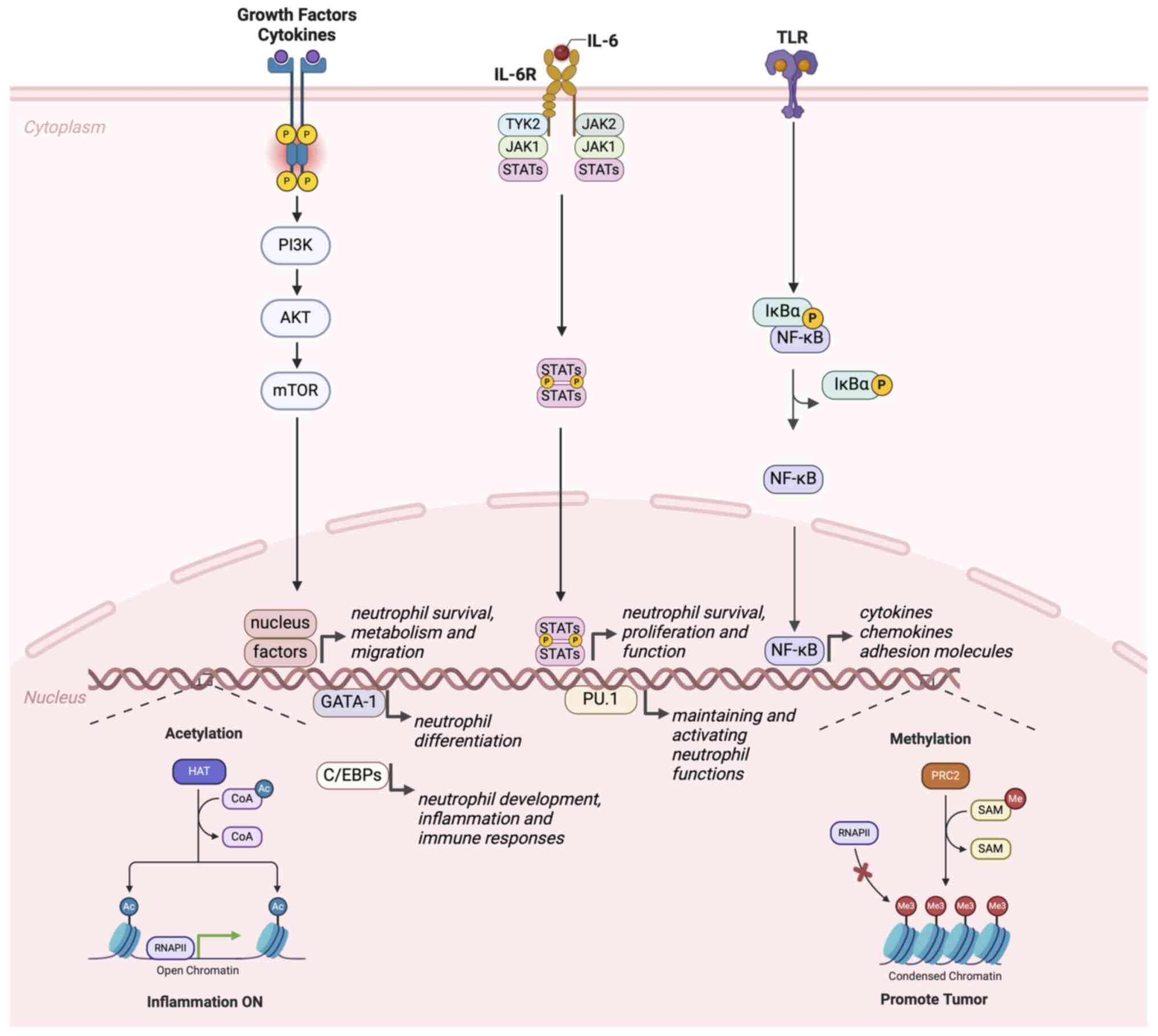
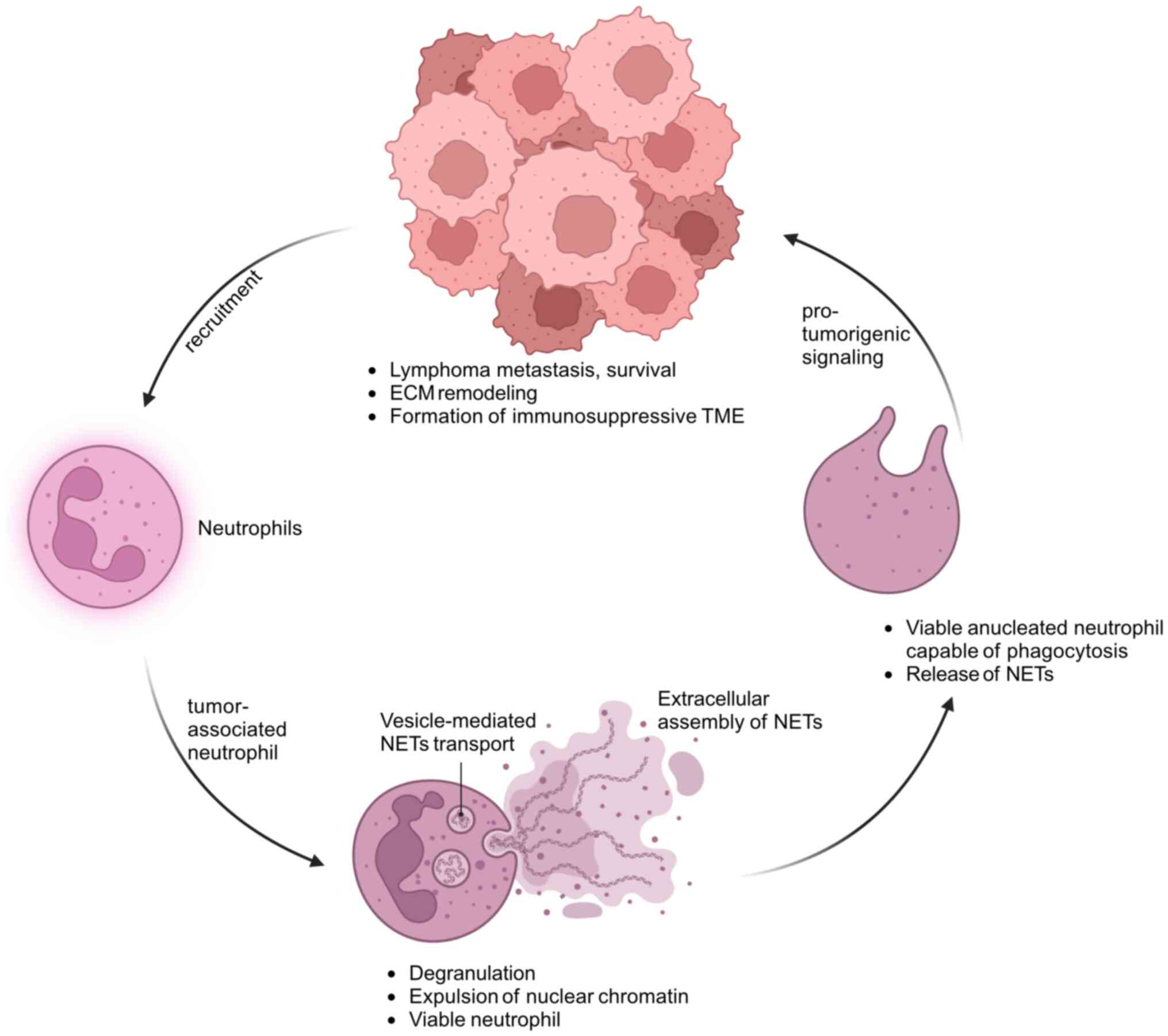
|
1
|
Rapoport BL, Steel HC, Theron AJ, Smit T
and Anderson R: Role of the neutrophil in the pathogenesis of
advanced cancer and impaired responsiveness to therapy. Molecules.
