1. Introduction
Cervical cancer (CC) is one of the most common
cancers in women of reproductive age, with 342,000 deaths and
604,000 new cases in 2020. Nearly 90% of these deaths occur in
middle- and low-income nations (1). The primary cause of CC is the chronic
persistent infection of high-risk human papillomavirus (HPV), which
is present in over 90% of cases. However, it is important to note
that only 1% of high-risk HPV-infected women develop CC (2). This indicates the presence of
additional factors, such as gene mutations and chromosome
rearrangements, that contribute to the development of CC. Numerous
studies have identified genetic alterations, such as mutations and
amplifications, that contribute to the oncogenic process in
HPV-positive CC. For example, recent observations have shown that
HPV integration leads to various genomic changes in cervical
adenocarcinoma (3). Similarly, a
previous study identified the FGFR3-TACC3 fusion in HPV-positive CC
(4). Therefore, it is urgent to
gain a deeper understanding of new fusion genes responsible for
molecular heterogeneity in HPV-related CC for improved clinical
outcomes.
The neurotrophic tyrosine receptor kinase (NTRK)
genes encode tropomyosin receptor kinases (TRK). NTRK genes are
essential for nerve cell development and function and may fuse with
different genes. When NTRK genes fuse, they produce constitutively
activated chimeric TRK receptors. These receptors can lead to
cancer invasion, angiogenesis, growth, survival and activate the
mitogen-activated protein kinase (MAPK) and phosphoinositide
3-kinase (PI3K) pathways (5).
NTRK1-3 fusions have now been recognized in multiple cancer types.
They are highly prevalent (~90%) in some rare cancers such as
mammary analogue secretory carcinoma, secretory breast carcinoma
and congenital infantile fibrosarcoma, and less common (<1%) in
numerous types of adult cancers including salivary gland cancers,
thyroid, colorectal and non-small cell lung cancers (6). The prevalence of NTRK fusion genes in
CC is low (0.36-1.88%). However, NTRK-fusion gene positive cancers
caused by these genetic alterations have a different tumor
microenvironment and do not respond to conventional treatments like
radiotherapy/chemotherapy (7).
Therefore, there is a dire need for in-depth studies of NTRK-fusion
positive CC to identify more potent signaling molecules associated
with dysregulation of immune cells and activation of oncogenic
pathways.
The role of HPV infection in the development of NTRK
fusion genes is unknown. Both factors independently contribute to
the risk of developing CC and contribute to CC heterogeneity. The
authors' hypothesis is that CC that is positive for NTRK fusion
genes and has HPV infection may have more severe outcomes and
require more effective treatments. Therefore, it is crucial to
review the interaction between HPV and NTRK fusion genes in CC. The
present review is pioneering because it focuses on the combined
effect and interaction of HPV and NTRK fusion genes in the
progression of CC.
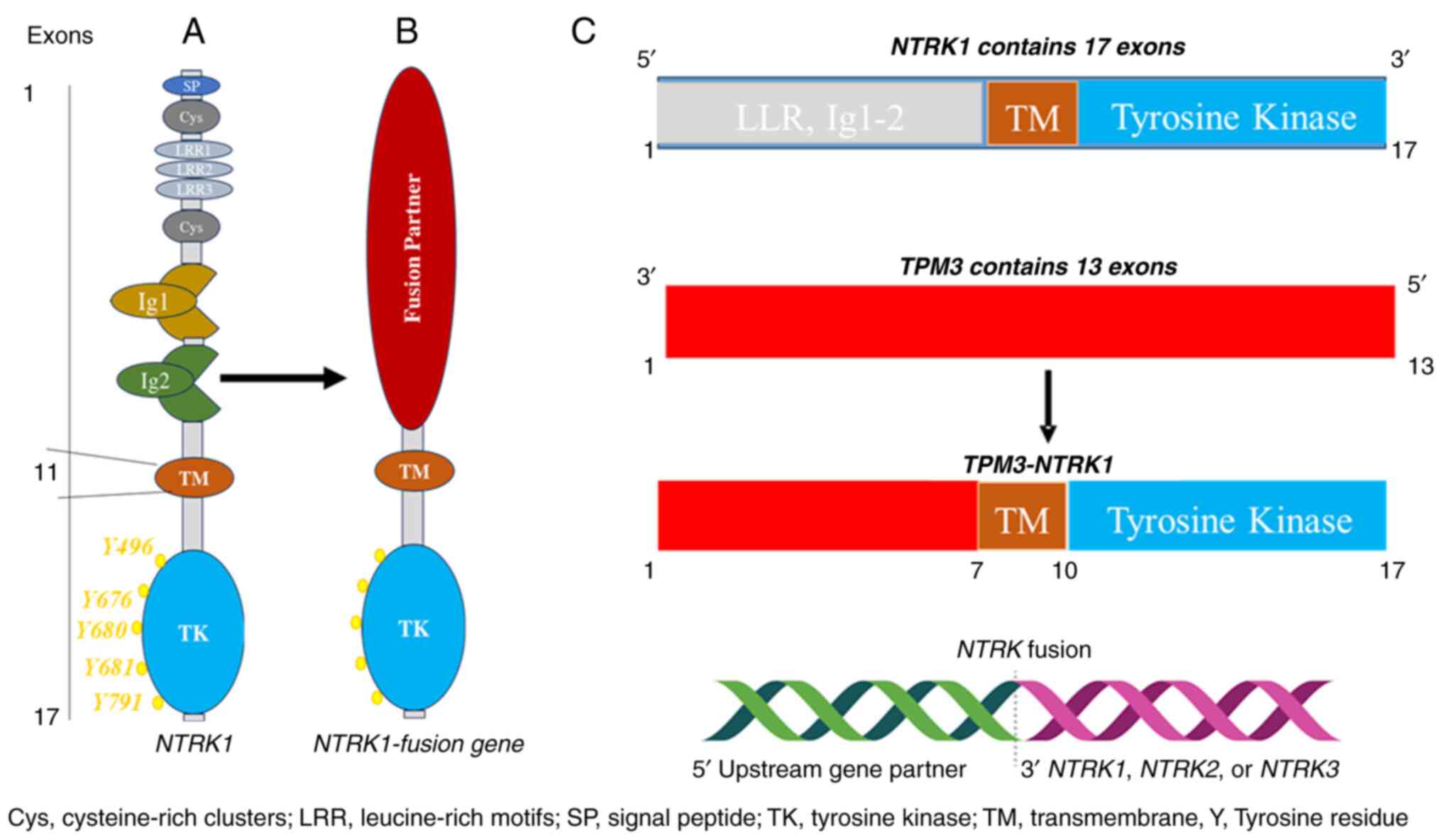
2. NTRK fusion genes
The NTRK1, 2 and 3 genes are located on chromosomes
1q21-q22, 9q22.1 and 15q25, respectively. They code for TRKA (140
kDa), TRKB (145 kDa) and TRKC (145 kDa) proteins, respectively.
