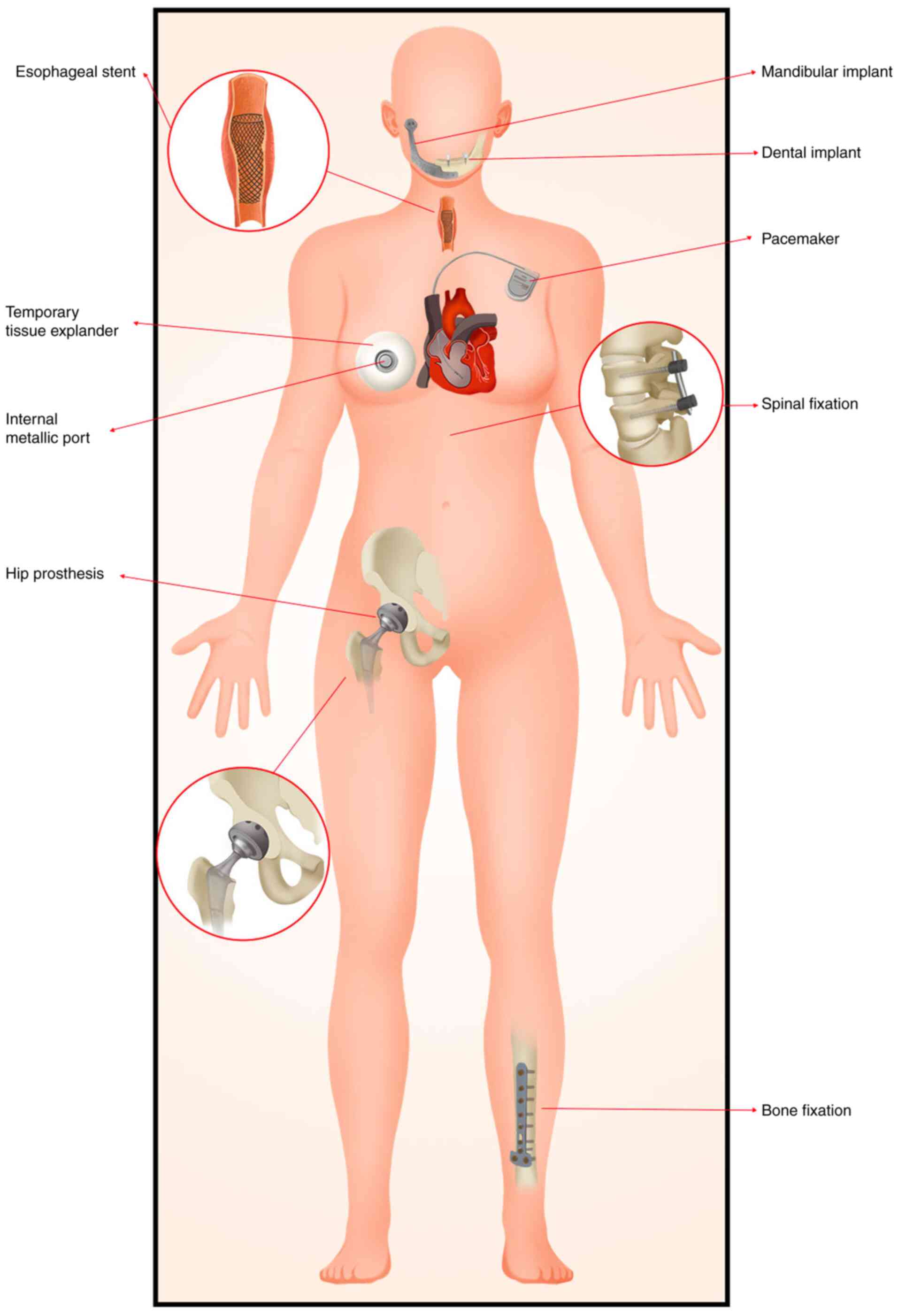
Biomaterials are commonly defined as non-viable
materials intended to interface with biological systems to
evaluate, treat, augment or replace any tissue, organ, or function
of the body (2). Metallic implants
are types of biomaterials, commonly used for reconstructing certain
important structures or alleviating symptoms. According to
statistics, ~4% of patients undergoing RT have metal implants in
their bodies, such as in the teeth, esophagus, breast, spine, hip
and other areas (3) (Fig. 1). These metal implants are usually
present around the tumor. For instance, self-expandable metal
stents are often used to palliate malignant dysphagia, either alone
or before definitive or preoperative CRT for esophageal cancer
(4-6).
In addition, metal implants are often present in patients with head
and neck cancer (7). However,
these metal implants have a non-negligible effect on the dose
distribution and delineation of target volumes during RT.
Due to the dose perturbation, the local control of
the tumor would be affected and cause excessive damage to the
normal tissues and organs at risk surrounding the tumor, resulting
in short- or long-term toxicity (8,9). In
addition, metal implants tend to be markedly denser than tissue or
bone (10); therefore, they
produce more severe artifacts when computer irradiation is
performed for treatment planning. Although several algorithms exist
to reduce metal artifacts, they still impact the accuracy of RT
(11,12). Therefore, the present study
reviewed the effects of different types of metal implants on
radiation dose and clinical outcomes. It also explored methods to
minimize the impact of metal implants on RT.
2. The mechanism of metal implants affecting
RT
The presence of metallics compromises computed
tomography (CT) image quality by generating metal artifacts mainly
through beam hardening, noise and scattering (13,14)
and thus affecting the accuracy of target volumes. Beam hardening
is due to the fact that metals with high atomic numbers absorb
photons more strongly, which ultimately results in a higher average
energy. Absorption of photons by metallic implants results in a
significant reduction in the number of photons detected by the
photon detector, which manifests as bright streaks and thin, dark,
areas in the image, defined as noise. As the predominant type of
interaction in CT, Compton scattered X-rays are the signals usually
detected by detectors. This scattering changes the direction of the
incident beam away from the center axis. This causes the metal to
appear white, with dark streaks along the axis of maximum
attenuation (15). Furthermore,
metal implants also have an effect on the delivery and distribution
of the dose during the course of RT. When photons or electrons pass
through a metal implant, secondary electrons or scattering can
cause dose perturbation, resulting in an overdose on the front
surface of the metal and tissue and a lower dose on the rear
surface (16). The metal
prosthesis is commonly made from ‘high-Z’ elements, which are
defined as material with an atomic number greater than that of
cortical bone. The presence of high-Z material during the
administration of an RT schedule can lead to local perturbations
through interface effects (17)
and distort dose distributions from therapeutic beams (18). The scattered radiation caused by
high atomic number materials when introduced into the photon beam
from megavoltage RT consists of both scattered photons and
electrons. The backscatter is especially important to be aware of
when the tumor is placed between the beams and metal implant, as it
can cause the dose to reflect and then build up on the surface,
resulting in an unplanned escalation in the dose, which can
increase the side effects of RT (19). In the study of Dietlicher et
al (20), the dose of
backscattered radiation is related to the angle between the axial
beam and the scattering material; however, not all metals can be
detected with such a significant relationship, such as silver.