25(1618)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sounbuli K, Mironova N and Alekseeva L:
diverse neutrophil functions in cancer and promising
neutrophil-based cancer therapies. Int J Mol Sci.
23(15827)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Borregaard N: Neutrophils, from marrow to
microbes. Immunity. 33:657–670. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nauseef WM and Borregaard N: Neutrophils
at work. Nat Immunol. 15:602–611. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hidalgo A, Chilvers ER, Summers C and
Koenderman L: The neutrophil life cycle. Trends Immunol.
40:584–597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kolaczkowska E and Kubes P: Neutrophil
recruitment and function in health and inflammation. Nat Rev
Immunol. 13:159–175. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ley K, Hoffman HM, Kubes P, Cassatella MA,
Zychlinsky A, Hedrick CC and Catz SD: Neutrophils: New insights and
open questions. Sci Immunol. 3(eaat4579)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sionov RV, Fridlender ZG and Granot Z: The
multifaceted roles neutrophils play in the tumor microenvironment.
Cancer Microenviron. 8:125–158. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Powell DR and Huttenlocher A: Neutrophils
in the tumor microenvironment. Trends Immunol. 37:41–52.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mantovani A, Cassatella MA, Costantini C
and Jaillon S: Neutrophils in the activation and regulation of
innate and adaptive immunity. Nat Rev Immunol. 11:519–531.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dhodapkar MV, Borrello I, Cohen AD and
Stadtmauer EA: Hematologic malignancies: Plasma cell disorders. Am
Soc Clin Oncol Educ Book. 37:561–568. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Parente P, Zanelli M, Sanguedolce F,
Mastracci L and Graziano P: Hodgkin Reed-Sternberg-like cells in
non-hodgkin lymphoma. Diagnostics (Basel). 10(1019)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Armitage JO, Gascoyne RD, Lunning MA and
Cavalli F: Non-Hodgkin lymphoma. Lancet. 390:298–310.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matasar MJ and Zelenetz AD: Overview of
lymphoma diagnosis and management. Radiol Clin North Am.
46:175–198, vii. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xing AY, Dong XZ, Zhu LQ, Liu L, Sun D and
Guo S: Clinicopathological characteristics and molecular phenotypes
of primary hepatic lymphoma. Front Oncol. 12(906245)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang HW, Balakrishna JP, Pittaluga S and
Jaffe ES: Diagnosis of Hodgkin lymphoma in the modern era. Br J
Haematol. 184:45–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liew PX and Kubes P: The Neutrophil's role
during health and disease. Physiol Rev. 99:1223–1248.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sureda A and Martinez C: Classical
Hodgkin's lymphoma. In: The EBMT Handbook: Hematopoietic Stem Cell
Transplantation and Cellular Therapies. Carreras E, Dufour C, Mohty
M and Kroger N (eds): 7th edition. Springer, Cham, CH, pp653-662,
2019.
|
|
23
|
Euler M and Hoffmann MH: The double-edged
role of neutrophil extracellular traps in inflammation. Biochem Soc
Trans. 47:1921–1930. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pillay J, Kamp VM, van Hoffen E, Visser T,
Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P and Koenderman
L: A subset of neutrophils in human systemic inflammation inhibits
T cell responses through Mac-1. J Clin Invest. 122:327–336.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Upadhyay R, Hammerich L, Peng P, Brown B,
Merad M and Brody JD: Lymphoma: Immune evasion strategies. Cancers
(Basel). 7:736–762. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hirz T, Matera EL, Chettab K, Jordheim LP,
Mathé D, Evesque A, Esmenjaud J, Salles G and Dumontet C:
Neutrophils protect lymphoma cells against cytotoxic and targeted
therapies through CD11b/ICAM-1 binding. Oncotarget. 8:72818–72834.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu S, Wu W, Du Y, Yin H, Chen Q, Yu W,
Wang W, Yu J, Liu L, Lou W and Pu N: The evolution and
heterogeneity of neutrophils in cancers: Origins, subsets,
functions, orchestrations and clinical applications. Mol Cancer.