Despite their different positions on different chromosomes and
different mechanisms of activation and regulation, they are highly
homologous and have similar structural domains, including
intracellular kinase domains and extracellular ligand binding
(8). The extracellular domain
contains two immunoglobulin-like (Ig1-2) high-affinity receptors
that interact with cognate ligands, predominantly via Ig-2.
Specifically, TRK proteins have three leucine-rich 24-residue
motifs that are flanked by two cysteine clusters (C1-2). Meanwhile,
the intracellular domain contains a kinase domain and is linked to
the extracellular domain through a transmembrane structure
(9) (Fig. 1A).
The fusion of NTRK1-3 genes is a common occurrence
that leads to the oncogenic activation of TRK. This happens when
the 3' region of the NTRK gene combines with a 5' sequence of a
fusion partner gene through rearrangement, either within the same
chromosome or between different chromosomes (Fig. 1B and C). In all TRK oncogenic fusions, the TRK
protein kinase domain is always present. Therefore, TRK fusion
proteins always contain the TRK kinase domain. As a result, the
resulting protein from the fusion, known as a chimeric oncoprotein,
is characterized by continuous activation and overexpression of the
TRK protein kinase, independent of any ligand (10). Because of their strong oncogenic
effects and potential for targeted therapy, TRK fusions have
received significant attention as promising therapeutic targets in
cancer treatment (11).
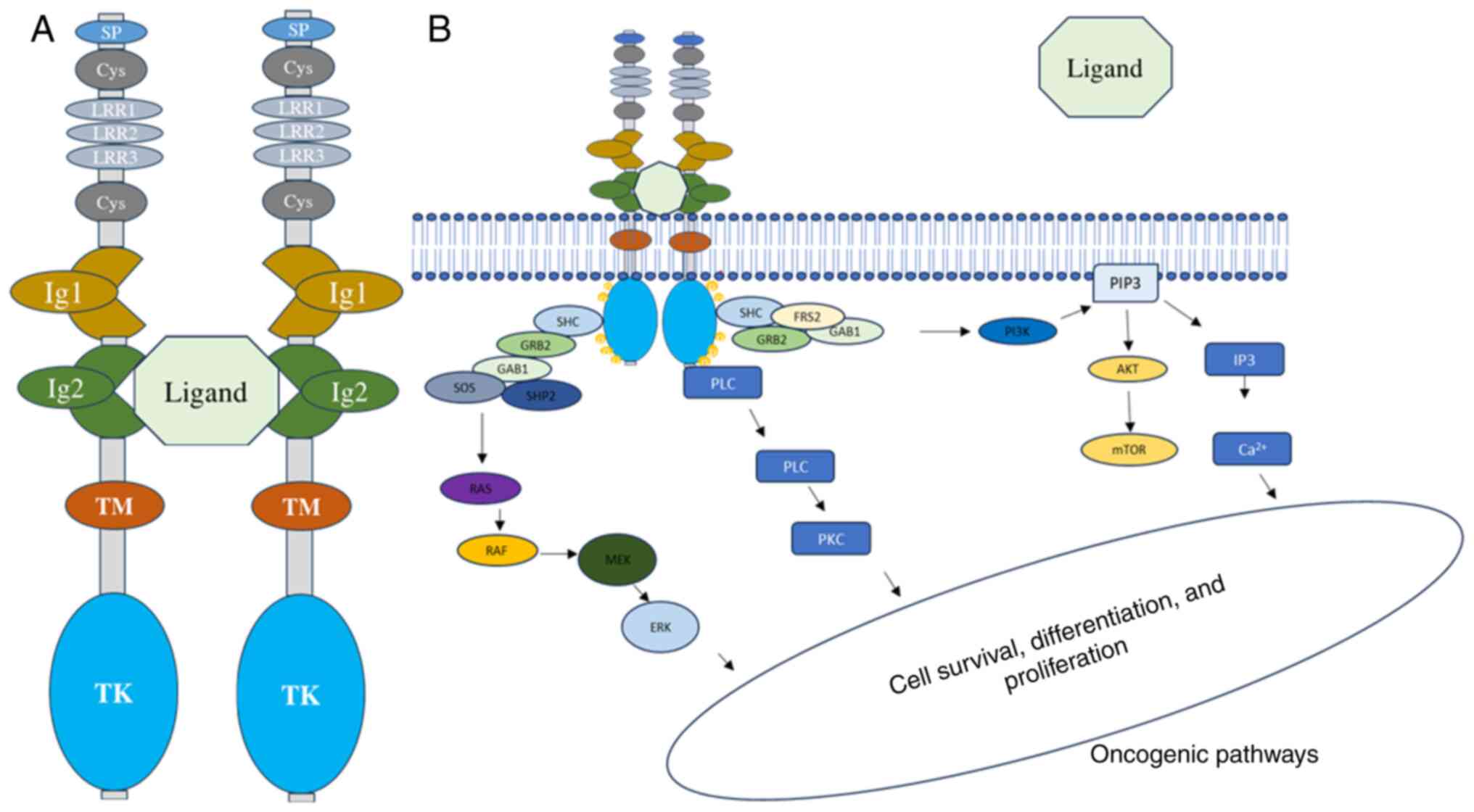
3. TRK signaling pathways
Binding of ligands to extracellular domains of Ig
receptors leads to autophosphorylation of intracellular tyrosine
(Y) residues. The most common ligand for TRKA is nerve growth
factor (NGF), while brain-derived growth factor and neurotrophin
(NT)-4/5 bind to TRKB, and NT-3 binds to TRKC. NGF binding to TRKA
triggers receptor homodimerization and transphosphorylation of
crucial tyrosine residues (Y496, Y676, Y680, Y681 and Y791)
(Fig. 2A). Specifically, Y496 and
Y791 serve as phosphorylation-dependent binding sites for adaptor
proteins with phosphotyrosine binding or src homology 2 (SH2)
domains, such as GRB2-associated-binding protein 1 (GAB1),
phospholipase C-γ (PLCγ) and SHC adaptor protein 1. Other adaptor
proteins involved are insulin receptor substrate (IRS)1-2, growth
factor receptor bound protein 2 (GRB2), SH2B and fibroblast growth
factor receptor substrate 2 (FRS2). Multiple studies have suggested
that RAS or GAB1 activates the PI3K signaling pathway, although
other mechanisms may also activate it (12). Once activated, the three wildtype
TRK family members commonly activate multiple downstream signaling
pathways, including PI3K-AKT, PLCγ-PKC, or SHC-RAS-MAPK, depending
on which docking protein(s) are bound to phosphorylated Y496 and
Y791(12). Activation of these
molecular pathways leads to various cellular processes, such as
transcriptional regulation, neurite outgrowth, synaptic plasticity,
cellular proliferation, repair or prevention of neurodegeneration,
maintenance of sensory neurons, or apoptosis (Fig. 2B) (12). Previous studies have also revealed
that the reduced isoforms of TRK proteins can act as active
signaling molecules by recruiting scaffolding proteins like Rho
GDP-dissociation inhibitor 1 and GRP1-associated scaffold protein
(13).