Moreover, the sharp density interfaces of a metal prothesis with
the surrounding tissues can degrade the homogeneity of the
delivered target dose (21).
3. The effect of different kind of metal
implants on dosage
Since the density of metallic implants differs
significantly from that of human tissue, there is an effect on the
dose transmitted to the surrounding tissue as the beam passes
through the metal implants. Several studies have investigated the
dose perturbation scenarios by means of phantom measurements,
algorithmic simulations and other methods. Type of metal, shape of
the implant and energy of the radiation have an effect on the dose
distribution (5,8,19,22-29)
(Table I). The measured distance
is the distance from the film to the surface (ray incident surface
or ray exit surface) of the metal implant, and the film is used to
detect the dose of RT. As for the geometry of the implants, this is
more prominent in the esophageal stent, such as the size of the
stent mesh, the thickness of the line that constitutes the mesh. In
other types of implants, it is mostly manifested as the thickness
or length of the implant.
 | Table IEffect of metal implants on
radiotherapy dose under different conditions. |
Table I
Effect of metal implants on
radiotherapy dose under different conditions.
| First author,
year | Material | Method | Location | Energy | Radiation
source | Technology of
radiation | Increase the dose
in incident surface | Reduce the dose in
exit surface | PTV Dose (Gy) | (Refs.) |
|---|
| Tsuji et al,
2003 | Stainless
steel | Phantom | Tracheal | 10 MV | X-ray | Single beams | 9% | 8% | 6 | (22) |
| | | Phantom | Lower limb
arteries | 10 MV | X-ray | Single beams | 3% | 3% | | |
| | | Phantom | Coronary
artery | 10 MV | X-ray | Single beams | - | 2% | | |
| Liu et al,
2010 | | Monte Carlo | Femur | 6 MV | X-ray | Single beams | 21.6% | 8.42% | - | (23) |
| Chen et al,
2011 | | Phantom | Esophageal | 6 MV | Photon beams | Single beams | 3.5-7.8% | negligible | 0.05 | (5) |
| | | Monte Carlo | Esophageal | 6 MV | Photon beams | Single beams | 6.2% | <1.0% | | |
| | | Monte Carlo | Esophageal | 6 MV | Photon beams | Dual beams | 3.0% | 3.0% | | |
| Lin et al,
2013 | | Monte Carlo | Dental | 6 MV | X-ray | Volumetric
modulated arc therapy | 0.8% | 10% | - | (24) |
| Mahuvava and
Du | | Monte Carlo | Unilateral hip | 6 MV | Photon beams | Six fields | - | 10.3% | 75 | (25) |
| Plessis, 2018 | | | | 10 MV | Photon beams | Six fields | - | 6.9% | | |
| | | | | 15 MV | Photon beams | Six fields | - | 3.5% | | |
| | | | | 20 MV | Photon beams | Six fields | - | 2.1% | | |
| He and Ni,
2018 | | Monte Carlo | Water | 6 MV | X-ray | Single beams | 24% | 16.2~55.1% | - | (26) |
| Bhushan et
al, 2020 | | Phantom | Unilateral hip | 6 MV | Photon beams | Single beams | - | 7.5-8.3% | - | (27) |
| | | | | 15 MV | Photon beams | Single beams | - | 5.0-7.6% | | |
| Ozen et al,
2005 | Titanium | Phantom | Lower jaw | Co-60 | Gamma ray | Single beams | 17-21% | - | 2 | (28) |
| | | | | 6 MV | X-ray | Single beams | 17-18% | - | | |
| | | | | 24 MV | X-ray | Single beams | 15-16% | - | | |
| Liu et al,
2010 | | Monte Carlo | Femur | 6 MV | X-ray | Single beams | 15.46% | 5.26% | - | (23) |
| Ade and du Plessis,
2017 | | Phantom | Hip | 6 MV | Photon beams | Single AP
beams | 21-23% | 18-21% | - | (29) |
| | | | | 15 MV | Photon beams | Single AP
beams | 25-30% | 15-18% | | |
| Mahuvava and and Du
Plessis, 2018 | | Monte Carlo | Unilateral hip | 6 MV | Photon beams | Six fields | - | 6.2% | 75 | (25) |
| | | | | 10 MV | Photon beams | Six fields | - | 4.2% | | |
| | | | | 15 MV | Photon beams | Six fields | - | 2.4% | | |
| | | | | 20 MV | Photon beams | Six fields | - | 1.0% | | |
| He and Ni,
2018 | | Monte Carlo | Water | 6 MV | X-ray | Single beams | 20% | 11.5~35% | - | (26) |
| Akyol et al,
2019 | | Monte Carlo | Dental | 6 MV | Photon beams | Single beams | 11.2% | 15.5% | - | (8) |
| Dayyeh et
al, 2012 | Nitinol | Phantom | Esophageal | 6 MV | Photon beams | Perpendicular
beam | 4.2% | 0 | 0.3 | (19) |
| | | | | 10 MV | Photon beams | Perpendicular
beam | 5.2% | 1.0% | | |
| | | | | 18 MV | Photon beams | Perpendicular
beam | 6.7% | 1.3% | | |
| Akyo et al,
2019 | Ti-6Al-4V | Monte Carlo | Dental | 6 MV | Photon beams | Single beams | 10.7% | 15.4% | - | (8) |
| Akyol et al,
2019 | Al2O3 | Monte Carlo | Dental | 6 MV | Photon beams | Single beams | 3.3% | 7.0% | - | (8) |
Stainless steel
Stainless steel is widely used in bone fixation,
cardiovascular systems, catheters, surgical instruments and dental
crowns (30). Furthermore, Bhushan
et al (27) studied the
effect of stainless-steel hip prosthesis on radiation using a
customized prosthesis containing wrought austenitic stainless
steel. It was observed that for 6 MV of photon irradiation, at a
depth of 10 cm below the prosthesis, with field sizes of 5x5, 10x10
and 20x20, the dose attenuation was 8.3, 7.4 and 7.5% when the
prosthesis was present compared with in its absence. In addition,
when the energy was increased to 15 MV, the dose attenuations were
7.6, 7.1 and 5.0% for the same distances 5x5, 10x10 and 20x20,
respectively, of the field sizes. Moreover, Mahuvava and Du Plessis
(25) observed that when bilateral
stainless steel hips were present, the attenuation of radiation by
a prosthesis was 22.8, 20.4, 18.5 and 16.9% with photon
irradiations at 6, 10, 15 and 20 MV, respectively. Furthermore, Liu
et al (23) used human
cadavers to simulate tumor resection for internal fixation surgery
by placing stainless steel plates in the anterior and upper 1/3 of
the human femur with a muscle strip of the same size and thickness
for control purposes. It was observed that the absorbed dose at the
incident surface increased by 21.65%. Conversely, the absorbed dose
at the exit surface was attenuated by 8.42% compared with the
control group, as measured with a pyroelectric dosimeter under 6 MV
X-ray irradiation. Additionally, their experiments also used the
treatment planning system (TPS). It was observed that the distance
from the tissue to the metal surface was an important factor
affecting dose absorption, and this effect was greatest at a
distance of 0.5 cm from the metal surface, resulting in a 6.1%
increase in dose upstream and a 2.2% dose attenuation downstream.