22(148)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang X, Qiu L, Li Z, Wang XY and Yi H:
Understanding the multifaceted role of neutrophils in cancer and
autoimmune diseases. Front Immunol. 9(2456)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Heshmat-Ghahdarijani K, Sarmadi V, Heidari
A, Falahati Marvasti A, Neshat S and Raeisi S: The
neutrophil-to-lymphocyte ratio as a new prognostic factor in
cancers: A narrative review. Front Oncol.
13(1228076)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ohashi K, Nishito Y, Fukuda H, Sadahiro R,
Yoshida Y, Watanabe SI, Motoi N, Sonobe Y, Mizuno H, Tsunoda H, et
al: Neutrophil-to-lymphocyte ratio is a prognostic factor
reflecting immune condition of tumor microenvironment in squamous
cell lung cancer. Sci Rep. 14(429)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim SI, Cassella CR and Byrne KT: Tumor
burden and immunotherapy: Impact on immune infiltration and
therapeutic outcomes. Front Immunol. 11(629722)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pradeep U, Chiwhane A, Acharya S, Kumar S,
Daiya V, Kasat PR, Gupta A and Bedi GN: The role of
neutrophil-to-lymphocyte ratio in predicting outcomes of acute
organophosphorus poisoning: A comprehensive review. Cureus.
16(e60854)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang G, Yang C, Zhao C, Xian F, Qing D,
Guo Q, Song J, Liu X and Bie J: Prognostic value of the
neutrophil-to-lymphocyte ratio in patients treated with definitive
chemoradiotherapy for locally advanced oesophageal squamous cell
carcinoma. Cancer Manag Res. 15:101–112. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Masucci MT, Minopoli M and Carriero MV:
Tumor associated neutrophils. Their role in tumorigenesis,
metastasis, prognosis and therapy. Front Oncol.
9(1146)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Quintero-Fabian S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9(1370)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Christoffersson G, Vagesjo E, Vandooren J,
Lidén M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G
and Phillipson M: VEGF-A recruits a proangiogenic MMP-9-delivering
neutrophil subset that induces angiogenesis in transplanted hypoxic
tissue. Blood. 120:4653–4662. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu X, Li C, Wang Z, Xu Y, Shao S, Shao F,
Wang H and Liu J: Neutrophils in cancer: Dual roles through
intercellular interactions. Oncogene. 43:1163–1177. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kwantwi LB: Interplay between
tumor-derived factors and tumor-associated neutrophils:
Opportunities for therapeutic interventions in cancer. Clin Transl
Oncol. 25:1963–1976. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xiong X, Liao X, Qiu S, Xu H, Zhang S,
Wang S, Ai J and Yang L: CXCL8 in tumor biology and its
implications for clinical translation. Front Mol Biosci.
9(723846)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Teijeira A, Garasa S, Ochoa MC, Villalba
M, Olivera I, Cirella A, Eguren-Santamaria I, Berraondo P, Schalper
KA, de Andrea CE, et al: IL8, Neutrophils, and NETs in a collusion
against cancer immunity and immunotherapy. Clin Cancer Res.
27:2383–2393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
De Meo ML and Spicer JD: The role of
neutrophil extracellular traps in cancer progression and
metastasis. Semin Immunol. 57(101595)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang X, Nepovimova E, Adam V, Sivak L,
Heger Z, Valko M, Wu Q and Kuca K: Neutrophils in cancer
immunotherapy: Friends or foes? Mol Cancer. 23(107)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Y, Guoqiang L, Sun M and Lu X:
Targeting and exploitation of tumor-associated neutrophils to
enhance immunotherapy and drug delivery for cancer treatment.