4. TRK activation in cancer
TRK proteins can be activated through various
mechanisms, including somatic NTRK mutations, activation of NTRK
splice variants and TRK overexpression. Somatic NTRK mutations have
been observed in different types of tumors, such as colorectal
cancer, lung cancer, acute myeloid leukemia and melanoma. Studies
have investigated mutations affecting Ig2, kinase activity,
activation loop residues and inhibitor efficiency (14). The exact role of NTRK mutants in
cancer development remains unclear. However, the NTRK1 splice
variant (TRKAIII) and a genomic in-frame deletion mutant (ΔTRKA)
are known to be oncogenic. Both variants lack glycosylated regions
in the ligand binding domain and have a constitutively active
kinase domain (14). Additionally,
TRKA-C is overexpressed in various cancers and is associated with
tumor aggressiveness. In breast cancer models, for instance, TRKA
overexpression leads to increased tumor cell migration, invasion
and proliferation through activation of the PI3K and MAPK pathways.
Overexpression of TRKB and/or TRKC has also been observed in
patients with cylindroma, as well as in sporadic basal cell
carcinomas (14).
5. TRK fusions oncogenic activation
In fusion biology, it is observed that upstream gene
partners in NTRK fusion events often possess WD repeats, zinc
finger domains, or oligomerization domains such as coiled-coil
domains. These domains are crucial for the full activation of
downstream kinase. Most NTRK fusion partners typically have
oligomerization domains, although there are exceptions in which
fusion partners do not possess known dimerization domains (15). In such cases, it remains unclear
how the upstream partner contributes to the downstream TRK kinase
activation. Immunohistochemical analyses of tumors with NTRK
fusions suggest that the fusion protein's subcellular localization
can be determined by the kinase partner. This emphasizes the varied
and crucial roles of upstream partners in the oncogenic activation
of various TRK fusion proteins. These fusion proteins, even without
ligand signaling, can still activate the same downstream pathways
as full-length TRK proteins. For instance, fusion oncoproteins,
tropomyosin 3 (TPM3)-TRKA and translocated promoter region
(TPR)-TRKA, were able to bind SHC, IRS1, IRS2, FRS2 and FRS3,
similar to the full-length TRK protein (16). Moreover, such activated adaptors
facilitate the recruitment of p85, SH2 domain containing protein
tyrosine phosphatase (SH-PTP2) and GRB2, leading to PI3K and MAPK
signaling network activation (16). Although TRK fusions signal through
the same pathways as full-length TRK proteins, the downstream
signaling can also be affected by the subcellular localization of
TRK receptors driven by the fusion partner and the specific
histology of the tumor tissue (14).
6. Mutations in NTRK fusion gene and drug
resistance
The kinase domains of NTRK exhibit structural
flexibility and undergo various conformational transitions that
directly affect how inhibitors bind. These domains primarily exist
in two conformations, which are determined by the position of three
specific residues: Aspartic acid (D), phenylalanine (F) and glycine
(G)-known as the DFG motif. This activation loop in the kinase
domain is flexible and determines whether the kinase is in an
active state (in conformation) or an inactive state
(out-conformation) (7). Crucial
mutations in the catalytic region of the kinase domain have been
identified through clinical screenings. These mutations can occur
in the solvent front of the ATP-binding pocket (solvent-front
mutations), the amino acid preceding the activation loop DFG motif
(xDFG mutation), or the gatekeeper residue (a conserved hydrophobic
amino acid in the active site). Somatic point mutations at these
sites in the NTRK kinase domain led to resistance against inhibitor
drugs such as larotrectinib and entrectinib (7). These mutations often impede inhibitor
binding and boost catalytic function by reducing the KM value for
ATP, thus increasing rivalry between inhibitors and ATP. For
example, the TPM3-NTRK1 fusion includes G595R, F589L, as well as
G667C mutations, while the ETV6-NTRK3 fusion contains G623R, F617L
and G696A (7,17).
The aforementioned data showed that somatic NTRK
mutations and gene fusions play a crucial role in activating TRK
proteins in a cancerous manner. Furthermore, these mutations and
fusions can significantly change the 3D structure of the kinase
domain. This change not only affects the recruitment of adaptor
proteins, which leads to false signals and the activation of
cancer-causing pathways, but also reduces the binding of TRK
inhibitor drugs, resulting in increased drug resistance (7). However, current research does not
fully understand how different mutations and NTRK gene fusions
contribute to the activation of cancer-causing pathways. Therefore,
further research is needed to investigate their role in CC and
identify the most promising abnormalities that can be targeted for
therapy.
7. NTRK fusion genes and cervical
cancer
NTRK gene fusions occur in various tumors in both
children and adults, across different tissues and cell lineages. A
recent study analyzed >295,000 patients with cancer and found
NTRK gene fusions in 889 cases, representing a prevalence of 0.30%
across 45 different tumor types. The prevalence of NTRK gene
fusions varied significantly depending on age, cancer type and
histology. These fusions were commonly found in both adult and
pediatric tumors, with NTRK1 and NTRK3 being the most frequent
partner genes and the ETV6-NTRK3 fusion being the most frequently
observed (6).