In addition, He and Ni (26) used
the Monte Carlo (MC) algorithm to simulate 6 MV X-ray irradiation
and observed that the incident surface dose of stainless steel
implants with thicknesses of 1, 2 and 4 cm increased by 23.8, 24.0
and 24.3%, respectively, compared with the dose without the
implant; by contrast, the dose at the exit surface decreased by
23.0, 35.2 and 55.1%, respectively. This indicates that the
thickness of the implant did not significantly affect the incident
radiation dose; however, the dose attenuated more with increasing
metal thickness at the exit surface.
Stainless steel is majorly used in stents. Chen
et al (5) used a solid
water phantom to simulate the tissue environment of the human
esophagus and measured the surrounding irradiation dose using
thermo-luminescent dosimeters. It was observed that the increase in
dose to the Z-stent's (stainless steel) anterior surface was
3.5-7.8% when using single beams. Abu Dayyeh et al (19) used a solid water phantom irradiated
with 6, 10 and 18 MV photons and observed that the dose
enhancements of the stainless-steel stent Wallflex front upstream
were 4.2, 5.2 and 6.7%, respectively, at three different photon
energy irradiations. The aforementioned experiments revealed that
the presence of stainless-steel implants could have a
non-negligible effect on the accuracy of the RT dose; therefore,
when stainless steel implants are present in the irradiation field,
this dose perturbation should be considered when making RT
plans.
Titanium and its alloys
Since the introduction of pure titanium for oral
implants in the 1960s, it has been widely used as a material for
surgical implants. Subsequently, Ti-3Al-2.5V and Ti-6Al-4V were
gradually used as femoral and tibial replacement materials.
Ti6Al4V is an important titanium alloy widely
used as a material in surgical implants (31).
External irradiation is a common treatment modality
for prostate cancer. With an aging population, several patients
treated for prostate or pelvic tumors undergo partial or total hip
replacement because of osteoarthritis and hip dysfunction (27). Titanium is often used for hip
implants because of its excellent biomedical properties, however it
inevitably may affect dose delivery (32).
Ade and du Plessis (29) investigated the perturbation effect
of a unilateral titanium prosthesis on the dose distribution of 6
MV and 15 MV photon beams. Using a built-in titanium hip
prosthesis, it was observed that the proximal dose enhancement of
the prosthesis ranged as 21-23%, and the distal dose reduction of
the prosthesis was 18-21% with 6 MV photon beam irradiation.
However, when the radiation energy was increased to 25 MV, the
proximal dose enhancement and distal dose attenuation were 25-30
and 15-18%, respectively. It could be inferred from their
experiments that the field size does not significantly affect the
dose, and that the most significant dose change is within 1.0 cm
from the implant surface. Additionally, Akyol et al
(8) used the pencil beam
convolution (PBC) algorithm of the TPS and MC simulation techniques
for their study. They simulated a linear accelerator to produce a
6-MV photon beam and observed an 11.2% increase in dose anterior to
the titanium dental implant and a 15.5% decrease in dose
posteriorly.
Furthermore, He and Ni (26) used an MC algorithm to simulate 6-MV
X-ray irradiation and compared the effect of different thicknesses
of stainless-steel plates with that of titanium metal implants on
the radiation dose. It was observed that titanium implants
increased the upstream dose by 19.8, 20.3 and 20.6% at the
thicknesses of 1, 2 and 4 cm, respectively, while decreasing the
downstream dose by 18.4, 23.6 and 35.0%, respectively.
Additionally, it was observed that the effect of titanium implants
on radiation dose was less than that of stainless-steel implants.
Similarly, experiments by Liu et al (23) on human cadavers revealed that the
effect of stainless-steel plates on the radiation dose distribution
was more pronounced than that of titanium plates under the same
conditions.
Titanium and its alloys are also often used in oral
implants, which may have an impact on the radiation dose to
patients with nasopharyngeal tumors. Lin et al (24) observed that titanium used as a
dental implant in head-and-neck volumetric modulated arc therapy
(VMAT) had clinically significant effects on the dose. They used the
MC and TPS methods and observed that at a distance of 2 mm from the
implant surface, the upstream dose increased by 0.8%; by contrast,
the downstream dose was attenuated by 10%. In addition, Ozen et
al (28) implanted titanium
dental implants of different diameters and lengths into the human
mandible and irradiated them with 6 MV X, 25 MV X and Co-60 gamma
rays. At the proximity to the titanium, the different sizes of
titanium implants increased the dose of Co-60 gamma rays by 17-21%,
and the same dose was increased by 17%. However, for 6 MV and 25 MV
X-ray irradiation, the dose increased by 17-18% and 15-16%,
respectively. Therefore, it could be inferred that the dose
increase of 25 MV energy X-rays was slightly lower than that of
other energy rays. Nevertheless, there was no significant
difference in the dose effect due to the difference in implant
size.
Nitinol, a titanium alloy, is widely used to
fabricate several types of stents, such as esophageal and tracheal
stents. Abu Dayyeh et al (19) used a solid water phantom irradiated
with 6, 10 and 18 MV photons. The nitinol stent and wall stent were
used; the anterior surface dose was increased by 4.1, 7.1 and 3.2%,
respectively; and the other nitinol stent ultraflex was irradiated
with the anterior surface dose enhancements of 4.7, 6.1 and 3.7%,
respectively. The stainless-steel stent was also used in the
aforementioned study, and the effect of the different stent
materials on the radiation dose was similar. The main determinant
of the dose effect in metallic stents was not the stent material,
but the mesh density of the stent. From extensive studies, it was
observed that the widely used implants made of nitinol could reduce
the effect on RT dose more than the stainless-steel stents;
however, caution should be exercised when choosing metal implants
because the shape of the stent itself can also affect radiation
dose.
Other metals
In addition to the commonly used stainless steel,
titanium and its alloys, several other metals such as gold, ZrO2,
and Al2O3 are used as materials for
artificial implants. These metallic materials are more widely used
in the field of dental implants. As reported by Akyol et al
(8) the calculated dose increment
by MC simulation in front of a dental implant was 15.5 and 3.3% for
ZrO2 and Al2O3, respectively. The dose
decrease behind the dental implant for ZrO2 and
Al2O3 was 22.2 and 7.0%, respectively. The
authors also calculated the change in dose after implantation of
metal implants such as titanium. The aforementioned study thus
revealed that the density of the implants has an effect on the dose
increase in the front of the material, with the higher density
increasing the dose to the front surface.