Cancer Biol Med. 17:32–43. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Armstrong H, Bording-Jorgensen M, Dijk S
and Wine E: The Complex Interplay between chronic inflammation, the
microbiome, and cancer: Understanding disease progression and what
we can do to prevent it. Cancers (Basel). 10(83)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu TH, Hsieh SC, Li TH, Lu CH, Liao HT,
Shen CY, Li KJ, Wu CH, Kuo YM, Tsai CY and Yu CL: Molecular basis
for paradoxical activities of polymorphonuclear neutrophils in
inflammation/anti-inflammation, bactericide/autoimmunity,
pro-cancer/anticancer, and antiviral infection/SARS-CoV-II-induced
immunothrombotic dysregulation. Biomedicines.
10(773)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Coletto LA, Rizzo C, Guggino G, Caporali
R, Alivernini S and D'Agostino MA: The role of neutrophils in
spondyloarthritis: A journey across the spectrum of disease
manifestations. Int J Mol Sci. 24(4108)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Herrero-Cervera A, Soehnlein O and Kenne
E: Neutrophils in chronic inflammatory diseases. Cell Mol Immunol.
19:177–191. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y
and Li Y: Inflammation and tumor progression: Signaling pathways
and targeted intervention. Signal Transduct Target Ther.
6(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xiong S, Dong L and Cheng L: Neutrophils
in cancer carcinogenesis and metastasis. J Hematol Oncol.
14(173)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rosales C: Neutrophil: A cell with many
roles in inflammation or several cell types? Front Physiol.
9(113)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tu Z, Zhong Y, Hu H, Shao D, Haag R,
Schirner M, Lee J, Sullenger B and Leong KW: Design of therapeutic
biomaterials to control inflammation. Nat Rev Mater. 7:557–574.
2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mata R, Yao Y, Cao W, Ding J, Zhou T, Zhai
Z and Gao C: The dynamic inflammatory tissue microenvironment:
Signality and disease therapy by biomaterials. Research (Wash D C).
2021(4189516)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hannoodee S and Nasuruddin DN: Acute
Inflammatory Response. StatPearls, Treasure Island, FL, 2023.
|
|
56
|
Ward PA and Lentsch AB: The acute
inflammatory response and its regulation. Arch Surg. 134:666–669.
1999.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Filep JG and Ariel A: Neutrophil
heterogeneity and fate in inflamed tissues: Implications for the
resolution of inflammation. Am J Physiol Cell Physiol.
319:C510–C532. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hirayama D, Iida T and Nakase H: The
phagocytic function of macrophage-enforcing innate immunity and
tissue homeostasis. Int J Mol Sci. 19(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lawrence T and Gilroy DW: Chronic
inflammation: A failure of resolution? Int J Exp Pathol. 88:85–94.
2007.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Megha KB, Joseph X, Akhil V and Mohanan
PV: Cascade of immune mechanism and consequences of inflammatory
disorders. Phytomedicine. 91(153712)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang JM and An J: Cytokines,
inflammation, and pain. Int Anesthesiol Clin. 45:27–37.
2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Altan-Bonnet G and Mukherjee R:
Cytokine-mediated communication: A quantitative appraisal of immune
complexity. Nat Rev Immunol. 19:205–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fajgenbaum DC and June CH: Cytokine Storm.
N Engl J Med. 383:2255–2273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Prame Kumar K, Nicholls AJ and Wong CHY:
Partners in crime: Neutrophils and monocytes/macrophages in
inflammation and disease. Cell Tissue Res. 371:551–565.
2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Shao S, Miao H and Ma W: Unraveling the
enigma of tumor-associated macrophages: Challenges, innovations,
and the path to therapeutic breakthroughs. Front Immunol.
14(1295684)2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Khilwani R and Singh S: Systems biology
and cytokines potential role in lung cancer immunotherapy targeting
autophagic axis. Biomedicines. 11(2706)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yang L, Xie X, Tu Z, Fu J, Xu D and Zhou
Y: The signal pathways and treatment of cytokine storm in COVID-19.