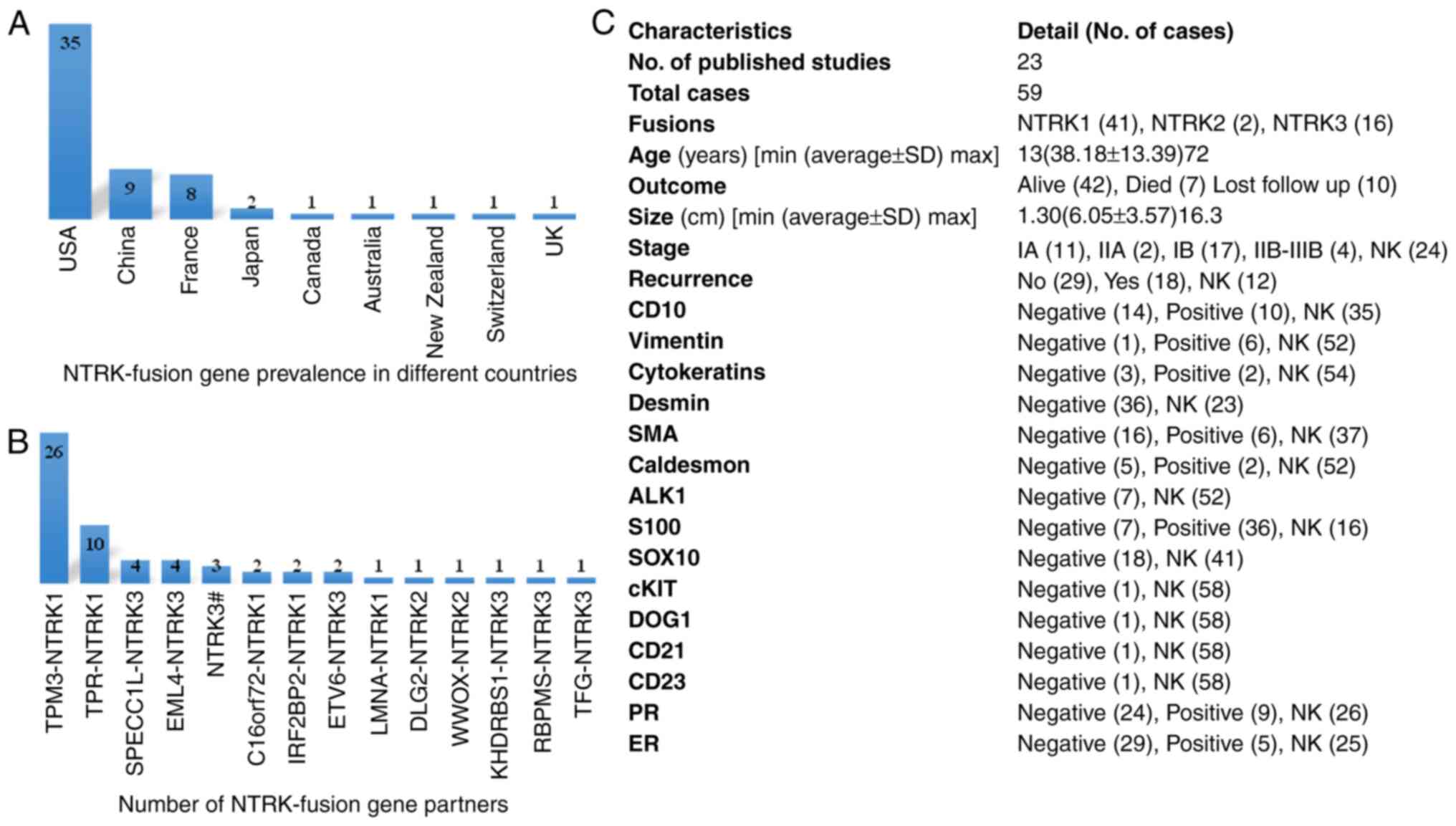
A total of 23 published case reports of NTRK fusion
genes in patients with CC (59 individuals) were obtained through a
literature survey conducted in December 2023 (Table I). Among the 59 cases, 35 cases
(59.32%) were reported in the United States of America, 9 cases
(15.25%) were reported in China, 8 cases (13.56%) were reported in
France and only 2 (3.39%) cases were reported in Japan. Australia,
Canada, Switzerland, the United Kingdom and New Zealand each
reported 1 case (1.7%) (Fig. 3A).
The highest number (41; 69.49%) of fusions were observed with
NTRK1, with TPM3 being the most frequent partner of NTRK1 in 26
cases (63.41%), followed TPR (10 cases; 24.39%). C16orf72 and
IRF2BP2 were each observed in 2 cases (4.87%). NTRK3 fusions were
observed in 16 cases (27.12%), with the partner genes sperm antigen
with calponin homology and coiled-coil domains 1-like (SPECC1L),
EMAP like 4 (EML4), ETS variant transcription factor 6 (ETV6),
RNA-binding protein with multiple splicing (RBPMS), trafficking
from endoplasmic reticulum to Golgi regulator (TFG) and KH
RNA-binding domain containing, signal transduction associated 1
(KHDRBS1) in 4, 4, 2, 1, 1 and 1 case, respectively. However, 3
cases showed NTRK3 rearrangements, but no fusion partner was
mentioned. There were only 2 (3.39%) reported cases of NTRK2
fusions, 1 case of discs large MAGUK scaffold protein 2 (DLG2) and
1 case of WW domain-containing oxidoreductase (WWOX) (Fig. 3B). The average age (38.18 years),
size of tumor (6.05 cm) and a high recurrence rate among 18
(30.51%) individuals were observed. The expression levels of
different proteins and other clinical characteristics are revealed
in Fig. 3C.
 | Table IList of included articles. |
Table I
List of included articles.
| First author,
year | Region | Cases | Case number | Age | Fusion type | (Refs.) |
|---|
| Boyle et al,
2020 | UK | 1 | 1 | 42 | TPM3-NTRK1 | (26) |
| Chiang et
al, 2018 | USA | 4 | 2 | 46 | RBPMS-NTRK3 | (27) |
| | | | 3 | 27 | TPR-NTRK1 | |
| | | | 4 | 47 | LMNA-NTRK1 | |
| | | | 5 | 42 | TPM3-NTRK1 | |
| Costigan et
al, 2022 | USA | 13 | 6 | 35 | C16orf72-NTRK1 | (28) |
| | | | 7 | 35 | TPM3-NTRK1 | |
| | | | 8 | 47 | TPR-NTRK1 | |
| | | | 9 | 30 | TPR-NTRK1 | |
| | | | 10 | 39 | TPM3-NTRK1 | |
| | | | 11 | 16 | TPR-NTRK1 | |
| | | | 12 | 26 | EML4-NTRK3 | |
| | | | 13 | 26 | TFG-NTRK3 | |
| | | | 14 | 61 | SPECC1L-NTRK3 | |
| | | | 15 | 24 | TPM3-NTRK1 | |
| | | | 16 | 42 | TPR-NTRK1 | |
| | | | 17 | 46 | IRF2BP2-NTRK1 | |
| | | | 18 | 26 | TPM3-NTRK1 | |
| Bühler et
al, 2023 | Switzerland | 1 | 19 | 24 | TPM3-NTRK1 | (29) |
| Wells et al,
2019 | USA | 1 | 20 | 30 | TPM3-NTRK1 | (30) |
| Croce et al,