A temporary tissue expander (TTE) is commonly used
in patients who require post-mastectomy RT to maintain breast shape
and create space between the chest wall and skin. TTE contains an
internal metallic port (IMP) used as the injection port for saline
injection, which is usually composed of high-density rare-earth
magnets, and inevitably perturbs the RT dose (33-35).
Using a film dosimetry phantom experiment, Shankar et al
(36) observed that the dose
attenuation measured at a depth of 22 mm was 22% when irradiated
with a single photon beam of 6 MV light. When the energy was raised
to 15 MV, the dose attenuation at the same depth was 16%.
Furthermore, Gee et al (37) performed in vitro water
phantom measurements, measuring the dose distribution at 0.5, 50.0
and 100.0 mm downstream from the IMP, and revealed that the angle
of the rays to the IMP had a different effect on the dose, with a
28% metric attenuation when the rays were parallel to one another
and a 16% dose attenuation when they were perpendicular to one
another. Various degrees of dose reduction were observed downstream
in IMP studies (38,39); however, some researchers consider
that such dose reduction falls into the saline of TTE and does not
significantly affect the surrounding tissues (40).
4. Target delineation
Accurate delineation of the target area and organs
at risk is crucial for ensuring the efficacy of RT and controlling
the occurrence of toxic reactions. Incorrect delineation may lead
to under-dosage of the treatment and over-dosage in the target area
and organs at risk. A previous study revealed that a dose deficit
of 1% volume of the target that is >20% of the prescription dose
may lead to serious loss of tumor control probability with
intensity-modulated RT (IMRT) (41). Due to the current target
delineation in RT being primarily based on CT images, the presence
of metal implants in CT images may negatively impact the image
quality and accuracy of the target delineation. Metal implants
mainly exhibit white and dark stripes along the maximum attenuation
axis on CT images, which are caused by a combination of beam
hardening and scattering (15). In
addition, photon starvation caused by strong attenuation can lead
to statistical errors, which manifest as thin dark and bright
stripes around metal implants in CT images (42). The presence of artifacts on CT
images is challenging for delineating target areas and organs at
risk, especially when there is a lack of prior knowledge about the
type of implant (shape, size, metal or alloy composition, and
effective atomic number of metals), resulting in increased
uncertainty in the delineation of target areas and organs at risk
(42,43).
5. Methods to reduce the influence of metal
implants during RT
Metal implants can generate metal artifacts which
can increase the error of structure visualization and reduce the
accuracy of radiation oncologists' delineating targets and that of
radiation dose calculation, which can result in damage to the
adjacent normal tissues and reduced control rate of the tumor.
There are various methods to reduce metal artifacts. Dose
calculation algorithms can override the adverse impact of metal
implants on RT.
Methods to reduce metal artifacts
Various strategies to minimize metal artifacts and
improve image quality techniques have been investigated and
developed over the years. Dual energy CT is a common method to
reduce metal artifacts. It was reported to reduce beam hardening
artifacts between 95 and 150 kilo electron volt levels (44,45).
Additionally, the use of iterative metal artifact reduction
algorithms can reduce metal artifacts and improve dose calculation
accuracy, which enables the precise irradiation of tumors (42,46).
These techniques are based on projection data and the image-based
metal segmentation method that was used as a start (47). There are also commercially
available techniques to minimize metal artifacts such as iterative
metal artifact reduction (IMAR; Siemens Healthineers) (48), O-MAR (Philips Medical Systems,
Inc.) (49), single-energy metal
artifact reduction (SEMAR; Toshiba Medical Systems; Canon Medical
Systems) (50), and smart metal
artifact reduction (Smart MAR (General Electric Healthcare)
(51,52). The technique based on projection
data and image-based metal segmentation method was used recently,
and VM imaging with projection-based material decomposition
algorithm can not only reduce metal artifacts effectively, but also
simultaneously prevent object blurring at the metal artifact
position and image distortion of the metal implants (53,54).
Ceccarelli et al (55)
considered that combining information from virtual monoenergetic
reconstructions and MAR software images could be the best way to
solve the issue of metal artifacts on CT images. Those tools were
helpful in reducing metal artifacts; however, improved methods need
to be further explored. First of all, most of the current research
is carried out on the phantom, and it is necessary to verify the
effectiveness of its application in the human body, so as to
provide a reliable basis for clinical application. In addition,
numerous reconstruction algorithms are time-consuming and need to
be further optimized to improve the reconstruction efficiency.
Finally, the algorithm based on deep learning to reduce metal
artifacts has gradually attracted attention. Compared with
non-machine learning algorithms, it has advantages in reducing
signal-to-noise ratio. In the future, combining big data, deep
learning and digital twin technology with increasingly enhanced
computer algorithms may improve methods that reduce metal
artifacts.
The methods of dose calculation
algorithms
Using the aforementioned techniques, oncologists can
obtain a relatively precise processed CT image, making target
delineation more accurate. However, it is only through an accurate
and fast dose distribution calculation that oncologists can be more
confident in RT delivery and avoid unnecessary harm to patients in
advance. The algorithm for simulating photon dosage focuses on
modeling the deposition pattern of X-rays generated by a linear
accelerator in the patient. The common dose calculation algorithms
included PBC, analytical anisotropic algorithm (AAA), collapsed
cone convolution (CCC) and MC. PBC is the simplest and fastest
kernel-based dose computation method. Kernel-based algorithms make
use of kernels and ray tracing to model the dose deposition
resulting from interactions at a given point. The kernel represents
the spread of energy resulting from an interaction at a given point
or line, and the ray tracing algorithm represents the energy that
passes through the tissue from the energy source. AAA is a
convolution-based algorithm which was released in 2005 and
implemented in the Eclipse (Varian Medical Systems, Inc.)
Integrated TPS (56). CCC
algorithm uses one or more-point kernels rather than a line kernel
which could accurately model beam hardening as the beam traverses
the medium for multiple point kernels. MC is a method of finding
numerical solutions to a problem by random simulation which may be
used to compute dose distributions by simulating the interactions
of a large number of particles (including photons, electrons and
protons), as they travel through a medium. It is both the most
accurate and computationally intensive method of dose calculations
on account of large number of simulated interactions at an atomic
level (57).
PBC is only suitable for homogeneous media; its
accuracy in non-homogeneous media is poor, and therefore, there are
limitations to using it to simulate dosage in the presence of metal
implants. A CCC algorithm was used in several TPSs because of its
accuracy in homogeneous tissues. In a study by Panettieri et
al (58), for the modeled 6-MV
photon beams, both the PBC algorithm and the AAA tended to
underestimate the absorbed dose in the build-up region compared
with the MC results. Paulu and Alaei (59) studied the results of three common
dose calculation algorithms in the presence of a hip prosthesis.