Signal Transduct Target Ther. 6(255)2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Anderson NM and Simon MC: The tumor
microenvironment. Curr Biol. 30:R921–R925. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yan M, Zheng M, Niu R, Yang X, Tian S, Fan
L, Li Y and Zhang S: Roles of tumor-associated neutrophils in tumor
metastasis and its clinical applications. Front Cell Dev Biol.
10(938289)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther.
221(107753)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Xiong T, He P, Zhou M, Zhong D, Yang T, He
W, Xu Z, Chen Z, Liu YW and Dai SS: Glutamate blunts cell-killing
effects of neutrophils in tumor microenvironment. Cancer Sci.
113:1955–1967. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Giese MA, Hind LE and Huttenlocher A:
Neutrophil plasticity in the tumor microenvironment. Blood.
133:2159–2167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
McFarlane AJ, Fercoq F, Coffelt SB and
Carlin LM: Neutrophil dynamics in the tumor microenvironment. J
Clin Invest. 131(e143759)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Galdiero MR, Garlanda C, Jaillon S, Marone
G and Mantovani A: Tumor associated macrophages and neutrophils in
tumor progression. J Cell Physiol. 228:1404–1412. 2013.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Di Carlo E, Forni G, Lollini P, Colombo
MP, Modesti A and Musiani P: The intriguing role of
polymorphonuclear neutrophils in antitumor reactions. Blood.
97:339–345. 2001.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li MY, Chong LC, Duns G, Lytle A, Woolcock
B, Jiang A, Telenius A, Ben-Neriah S, Nawaz W, Slack GW, et al:
TRAF3 loss-of-function reveals the noncanonical NF-κB pathway as a
therapeutic target in diffuse large B cell lymphoma. Proc Natl Acad
Sci USA. 121(e2320421121)2024.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Mondragon L, Mhaidly R, De Donatis GM,
Tosolini M, Dao P, Martin AR, Pons C, Chiche J, Jacquin M, Imbert
V, et al: GAPDH Overexpression in the T cell lineage promotes
angioimmunoblastic T cell lymphoma through an NF-κB-Dependent
Mechanism. Cancer Cell. 36:268–287 e10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
von Hoff L, Kargel E, Franke V, McShane E,
Schulz-Beiss KW, Patone G, Schleussner N, Kolesnichenko M, Hübner
N, Daumke O, et al: Autocrine LTA signaling drives NF-kappaB and
JAK-STAT activity and myeloid gene expression in Hodgkin lymphoma.
Blood. 133:1489–1494. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Gluud M, Pallesen EMH, Buus TB, Gjerdrum
LMR, Lindahl LM, Kamstrup MR, Bzorek M, Danielsen M, Bech R,
Monteiro MN, et al: Malignant T cells induce skin barrier defects
through cytokine-mediated JAK/STAT signaling in cutaneous T-cell
lymphoma. Blood. 141:180–193. 2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ramis-Zaldivar JE, Gonzalez-Farre B,
Nicolae A, Pack S, Clot G, Nadeu F, Mottok A, Horn H, Song JY, Fu
K, et al: MAPK and JAK-STAT pathways dysregulation in plasmablastic
lymphoma. Haematologica. 106:2682–2693. 2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Gehringer F, Weissinger SE, Moller P,
Wirth T and Ushmorov A: Physiological levels of the PTEN-PI3K-AKT
axis activity are required for maintenance of Burkitt lymphoma.
Leukemia. 34:857–871. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Takashima Y, Hayano A and Yamanaka R:
Metabolome analysis reveals excessive glycolysis via PI3K/AKT/mTOR
and RAS/MAPK signaling in methotrexate-resistant primary CNS
Lymphoma-Derived Cells. Clin Cancer Res. 26:2754–2766.
2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wang G, Liu H, An L, Hou S and Zhang Q:
CAPG facilitates diffuse large B-cell lymphoma cell progression
through PI3K/AKT signaling pathway. Hum Immunol. 83:832–842.