2019 | France | 8 | 21 | 39 | TPM3-NTRK1 | (31) |
| | | | 22 | 44 | TPM3-NTRK1 | |
| | | | 23 | 26 | EML4-NTRK3 | |
| | | | 24 | 23 | TPM3-NTRK1 | |
| | | | 25 | 30 | TPM3-NTRK1 | |
| | | | 26 | 60 | TPM3-NTRK1 | |
| | | | 27 | 33 | TPM3-NTRK1 | |
| | | | 28 | 23 | TPM3-NTRK1 | |
| Dang et al,
2022 | China | 1 | 29 | 33 | EML4–NTRK3 | (32) |
| Devereaux et
al, 2021 | USA | 9 | 30 | 39 | TPM3-NTRK1 | (33) |
| | | | 31 | 66 | TPM3-NTRK1 | |
| | | | 32 | 30 | TPM3-NTRK1 | |
| | | | 33 | 32 | TPM3-NTRK1 | |
| | | | 34 | 21 | TPM3-NTRK1 | |
| | | | 35 | 40 | TPR-NTRK1 | |
| | | | 36 | 37 | IRF2BP2-NTRK1 | |
| | | | 37 | 35 | C16orf72-NTRK1 | |
| | | | 38 | 24 | SPECC1L-NTRK3 | |
| Fang et al,
2023 | China | 1 | 39 | 49 | NTRK3# | (34) |
| Gatalica et
al, 2019 | USA | 1 | 40 | NK | TPM3-NTRK1 | (35) |
| Goulding et
al, 2021 | New Zealand | 1 | 41 | 13 | TPM3-NTRK1 | (36) |
| Xiaoqing et
al, 2023 | China | 2 | 42 | 55 | KHDRBS1-NTRK3 | (37) |
| | | | 43 | 46 | TPR-NTRK1 | |
| Wong et al,
2020 | Australia | 1 | 44 | 31 | NTRK3# | (38) |
| Tsaiet al,
2022 | China | 2 | 45 | 47 | TPM3-NTRK1 | (39) |
| | | | 46 | 53 | TPM3-NTRK1 | |
| Takahashi et
al, 2018 | Japan | 1 | 47 | 44 | ETV6-NTRK3 | (40) |
| Rabban et
al, 2020 | USA | 3 | 48 | 24 | TPM3-NTRK1 | (41) |
| | | | 49 | 30 | TPR-NTRK1 | |
| | | | 50 | 49 | TPR-NTRK1 | |
| Nilforoushan et
al, 2022 | USA | 2 | 51 | 54 | SPECC1L-NTRK3 | (42) |
| | | | 52 | 52 | TPM3-NTRK1 | |
| Munkhdelger et
al, 2021 | Japan | 1 | 53 | 72 | DLG2-NTRK2 | (43) |
| Moh et al,
2021 | USA | 1 | 54 | 69 | WWOX-NTRK2 | (44) |
| Hanhan et
al, 2021 | China | 1 | 55 | 33 | ETV6-NTRK3 | (45) |
| Xiaoqi et
al, 2023 | China | 2 | 56 | 21 | EML4-NTRK3 | (46) |
| | | | 57 | 28 | NTRK3# | |
| Hodgson et
al, 2021 | Canada | 1 | 58 | 60 | SPECC1L-NTRK3 | (47) |
| Hartmaier et
al, 2017 | USA | 1 | 59 | - | TPR-NTRK1 | (48) |
8. HPV and cervical cancer
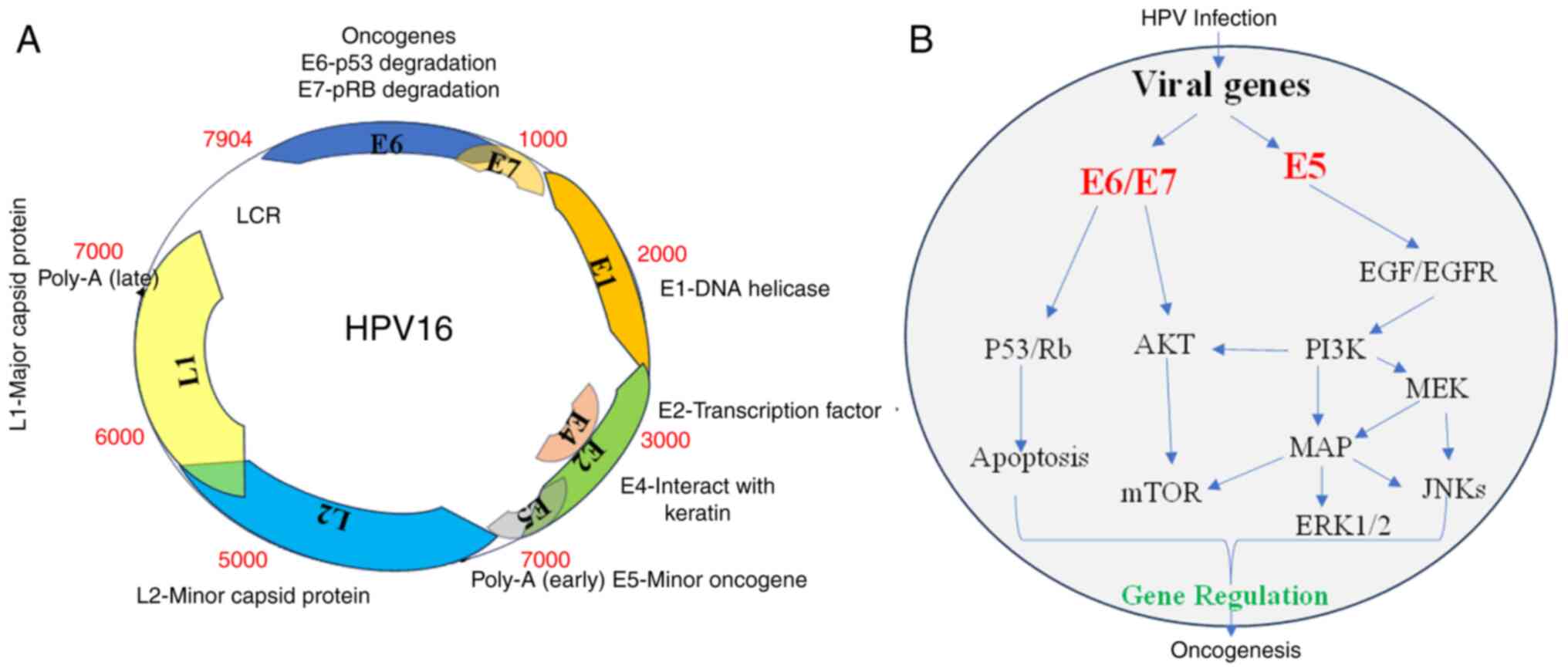
HPV is a virus that belongs to the
Papovaviridae family and has a double-stranded DNA. It has a
small, highly conserved DNA with ~8,000 base pairs (bp), which is
divided into three regions. The genome encodes eight open reading
frames that are arranged on one DNA strand. These include six early
proteins, three regulatory proteins (E1, E2 and E4), and three
oncoproteins (E5, E6 and E7). These proteins, which are encoded in
4,000 bp, play a role in viral replication and cell transformation.
An additional 3,000 bp region of the DNA molecule encodes two
structural proteins, L1 and L2, which make up the capsid of the
virus. The replication and transcriptional regulatory elements of
the viral DNA are controlled by a long control region that is
encoded within a 1,000 bp region (Fig.