The aforementioned study found that near the surface of the
prosthesis for all energies, a Pinnacle collapse cone convolution
algorithm created a 5-22% higher measured dose than calculated, and
for the Eclipse Acuros XB and the Eclipse AAA the overestimation of
dose was 2-23% and 6-25%, respectively. MC methods are regarded as
the ‘gold standard’ for patient dose calculations and are widely
used in clinical practice. Ade and Plessis (60) revealed that the MC algorithm used
in Monaco was significantly more accurate than the CCC algorithm
used in XiO. As Parenica et al (61) reported, the CCC algorithm in
Pinnacle demonstrated a significant 9.2% error in calculating the
dose and for the MC algorithm in the Monaco TPS the error was 3.6%.
Therefore, to the best of our knowledge, the MC algorithm can be
used to calculate the dose for the tumor and its surroundings more
accurately in the presence of metal implants. Additionally, it can
be used as a second check to ensure the accuracy of the RT plan.
However, the MC algorithm has some drawbacks, such as the long time
period required for computation and the statistical noise when the
number of simulated particles is insufficient. Further optimization
of the simulation algorithm is needed. For example, in
heterogeneous medium, especially at the junction of different
density materials, the accuracy of the measurement simulation
algorithm needs to be improved. To improve application of these
algorithms to clinical practice, it is necessary to further
optimize the computational complexity and reduce the computational
time.
6. The dosimetric effect analysis of metal
implants in different RT modalities
Currently, RT techniques commonly used in clinical
practice include single-field techniques, three-dimensional
conformal RT (3D-CRT), IMRT and VMAT. The dosimetric effects when
applying different RT techniques need to be considered when
treating patients with metallic implants.
Given the accelerating aging population and the rise
in hip replacement surgeries, the incidence of patients with cancer
and metallic hip implants (MHI) undergoing pelvic RT has increased
over the past few decades. Su et al (62) compared RT plans for patients with
prostate cancer and bilateral MHI using IMRT vs. 3D-CRT. Their
findings indicated that IMRT provided improved protection for the
bladder and rectum across all treatment stages, particularly in
high-dose regions. Both RT strategies, 3D-CRT and IMRT, provided
adequate target coverage, and the dose-volume histograms (DVHs) for
the prostheses were similar. However, IMRT had a drawback of dose
inhomogeneity within the Planning Target Volume. Van Der Est et
al (63) proposed methods to
further optimize IMRT, which effectively reduced the radiation dose
to the bladder and rectum during pelvic irradiation, offering
enhanced protection for patients with either unilateral or
bilateral MHI.
VMAT is a technique that utilizes inverse planning
without restricting beam angles. Singh et al (64) developed IMRT and VMAT plans for
patients with MHI using various optimization methods. Their results
revealed that, regardless of the optimization method, VMAT
consistently outperformed IMRT, offering greater volumetric
coverage, fewer hotspots, and less heterogeneity. Koutsouvelis
et al (65) demonstrated
that standard 2-co-planar arc 360˚ VMAT treatment, when applying
artifact reduction algorithms, could mitigate errors induced by
prostheses during pelvic RT in patients with bilateral MHI. The
dose errors due to the MHI were between 0.3 and 0.5%. This
technique enabled effective treatment without avoiding the
prostheses, particularly when the distance between the prosthesis
and the target was >0.5 cm. Another study also revealed that
VMAT not only resulted in lower rates of acute and chronic
genitourinary and gastrointestinal adverse effects but also offered
an improved therapeutic option overall (66). Soda et al (67) directly compared the performances of
3D-CRT, IMRT and VMAT in treating patients with prostate cancer and
bilateral MHI. Their findings revealed that VMAT delivered improved
DVH and required shorter treatment times compared with the other
two methods. Additionally, VMAT significantly improved dose
distribution in the presence of MHI compared with 3D-CRT,
highlighting its advantage in managing the complexities introduced
by metal prostheses during RT.
Furthermore, Rana et al (68) conducted a dosimetric study
comparing uniform scanning proton therapy (USPT) and VMAT for
patients with prostate cancer and MHI. Their findings indicated
that USPT provided superior dose uniformity and improved protection
for the rectum and bladder. These results suggested that uniform
scanning proton therapy offers potential dosimetric advantages in
treating prostate cancer involving MHI.
Metal implants are also common in the oral cavity.
Shimamoto et al (69)
compared dose differences when using single-field RT, 3D-CRT and
IMRT in the presence of dental metal implants (DMI). The
aforementioned study employed various types of DMIs and revealed
that single-field RT resulted in a scatter dose increase of
3.7-19.3% due to the DMI, while 3D-CRT and IMRT demonstrated
increases of 1.4-6.9 and 1.4-4.3%, respectively. The results
indicated that both 3D-CRT and IMRT were superior to single-field
RT in mitigating the increase in scatter dose caused by DMI.
Additionally, there was no significant difference in scatter doses
between 3D-CRT and IMRT for metals other than gold.
Based on these findings, oncologists should consider
the type of metallic implant and the specific circumstances of
their treatment center when selecting the appropriate RT technique
for patients with metallic implants in the treatment area, aiming
to minimize the impact of metallic implants on RT dosing.
7. The effects of metal implants on clinical
outcomes of RT
Although different metal implants have different
effects on RT dose, the methods of reduce metal artifacts and dose
calculation algorithms can decrease the impact to minimum, which
result to a favorable clinical outcome.
The self-expanding metallic stents (FCSEMS) have
been widely used in patients with esophageal cancer and is often
combined with RT. Post-stenting external beam RT effectively
prolongs duration of dysphagia relief and improves overall survival
in inoperable esophageal cancer (70). A meta-analysis involving eight
randomized controlled trials enrolling 732 patients were included
with three distinct comparisons: Stents combination therapy (RT or
chemotherapy or both) vs. stents alone, stents alone vs.
brachytherapy alone, and stents + brachytherapy vs. brachytherapy
alone. This revealed that combination therapy significantly
improves the overall survival as well as demonstrated improvements
in the quality-of-life scores (71). Another study revealed that
palliation of dysphagia or fistulas with FCSEMS in patients with
incurable esophageal cancer before or after RT was not associated
with an increased risk of life-threatening complications (72). The latest research revealed that RT
treatment in patients with an esophageal stent increases the
frequency of minor, however not life-threatening adverse events
(73). Stents have also been used
in contact with biliary obstruction caused by tumors such as
pancreatic cancer. Hayakawa et al (74) retrospectively analyzed the impact
on the safety of receiving CCRT after stent implantation in 30
cases (seven patients had SEMS while 23 had plastic stents). It was
observed that patients with biliary stents had a higher CCRT
completion rate, and CCRT after stenting was not associated with
significant toxicity or side effects. Furthermore, SEMS may benefit
patients more than plastic stents by keeping the bile duct more
normal for an extended duration and reducing stent obstruction.