2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Sato A, Kamio N, Yokota A, Hayashi Y,
Tamura A, Miura Y, Maekawa T and Hirai H: C/EBPβ isoforms
sequentially regulate regenerating mouse hematopoietic
stem/progenitor cells. Blood Adv. 4:3343–3356. 2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Wang W, Xia X, Mao L and Wang S: The
CCAAT/Enhancer-Binding protein family: Its Roles in MDSC expansion
and function. Front Immunol. 10(1804)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Avellino R and Delwel R: Expression and
regulation of C/EBPα in normal myelopoiesis and in malignant
transformation. Blood. 129:2083–2091. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Hosokawa H, Koizumi M, Masuhara K,
Romero-Wolf M, Tanaka T, Nakayama T and Rothenberg EV:
Stage-specific action of Runx1 and GATA3 controls silencing of PU.1
expression in mouse pro-T cells. J Exp Med.
218(e20202648)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Inage E, Kasakura K, Yashiro T, Suzuki R,
Baba Y, Nakano N, Hara M, Tanabe A, Oboki K, Matsumoto K, et al:
Critical Roles for PU.1, GATA1, and GATA2 in the expression of
human FcƐRI on mast cells: PU.1 and GATA1 transactivate FCER1A, and
GATA2 transactivates FCER1A and MS4A2. J Immunol. 192:3936–3946.
2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zakrzewska A, Cui C, Stockhammer OW,
Benard EL, Spaink HP and Meijer AH: Macrophage-specific gene
functions in Spi1-directed innate immunity. Blood. 116:e1–e11.
2010.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wu S, Wang H, Yang Q, Liu Z, Du J, Wang L,
Chen S, Lu Q and Yang DH: METTL3 regulates M6A methylation-modified
EBV-pri-miR-BART3-3p to promote NK/T cell lymphoma growth. Cancer
Lett. 597(217058)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhao A, Zhou H, Yang J, Li M and Niu T:
Epigenetic regulation in hematopoiesis and its implications in the
targeted therapy of hematologic malignancies. Signal Transduct
Target Ther. 8(71)2023.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Zhuang S, Yang Z, Cui Z, Zhang Y and Che
F: Epigenetic alterations and advancement of lymphoma treatment.
Ann Hematol. 103:1435–1454. 2024.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Tecchio C and Cassatella MA:
Neutrophil-derived cytokines involved in physiological and
pathological angiogenesis. Chem Immunol Allergy. 99:123–137.
2014.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Shaul ME and Fridlender ZG: Neutrophils as
active regulators of the immune system in the tumor
microenvironment. J Leukoc Biol. 102:343–349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Jablonska J, Leschner S, Westphal K,
Lienenklaus S and Weiss S: Neutrophils responsive to endogenous
IFN-beta regulate tumor angiogenesis and growth in a mouse tumor
model. J Clin Invest. 120:1151–1164. 2010.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Matta B, Battaglia J and Barnes BJ:
Detection of neutrophil extracellular traps in patient plasma:
Method development and validation in systemic lupus erythematosus
and healthy donors that carry IRF5 genetic risk. Front Immunol.
13(951254)2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Demers M, Krause DS, Schatzberg D,
Martinod K, Voorhees JR, Fuchs TA, Scadden DT and Wagner DD:
Cancers predispose neutrophils to release extracellular DNA traps
that contribute to cancer-associated thrombosis. Proc Natl Acad Sci
USA. 109:13076–13081. 2012.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Saffarzadeh M, Juenemann C, Queisser MA,
Lochnit G, Barreto G, Galuska SP, Lohmeyer J and Preissner KT:
Neutrophil extracellular traps directly induce epithelial and
endothelial cell death: A predominant role of histones. PLoS One.
7(e32366)2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Fuchs TA, Abed U, Goosmann C, Hurwitz R,
Schulze I, Wahn V, Weinrauch Y, Brinkmann V and Zychlinsky A: Novel
cell death program leads to neutrophil extracellular traps. J Cell
Biol. 176:231–241. 2007.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 123:3446–3458.