4A) (18). More than 200 HPV
types have been recognized, with over 40 types that can colonize
the genital tract. HPV infection types are categorized into high
and low risk groups based on their ability to cause cancer. It is
well established that HPV-16 and 18 are the most dangerous
high-risk genotypes, responsible for ~70% of all cases of invasive
CC worldwide.
Multiple studies have confirmed that oncogenic HPV
infection is the main risk factor for the development of cervical
intraepithelial neoplasia (CIN). CIN can range from low-grade
squamous intraepithelial lesions (SIL) to high-grade SIL and
cancer. Persistent HPV infection can cause cellular changes in the
cervix, leading to precancerous lesions known as CIN, which are
classified into three grades: CIN1, CIN2 and CIN3. If left
untreated, CIN3 can progress to invasive CC. The VIVIANE study
found that HPV33 and HPV16 pose the highest risk for developing
CIN, followed by HPV18, HPV31 and HPV45(19). Additionally, HPV testing has proven
to be effective in detecting precancerous cervical lesions,
particularly in population-based cervical screening programs
(20). The role of different HPVs
in the progression of CC has been recently reviewed in studies
(21,22).
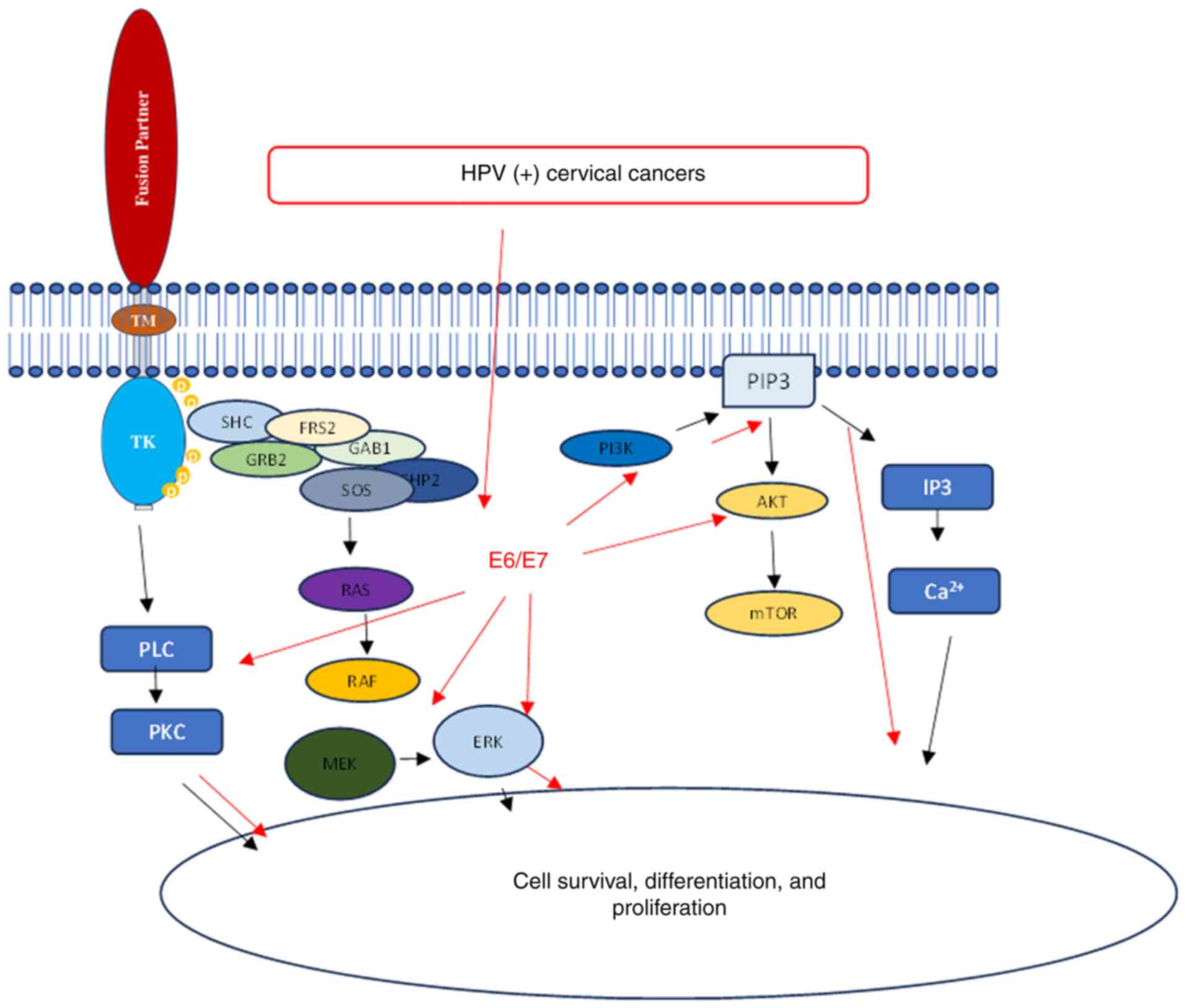
9. HPV activates the same oncogenic pathways
as NTRK fusion genes
The main reason why HPV is considered oncogenic is
due to the expression of viral oncoproteins E5, E6 and E7. These
oncoproteins disrupt normal cellular functions and promote
malignant transformation (18).
The role of E6 and E7 oncoproteins in the development of
HPV-associated CC has been extensively studied. It has been
observed that the E6 and E7 proteins interact with various
intracellular signaling pathways, leading to induced
carcinogenesis. The viral oncoprotein E6 interacts with the tumor
suppressor protein p53, causing its degradation and inhibiting
apoptosis. Similarly, the E7 protein binds to and inactivates the
tumor suppressor retinoblastoma protein (pRb), promoting cell cycle
progression and genomic instability. HPV infection leads to cell
immortalization and transformation, primarily through the viral
oncogenes E6, E7 and E5. These oncogenes have various effects on
cellular processes, such as inhibiting p53 and pRb (23), altering the expression of numerous
genes (~4% of the genes on the array) (24), and activating signaling pathways.
The virus utilizes various pathways (PI3K/Akt, Wnt/β-catenin,
ERK/MAPK and JAK/STAT) that transmit signaling through active
molecules such as MEK, ERK and Akt. Ultimately, all these
developments increase cell proliferation, leading to carcinogenesis
(18,23,25)
(Fig. 4B).