Similar clinical results also appear in pelvic RT
with metal hip prostheses. Fischer and Hoskin (75) reported that no significant
differences were observed in genitourinary and gastrointestinal
toxicity incidence between patients with bilateral hip prostheses
and a control group (75). A
multi-institutional retrospective study demonstrated that their hip
prostheses were not affecting the prognosis of patients with
prostate cancer (76). TTE with an
IMP was commonly used for breast reconstructions and was inserted
subcutaneously at the time of mastectomy. Most patients who undergo
mastectomy require postoperative RT. A study revealed that patients
with TTE completed RT and did not experience any unacceptable
adverse effects during RT. No manifestations of infection, tissue
necrosis, or hematoma were observed during the RT (36).
8. Conclusion
RT is an essential modality in cancer treatment.
With an aging population and advancements in surgical techniques,
an increasing number of patients undergoing RT have metallic
implants. These implants impact various aspects of RT, including
target delineation, dose calculation and dose delivery, which in
turn affect dosimetric outcomes, control rates and side effects. To
address the influence of metallic implants and improve the efficacy
of RT, researchers have made efforts in reducing metal artifacts,
optimizing algorithms, and enhancing RT techniques. However, when
metallic implants are present within the radiation field,
oncologists must carefully choose appropriate dose calculation
methods and RT strategies based on the type of implant to improve
control of the tumor and minimize complications.
Acknowledgements
Not applicable.
Funding
Funding: The present review was supported by the Projects of
National Natural Science Foundation of China-NSAF (grant no.
U2330122) and the National Health Commission Key Laboratory of
Nuclear Technology Medical Transformation Open Project (grant nos.
2022HYX008 and 2022HYX011).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL and HX drafted the manuscript. YL, HX and WT
reviewed and collected data for the study. XD conceived the study
and contributed in the review and edit of the manuscript. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chandra RA, Keane FK, Voncken FEM and
Thomas CR Jr: Contemporary radiotherapy: Present and future.
Lancet. 398:171–184. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen Q and Thouas GA: Metallic implant
biomaterials. Mater Sci Eng R Rep. 87:1–57. 2015.
|
|
3
|
Le Fèvre C, Lacornerie T, Noël G and
Antoni D: Management of metallic implants in radiotherapy. Cancer
Radiother. 26:411–416. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Spaander MCW, Van Der Bogt RD, Baron TH,
Albers D, Blero D, de Ceglie A, Conio M, Czakó L, Everett S,
Garcia-Pagán JC, et al: Esophageal stenting for benign and
malignant disease: European Society of Gastrointestinal Endoscopy
(ESGE) Guideline-Update 2021. Endoscopy. 53:751–762.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen YK, Schefter TE and Newman F:
Esophageal cancer patients undergoing external beam radiation after
placement of self-expandable metal stents: Is there a risk of
radiation dose enhancement? Gastrointest Endosc. 73:1109–1114.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Conio M and Sorbi D: Metal stents improve
dysphagia, nutrition and survival in malignant oesophageal
stenosis: A randomized controlled trial comparing modified
Gianturco Z-stents with plastic Atkinson tubes. Gastrointest
Endosc. 51:248–249. 2000.PubMed/NCBI
|
|
7
|
Hansen CR, Christiansen RL, Lorenzen EL,
Bertelsen AS, Asmussen JT, Gyldenkerne N, Eriksen JG, Johansen J
and Brink C: Contouring and dose calculation in head and neck
cancer radiotherapy after reduction of metal artifacts in CT
images. Acta Oncol. 56:874–878. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Akyol O, Dirican B, Toklu T, Eren H and
Olgar T: Investigating the effect of dental implant materials with
different densities on radiotherapy dose distribution using
Monte-Carlo simulation and pencil beam convolution algorithm.
Dentomaxillofac Radiol. 48(20180267)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Evans AJ, Lee DY, Jain AK, Razi SS, Park
K, Schwartz GS, Trichter F, Ostenson J, Sasson JR and Bhora FY: The
effect of metallic tracheal stents on radiation dose in the airway
and surrounding tissues. J Surg Res. 189:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Spadea MF, Verburg JM, Baroni G and Seco
J: The impact of low-Z and high-Z metal implants in IMRT: A Monte
Carlo study of dose inaccuracies in commercial dose algorithms. Med
Phys. 41(011702)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bazalova M, Beaulieu L, Palefsky S and
Verhaegena F: Correction of CT artifacts and its influence on Monte
Carlo dose calculations. Med Phys. 34:2119–2132. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paudel MR, Mackenzie M, Fallone BG and
Rathee S: Evaluation of normalized metal artifact reduction (NMAR)
in kVCT using MVCT prior images for radiotherapy treatment
planning. Med Phys. 40(081701)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park HS, Hwang D and Seo JK: Metal
Artifact Reduction for Polychromatic X-ray CT Based on a
Beam-Hardening Corrector. IEEE Trans Med Imaging. 35:480–487.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Praveenkumar RD, Santhosh KP and Augustine
A: Estimation of inhomogenity correction factors for a Co-60 beam
using Monte Carlo simulation. J Cancer Res Ther. 7:308–313.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Giantsoudi D, De Man B, Verburg J,
Trofimov A, Jin Y, Wang G, Gjesteby L and Paganetti H: Metal
artifacts in computed tomography for radiotherapy planning:
Dosimetric effects and impact of metal artifact reduction. Phys Med
Biol. 62:R49–R80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reft C, Alecu R, Das IJ, Gerbi BJ, Keall
P, Lief E, Mijnheer BJ, Papanikolaou N, Sibata C and Van Dyk J:
AAPM Radiation Therapy Committee Task Group 63. Dosimetric
considerations for patients with HIP prostheses undergoing pelvic
irradiation. Report of the AAPM Radiation Therapy Committee Task
Group 63. Med Phys. 30:1162–1182. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nevelsky A, Borzov E, Daniel S and
Bar-Deroma R: Perturbation effects of the carbon fiber-PEEK screws
on radiotherapy dose distribution. J Appl Clin Med Phys. 18:62–68.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mail N, Albarakati Y, Ahmad Khan M, Saeedi
F, Safadi N, Al-Ghamdi S and Saoudi A: The impacts of dental
filling materials on RapidArc treatment planning and dose delivery:
challenges and solution. Med Phys. 40(081714)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abu Dayyeh BK, Vandamme JJ, Miller RC and
Baron TH: Esophageal self-expandable stent material and mesh grid
density are the major determining factors of external beam
radiation dose perturbation: Results from a phantom model.