2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Berger-Achituv S, Brinkmann V, Abed UA,
Kühn LI, Ben-Ezra J, Elhasid R and Zychlinsky A: A proposed role
for neutrophil extracellular traps in cancer immunoediting. Front
Immunol. 4(48)2013.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Jehannin-Ligier K, Belot A, Guizard AV,
Bossard N, Launoy G and Uhry Z: FRANCIM network. Incidence trends
for potentially human papillomavirus-related and -unrelated head
and neck cancers in France using population-based cancer registries
data: 1980-2012. Int J Cancer. 140:2032–2039. 2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi
AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H and Tsung A:
Neutrophil extracellular traps promote the development and
progression of liver metastases after surgical stress. Cancer Res.
76:1367–1380. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Shimada K, Crother TR, Karlin J, Dagvadorj
J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et
al: Oxidized mitochondrial DNA activates the NLRP3 inflammasome
during apoptosis. Immunity. 36:401–414. 2012.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Brinkmann V and Zychlinsky A: Neutrophil
extracellular traps: Is immunity the second function of chromatin?
J Cell Biol. 198:773–783. 2012.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Papayannopoulos V, Metzler KD, Hakkim A
and Zychlinsky A: Neutrophil elastase and myeloperoxidase regulate
the formation of neutrophil extracellular traps. J Cell Biol.
191:677–691. 2010.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Kaplan MJ and Radic M: Neutrophil
extracellular traps: Double-edged swords of innate immunity. J
Immunol. 189:2689–2695. 2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Porto BN and Stein RT: Neutrophil
extracellular traps in pulmonary diseases: Too much of a good
thing? Front Immunol. 7(311)2016.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Schonrich G, Raftery MJ and Samstag Y:
Devilishly radical NETwork in COVID-19: Oxidative stress,
neutrophil extracellular traps (NETs), and T cell suppression. Adv
Biol Regul. 77(100741)2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Cedervall J, Zhang Y, Huang H, Zhang L,
Femel J, Dimberg A and Olsson AK: Neutrophil extracellular traps
accumulate in peripheral blood vessels and compromise organ
function in tumor-bearing animals. Cancer Res. 75:2653–2662.
2015.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Albrengues J, Shields MA, Ng D, Park CG,
Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A,
Küttner V, et al: Neutrophil extracellular traps produced during
inflammation awaken dormant cancer cells in mice. Science.
361(eaao4227)2018.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Demers M, Wong SL, Martinod K, Gallant M,
Cabral JE, Wang Y and Wagner DD: Priming of neutrophils toward
NETosis promotes tumor growth. Oncoimmunology.
5(e1134073)2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Najmeh S, Cools-Lartigue J, Rayes RF,
Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau
S, Ferri LE and Spicer JD: Neutrophil extracellular traps sequester
circulating tumor cells via β1-integrin mediated interactions. Int
J Cancer. 140:2321–2330. 2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Park J, Wysocki RW, Amoozgar Z, Maiorino
L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH,
et al: Cancer cells induce metastasis-supporting neutrophil
extracellular DNA traps. Sci Transl Med. 8(361ra138)2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Belaaouaj A, McCarthy R, Baumann M, Gao Z,
Ley TJ, Abraham SN and Shapiro SD: Mice lacking neutrophil elastase
reveal impaired host defense against gram negative bacterial
sepsis. Nat Med. 4:615–618. 1998.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743.
2006.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Allen TM: Ligand-targeted therapeutics in
anticancer therapy. Nat Rev Cancer. 2:750–763. 2002.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Mayadas TN, Cullere X and Lowell CA: The
multifaceted functions of neutrophils. Annu Rev Pathol. 9:181–218.
2014.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Nemeth T, Mocsai A and Lowell CA:
Neutrophils in animal models of autoimmune disease. Semin Immunol.
28:174–186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Tecchio C, Micheletti A and Cassatella MA:
Neutrophil-derived cytokines: Facts beyond expression. Front
Immunol. 5(508)2014.PubMed/NCBI View Article : Google Scholar
|