10. Conclusion
The aforementioned data suggested that both
NTRK-fusion genes and HPV regulate CC through the same signaling
pathways. The development of HPV-induced CC involves multiple
steps, including the accumulation of genetic and epigenetic
alterations in cervical cells. In cases of persistent HPV
infection, viral oncoproteins E6 and E7 contribute to the
progression of precancerous lesions and CC. Therefore, the presence
of NTRK-fusion genes in HPV-induced CC could potentially enhance
the impact on downstream signaling pathways, affecting cellular
functions such as cell survival, differentiation and proliferation,
and ultimately lead to oncogenesis. A hypothetical mechanism,
suggesting the existence of a synergistic relationship between NTRK
fusion genes and HPV, is demonstrated in Fig. 5. This emphasizes the complexity of
this scientific problem and highlights the need for further
in-depth research.
Based on the aforementioned literature surveys, it
was hypothesized that treating HPV-positive CC with NTRK fusion
genes may present more challenges. However, despite extensive
searches in online databases, data correlating HPV with NTRK fusion
genes could not be found. This further emphasizes the novelty of
the present review. One limitation of the present review is the
absence of statistical analysis or correlation due to the
unavailability of relevant data. Therefore, further investigation
is necessary to explore this area and contribute to the development
of personalized treatment strategies for patients with NTRK fusion
and HPV-positive CC. This could potentially lead to improved
patient outcomes and a reduction in mortality rates.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Innovation Funds for Dalian (grant no.
2020JJ27SN096).
Availability of data and materials
Not applicable.
Authors' contributions
AURA conceptualized the study, developed
methodology, performed software analysis and wrote the original
draft. JZ and CZ curated and validated data. XY and DW supervised
the study, acquired funding, and wrote, reviewed and edited the
manuscript. DW conducted project administration. All authors read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mittelstadt S, Kelemen O, Admard J,
Gschwind A, Koch A, Wörz S, Oberlechner E, Engler T, Bonzheim I,
Staebler A, et al: Detection of circulating cell-free HPV DNA of 13
HPV types for patients with cervical cancer as potential biomarker
to monitor therapy response and to detect relapse. Br J Cancer.
128:2097–2103. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou L, Qiu Q, Zhou Q, Li J, Yu M, Li K,
Xu L, Ke X, Xu H, Lu B, et al: Long-read sequencing unveils
high-resolution HPV integration and its oncogenic progression in
cervical cancer. Nat Commun. 13(2563)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carneiro BA, Elvin JA, Kamath SD, Ali SM,
Paintal AS, Restrepo A, Berry E, Giles FJ and Johnson ML:
FGFR3-TACC3: A novel gene fusion in cervical cancer. Gynecol Oncol
Rep. 13:53–56. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Amatu A, Sartore-Bianchi A, Bencardino K,
Pizzutilo EG, Tosi F and Siena S: Tropomyosin receptor kinase (TRK)
biology and the role of NTRK gene fusions in cancer. Ann Oncol. 30
(Suppl 8):viii5–viii15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Westphalen CB, Krebs MG, Le Tourneau C,
Sokol ES, Maund SL, Wilson TR, Jin DX, Newberg JY, Fabrizio D,
Veronese L, et al: Genomic context of NTRK1/2/3 fusion-positive
tumours from a large real-world population. NPJ Precis Oncol.
5(69)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Somwar R, Hofmann NE, Smith B, Odintsov I,
Vojnic M, Linkov I, Tam A, Khodos I, Mattar MS, de Stanchina E, et
al: NTRK kinase domain mutations in cancer variably impact
sensitivity to type I and type II inhibitors. Commun Biol.
3(776)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gambella A, Senetta R, Collemi G, Vallero
SG, Monticelli M, Cofano F, Zeppa P, Garbossa D, Pellerino A, Rudà
R, et al: NTRK fusions in central nervous system tumors: A rare,
but worthy target. Int J Mol Sci. 21(753)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Amatu A, Sartore-Bianchi A and Siena S:
NTRK gene fusions as novel targets of cancer therapy across
multiple tumour types. ESMO Open. 1(e000023)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ardini E, Bosotti R, Borgia AL, De Ponti
C, Somaschini A, Cammarota R, Amboldi N, Raddrizzani L, Milani A,
Magnaghi P, et al: The TPM3-NTRK1 rearrangement is a recurring
event in colorectal carcinoma and is associated with tumor
sensitivity to TRKA kinase inhibition. Mol Oncol. 8:1495–1507.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vaishnavi A, Le AT and Doebele RC: TRKing
down an old oncogene in a new era of targeted therapy. Cancer
Discov. 5:25–34. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Deinhardt K and Chao MV: Trk receptors.
Handb Exp Pharmacol. 220:103–119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cocco E, Scaltriti M and Drilon A: NTRK
fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin
Oncol. 15:731–747. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hechtman JF: NTRK insights: Best practices
for pathologists. Mod Pathol. 35:298–305. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ranzi V, Meakin SO, Miranda C, Mondellini
P, Pierotti MA and Greco A: The signaling adapters fibroblast
growth factor receptor substrate 2 and 3 are activated by the
thyroid TRK oncoproteins. Endocrinology. 144:922–928.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Drilon A, Nagasubramanian R, Blake JF, Ku
N, Tuch BB, Ebata K, Smith S, Lauriault V, Kolakowski GR,
Brandhuber BJ, et al: A next-generation TRK kinase inhibitor
overcomes acquired resistance to prior TRK kinase inhibition in
patients with TRK fusion-positive solid tumors. Cancer Discov.
7:963–972. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rasi Bonab F, Baghbanzadeh A, Ghaseminia
M, Bolandi N, Mokhtarzadeh A, Amini M, Dadashzadeh K,
Hajiasgharzadeh K, Baradaran B and Bannazadeh Baghi H: Molecular
pathways in the development of HPV-induced cervical cancer. EXCLI
J. 20:320–337. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Skinner SR, Wheeler CM, Romanowski B,
Castellsagué X, Lazcano-Ponce E, Del Rosario-Raymundo MR, Vallejos
C, Minkina G, Pereira Da Silva D, McNeil S, et al: Progression of
HPV infection to detectable cervical lesions or clearance in adult
women: Analysis of the control arm of the VIVIANE study. Int J
Cancer. 138:2428–2438. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ronco G, Dillner J, Elfström KM, Tunesi S,
Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi
P, et al: Efficacy of HPV-based screening for prevention of
invasive cervical cancer: Follow-up of four European randomised
controlled trials. Lancet. 383:524–532. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Haręża DA, Wilczyński JR and Paradowska E:
Human papillomaviruses as infectious agents in gynecological
cancers. Oncogenic properties of viral proteins. Int J Mol Sci.
23(1818)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chan CK, Aimagambetova G, Ukybassova T,
Kongrtay K and Azizan A: Human papillomavirus infection and
cervical cancer: epidemiology, screening, and vaccination-review of
current perspectives. J Oncol. 2019(3257939)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang L, Wu J, Ling MT, Zhao L and Zhao
KN: The role of the PI3K/Akt/mTOR signalling pathway in human
cancers induced by infection with human papillomaviruses. Mol
Cancer. 14(87)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Komatsu N, Aoki K, Yamada M, Yukinaga H,
Fujita Y, Kamioka Y and Matsuda M: Development of an optimized
backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell.