Endoscopy. 45:42–47. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dietlicher I, Casiraghi M, Ares C, Bolsi
A, Weber DC, Lomax AJ and Albertini F: The effect of surgical
titanium rods on proton therapy delivered for cervical bone tumors:
Experimental validation using an anthropomorphic phantom. Phys Med
Biol. 59:7181–7194. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Atwood TF, Hsu A, Ogara MM, Luba DG,
Tamler BJ, Disario JA and Maxim PG: Radiotherapy dose perturbation
of esophageal stents examined in an experimental model. Int J
Radiat Oncol Biol Phys. 82:1659–1664. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tsuji Y, Yoshimura H, Uto F, Tamada T,
Iwata K, Tamamoto T, Asakawa I, Shinkai T, Kichikawa K and Hasegawa
M: Physical and histopathological assessment of the effects of
metallic stents on radiation therapy. J Radiat Res. 48:477–483.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu M, Li X, Niu Q and Zhai F: Impact of
implanted metal plates on radiation dose distribution in vivo. Chin
J Rad Oncol. 19:459–462. 2010.
|
|
24
|
Lin MH, Li J, Price RA Jr, Wang L, Lee CC
and Ma CM: The dosimetric impact of dental implants on
head-and-neck volumetric modulated arc therapy. Phys Med Biol.
58:1027–1040. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mahuvava C and Du Plessis FCP: Dosimetry
effects caused by unilateral and bilateral hip prostheses: A monte
carlo case study in megavoltage photon radiotherapy for computed
tomography data without metal artifacts. J Med Phys. 43:236–246.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
He Xueping and Ni Xinye: Impact of Metal
Implants with Two Different Materials on Radiation Dose
Distribution. China Medical Devices. 33:54–69. 2018.
|
|
27
|
Bhushan M, Tripathi D, Yadav G, Kumar L,
Dewan A and Kumar G: Effect of Hip prosthesis on photon beam
characteristics in radiological physics. Asian Pac J Cancer Prev.
21:1731–1738. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ozen J, Dirican B, Oysul K, Beyzadeoglu M,
Ucok O and Beydemir B: Dosimetric evaluation of the effect of
dental implants in head and neck radiotherapy. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 99:743–747. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ade N and du Plessis FCP: Measurement of
the influence of titanium hip prosthesis on therapeutic electron
beam dose distributions in a novel pelvic phantom. Phys Med.
42:99–107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Warburton A, Girdler SJ, Mikhail CM, Ahn A
and Cho SK: Biomaterials in spinal implants: A review. Neurospine.
17:101–110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kaur M and Singh K: Review on titanium and
titanium based alloys as biomaterials for orthopaedic applications.
Mater Sci Eng C Mater Biol Appl. 102:844–862. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rana SB and Pokharel S: A dosimetric study
of volumetric modulated arc therapy planning techniques for
treatment of low-risk prostate cancer in patients with bilateral
hip prostheses. South Asian J Cancer. 3:18–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Koutcher L, Ballangrud A, Cordeiro PG,
McCormick B, Hunt M, Van Zee KJ, Hudis C and Beal K: Postmastectomy
intensity modulated radiation therapy following immediate
expander-implant reconstruction. Radiother Oncol. 94:319–323.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park SH, Kim YS and Choi J: Dosimetric
analysis of the effects of a temporary tissue expander on the
radiotherapy technique. Radiol Med. 126:437–444. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen SA, Ogunleye T, Dhabbaan A, Huang EH,
Losken A, Gabram S, Davis L and Torres MA: Impact of internal
metallic ports in temporary tissue expanders on postmastectomy
radiation dose distribution. Int J Radiat Oncol Biol Phys.
85:630–635. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shankar RA, Nibhanupudy JR, Sridhar R,
Ashton C and Goldson AL: Immediate breast reconstruction-impact on
radiation management. J Natl Med Assoc. 95:286–295. 2003.PubMed/NCBI
|
|
37
|
Gee HE, Bignell F, Odgers D, Gill S,
Martin D, Toohey J and Carroll S: In vivo dosimetric impact of
breast tissue expanders on post-mastectomy radiotherapy. J Med
Imaging Radiat Oncol. 60:138–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Da Silva MF, De Oliveira HF, Borges LF,
Carrara HHA and Farina JA Jr: Effects of the metallic port in
tissue expanders on dose distribution in postmastectomy
radiotherapy: A tridimensional experimental model of dosimetry in
breast reconstruction. Ann Plast Surg. 80:67–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mizuno N, Takahashi H, Kawamori J,
Nakamura N, Ogita M, Hatanaka S, Yamauchi R, Hariu M and Sekiguchi
K: Determination of the appropriate physical density of internal
metallic ports in temporary tissue expanders for the treatment
planning of post-mastectomy radiation therapy. J Radiat Res.
59:190–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Park JM, Kim K, Park JI, Shin KH, Jin US
and Kim JI: Dosimetric effect of internal metallic ports in
temporary tissue expanders on postmastectomy radiation therapy: A
Monte Carlo study. Phys Med Biol. 62:4623–4636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tomé WA and Fowler JF: On cold spots in
tumor subvolumes. Med Phys. 29:1590–1598. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kovacs DG, Rechner LA, Appelt AL,
Berthelsen AK, Costa JC, Friborg J, Persson GF, Bangsgaard JP,
Specht L and Aznar MC: Metal artefact reduction for accurate tumour
delineation in radiotherapy. Radiother Oncol. 126:479–486.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rousselle A, Amelot A, Thariat J, Jacob J,
Mercy G, De Marzi L and Feuvret L: Metallic implants and CT
artefacts in the CTV area: Where are we in 2020 ? Cancer Radiother.
24:658–666. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang Y, Qian B, Li B, Qin G, Zhou Z, Qiu
Y, Sun X and Zhu B: Metal artifacts reduction using monochromatic
images from spectral CT: Evaluation of pedicle screws in patients
with scoliosis. Eur J Radiol. 82:e360–e366. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhou C, Zhao YE, Luo S, Shi H, Li L, Zheng
L, Zhang LJ and Lu G: Monoenergetic imaging of dual-energy CT
reduces artifacts from implanted metal orthopedic devices in
patients with factures. Acad Radiol. 18:1252–1257. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Morsbach F, Bickelhaupt S, Wanner GA,
Krauss A, Schmidt B and Alkadhi H: Reduction of metal artifacts
from hip prostheses on CT images of the pelvis: Value of iterative
reconstructions. Radiology. 268:237–244. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Conti D, Baruffaldi F, Erani P, Festa A,
Durante S and Santoro M: Dual-Energy Computed Tomography
Applications to Reduce Metal Artifacts in Hip Prostheses: A Phantom
Study. Diagnostics (Basel). 13(50)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Axente M, Paidi A, Von Eyben R, Zeng C,
Bani-Hashemi A, Krauss A and Hristov D: Clinical evaluation of the
iterative metal artifact reduction algorithm for CT simulation in
radiotherapy. Med Phys. 42:1170–1183. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Metal Artifact Reduction for Orthopedic
Implants (O-MAR), Philips Healthc 1-12, 2011.
|
|
50
|
Gondim Teixeira PA, Meyer JB, Baumann C,
Raymond A, Sirveaux F, Coudane H and Blum A: Total hip prosthesis
CT with single-energy projection-based metallic artifact reduction:
impact on the visualization of specific periprosthetic soft tissue
structures. Skeletal Radiol. 43:1237–1246. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Guilfoile C, Rampant P and House M: The
impact of smart metal artefact reduction algorithm for use in
radiotherapy treatment planning. Australas Phys Eng Sci Med.