22:4647–4656. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bossler F, Hoppe-Seyler K and Hoppe-Seyler
F: PI3K/AKT/mTOR signaling regulates the virus/host cell crosstalk
in HPV-positive cervical cancer cells. Int J Mol Sci.
20(2188)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Boyle W, Williams A, Sundar S, Yap J,
Taniere P, Rehal P and Ganesan R: TMP3-NTRK1 rearranged uterine
sarcoma: A case report. Case Rep Womens Health.
28(e00246)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chiang S, Cotzia P, Hyman DM, Drilon A,
Tap WD, Zhang L, Hechtman JF, Frosina D, Jungbluth AA, Murali R, et
al: NTRK fusions define a novel uterine sarcoma subtype with
features of fibrosarcoma. Am J Surg Pathol. 42:791–798.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Costigan DC, Nucci MR, Dickson BC, Chang
MC, Song S, Sholl LM, Hornick JL, Fletcher CDM and Kolin DL:
NTRK-rearranged uterine sarcomas: Clinicopathologic features of 15
cases, literature review, and risk stratification. Am J Surg
Pathol. 46:1415–1429. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bühler MM, Honcharova-Biletska H, Pauli C,
Chronas D and Bolten K: Conservative surgical treatment with
fertility preservation in a young adult with NTRK rearranged
spindle cell neoplasm of the uterine cervix. Gynecol Oncol Rep.
48(101233)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wells AE, Mallen AM, Bui MM, Reed DR and
Apte SM: NTRK-1 fusion in endocervical fibroblastic malignant
peripheral nerve sheath tumor marking eligibility for larotrectinib
therapy: A case report. Gynecol Oncol Rep. 28:141–144.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Croce S, Hostein I, Longacre TA, Mills AM,
Pérot G, Devouassoux-Shisheboran M, Velasco V, Floquet A, Guyon F,
Chakiba C, et al: Uterine and vaginal sarcomas resembling
fibrosarcoma: A clinicopathological and molecular analysis of 13
cases showing common NTRK-rearrangements and the description of a
COL1A1-PDGFB fusion novel to uterine neoplasms. Mod Pathol.
32:1008–1022. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dang X, Xiang T, Zhao C, Tang H and Cui P:
EML4-NTRK3 fusion cervical sarcoma: A case report and literature
review. Front Med (Lausanne). 9(832376)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Devereaux KA, Weiel JJ, Mills AM, Kunder
CA and Longacre TA: Neurofibrosarcoma revisited: An institutional
case series of uterine sarcomas harboring kinase-related fusions
with report of a novel FGFR1-TACC1 fusion. Am J Surg Pathol.
45:638–652. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fang X, Huang R and Zhang Z:
NTRK-rearranged uterine sarcoma: A case report. Asian J Surg.
46:4764–4765. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gatalica Z, Xiu J, Swensen J and Vranic S:
Molecular characterization of cancers with NTRK gene fusions. Mod
Pathol. 32:147–153. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Goulding EA, Morreau P, De Silva M, Watson
M, van Vliet C, Leung B and Eva LJ: Case report: NTRK1-rearranged
cervical sarcoma with fibrosarcoma like morphology presenting in a
13-year-old managed with a neo-adjuvant TRK-inhibitor and surgical
excision. Gynecol Oncol Rep. 37(100845)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xiaoqing Z, Shan Z, Zhansan S, Xianyang Z,
Xiaoyan C and Qiong Z: The clinicopathological analysis of cervical
NTRK-rearranged spindle cell tumor. Mod Oncol. 31:2069–2072.
2023.
|
|
38
|
Wong DD, Vargas AC, Bonar F, Maclean F,
Kattampallil J, Stewart C, Sulaiman B, Santos L and Gill AJ:
NTRK-rearranged mesenchymal tumours: Diagnostic challenges,
morphological patterns and proposed testing algorithm. Pathology.
52:401–409. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tsai JW, Lee JC, Hsieh TH, Huang SC, Lee
PH, Liu TT, Kao YC, Chang CD, Weng TF, Li CF, et al: Adult
NTRK-rearranged spindle cell neoplasms of the viscera: With an
emphasis on rare locations and heterologous elements. Mod Pathol.
35:911–921. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Takahashi A, Kurosawa M, Uemura M,
Kitazawa J and Hayashi Y: Anaplastic lymphoma kinase-negative
uterine inflammatory myofibroblastic tumor containing the
ETV6-NTRK3 fusion gene: A case report. J Int Med Res. 46:3498–3503.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rabban JT, Devine WP, Sangoi AR, Poder L,
Alvarez E, Davis JL, Rudzinski E, Garg K and Bean GR: NTRK fusion
cervical sarcoma: a report of three cases, emphasising
morphological and immunohistochemical distinction from other
uterine sarcomas, including adenosarcoma. Histopathology.
77:100–111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nilforoushan N, Wethington SL, Nonogaki H,
Gross J, Vang R and Xing D: NTRK-fusion sarcoma of the uterine
cervix: Report of 2 cases with comparative clinicopathologic
features. Int J Gynecol Pathol. 41:642–648. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Munkhdelger J, Shimooka T, Koyama Y, Ikeda
S, Mikami Y, Fukuoka J, Hori T and Bychkov A: Basaloid squamous
cell carcinoma of the uterine cervix: Report of a case with
molecular analysis. Int J Surg Pathol. 29:770–774. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moh M, Johnson CM, Geurts JL and Bishop
EE: Uterine sarcoma with a novel WWOX-NTRK2 fusion in a
postmenopausal woman with li-fraumeni-like syndrome: A case that
expands the spectrum of NTRK-rearranged uterine tumors. AJSP Rev
Rep. 26:304–306. 2021.
|
|
45
|
Hanhan L: Clinicopathological analysis of
one case of cervical sarcoma with NTRK gene rearrangement. J Clin
Exp Pathol. 37:1240–1243. 2021.
|
|
46
|
Xiaoqi L: Case reports of NTRK-rearranged
cervical sarcomas and literature review. Anti-Tumor Pharm.
13:515–520. 2023.
|
|
47
|
Hodgson A, Pun C, Djordjevic B and
Turashvili G: NTRK-rearranged cervical sarcoma: Expanding the
clinicopathologic spectrum. Int J Gynecol Pathol. 40:73–77.
2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hartmaier RJ, Albacker LA, Chmielecki J,
Bailey M, He J, Goldberg ME, Ramkissoon S, Suh J, Elvin JA,
Chiacchia S, et al: High-throughput genomic profiling of adult
solid tumors reveals novel insights into cancer pathogenesis.
Cancer Res. 77:2464–2475. 2017.PubMed/NCBI View Article : Google Scholar
|