40:385–394. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Puvanasunthararajah S, Fontanarosa D,
Wille ML and Camps SM: The application of metal artifact reduction
methods on computed tomography scans for radiotherapy applications:
A literature review. J Appl Clin Med Phys. 22:198–223.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Katsura M, Sato J, Akahane M, Kunimatsu A
and Abe O: Current and novel techniques for metal artifact
reduction at CT: Practical guide for radiologists. Radiographics.
38:450–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chang CH, Wu HN, Hsu CH and Lin HH:
Virtual monochromatic imaging with projection-based material
decomposition algorithm for metal artifacts reduction in
photon-counting detector computed tomography. PLoS One.
18(e0282900)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ceccarelli L, Vara G, Ponti F, Miceli M,
Golfieri R and Facchini G: Reduction of metal artifacts caused by
titanium peduncular screws in the spine by means of monoenergetic
images and the metal artifact reduction software in dual-energy
computed tomography. J Med Phys. 47:152–158. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Fogliata A, Nicolini G, Vanetti E, Clivio
A and Cozzi L: Dosimetric validation of the anisotropic analytical
algorithm for photon dose calculation: fundamental characterization
in water. Phys Med Biol. 51:1421–1438. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Brualla L, Rodriguez M and Lallena AM:
Monte Carlo systems used for treatment planning and dose
verification. Strahlenther Onkol. 193:243–259. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Panettieri V, Barsoum P, Westermark M,
Brualla L and Lax I: AAA and PBC calculation accuracy in the
surface build-up region in tangential beam treatments. Phantom and
breast case study with the Monte Carlo code PENELOPE. Radiother
Oncol. 93:94–101. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Paulu D and Alaei P: Evaluation of dose
calculation accuracy of treatment planning systems at hip
prosthesis interfaces. J Appl Clin Med Phys. 18:9–15.
2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ade N and du Plessis FCP: Dose comparison
between Gafchromic film, XiO, and Monaco treatment planning systems
in a novel pelvic phantom that contains a titanium hip prosthesis.
J Appl Clin Med Phys. 18:162–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Parenica HM, Mavroidis P, Jones W, Swanson
G, Papanikolaou N and Stathakis S: VMAT Optimization and Dose
Calculation in the Presence of Metallic Hip Prostheses. Technol
Cancer Res Treat. 18(1533033819892255)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Su A, Reft C, Rash C, Price J and Jani AB:
A case study of radiotherapy planning for a bilateral metal hip
prosthesis prostate cancer patient. Med Dosim. 30:169–175.
2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Van Der Est H, Prins P, Heijmen BJ and
Dirkx ML: Intensity modulated radiation therapy planning for
patients with a metal hip prosthesis based on class solutions.
Pract Radiat Oncol. 2:35–40. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Singh PK, Tripathi D, Singh S, Bhushan M,
Kumar L, Raman K, Barik S, Kumar G, Shukla SK and Gairola M: To
study the impact of different optimization methods on
intensity-modulated radiotherapy and volumetric-modulated Arc
therapy plans for Hip prosthesis patients. J Med Phys. 47:262–269.
2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Koutsouvelis N, Dipasquale G, Rouzaud M,
Dubouloz A, Nouet P, Jaccard M, Miralbell R, Tsoutsou P and Zilli
T: Bilateral metallic hip implants: Are avoidance sectors necessary
for pelvic VMAT treatments? Z Med Phys. 31:420–427. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ng WL, Brunt J, Temple S, Saipillai M,
Haridass A, Wong H, Malik Z and Eswar C: Volumetric modulated arc
therapy in prostate cancer patients with metallic hip prostheses in
a UK centre. Rep Pract Oncol Radiother. 20:273–277. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Soda R, Hatanaka S, Hariu M, Shimbo M,
Yamano T, Nishimura K, Kondo S, Utsumi N and Takahashi T:
Evaluation of geometrical uncertainties on localized prostate
radiotherapy of patients with bilateral metallic hip prostheses
using 3D-CRT, IMRT and VMAT: A planning study. J Xray Sci Technol.
28:243–254. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Rana S, Cheng C, Zheng Y, His W, Zeidan O,
Schreuder N, Vargas C and Larson G: Dosimetric study of uniform
scanning proton therapy planning for prostate cancer patients with
a metal hip prosthesis, and comparison with volumetric-modulated
arc therapy. J Appl Clin Med Phys. 15(4611)2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Shimamoto H, Sumida I, Kakimoto N,
Marutani K, Okahata R, Usami A, Tsujimoto T, Murakami S, Furukawa S
and Tetradis S: Evaluation of the scatter doses in the direction of
the buccal mucosa from dental metals. J Appl Clin Med Phys.
16(5374)2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Javed A, Pal S, Dash NR, Ahuja V, Mohanti
BK, Vishnubhatla S, Sahni P and Chattopadhyay TK: Palliative
stenting with or without radiotherapy for inoperable esophageal
carcinoma: A randomized trial. J Gastrointest Cancer. 43:63–69.
2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lai A, Lipka S, Kumar A, Sethi S, Bromberg
D, Li N, Shen H, Stefaniwsky L and Brady P: Role of esophageal
metal stents placement and combination therapy in inoperable
esophageal carcinoma: A systematic review and meta-analysis. Dig
Dis Sci. 63:1025–1034. 2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Sasaki K, Osako Y, Urata M, Noda M,
Tsuruda Y, Uchikado Y, Omoto I, Kita Y, Matsushita D, Okubo K, et
al: Clinical outcomes of fully covered self-expanding metallic
stent placement for palliation of incurable esophageal cancer with
or without radiotherapy. Anticancer Res. 41:385–389.
2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Machado AA, Martins BC, Josino IR, Chen
ATC, Hong CBC, Santos ALDR, Lima GRA, Cordero MAC, Safatle-Ribeiro
AV, Pennacchi C, et al: Impact of radiotherapy on adverse events of
self-expanding metallic stents in patients with esophageal cancer.
Dis Esophagus. 36(doad019)2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hayakawa S, Ito K, Hayakawa J, Murofushi
KN and Karasawa K: Safety of biliary stent placement followed by
definitive chemoradiotherapy in patients with pancreatic cancer
with bile duct obstruction. J Gastrointest Oncol. 12:2260–2267.
2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Fischer AM and Hoskin PJ:
Radiotherapy-induced toxicity in prostate cancer patients with hip
prostheses. Radiat Oncol. 17(9)2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Sun L, Quon H, Tran V, Kirkby C and Smith
W: External beam radiation therapy treatment factors prognostic of
biochemical failure free survival: A multi-institutional
retrospective study for prostate cancer. Radiother Oncol.
173:109–118. 2022.PubMed/NCBI View Article : Google Scholar
|