Introduction
Neuroendocrine carcinoma (NEC) of the colon and
rectum is rare. The reported incidence of NEC in these regions
ranges from <0.6% to as high as 5% (1,2). NEC
is characterized as an epithelial cancer that is distinguished by
the expression of neuroendocrine markers (NEMs), such as
chromogranin A (CgA), synaptophysin (Syn), and
insulinoma-associated 1 (INSM1) (3). The 2019 World Health Organization
(WHO) update on colorectal cancer (CRC) classification emphasized
that NEC of the colon and rectum is distinctly classified as a
high-grade, poorly differentiated NEC, which is distinct from
low-grade grade 3 neuroendocrine tumors (NETs) (4). NEC of the colon and rectum has been
reported to have a poor prognosis (1). On the other hand, the most common
histological type of CRC is adenocarcinoma, which accounts for ~90%
of cases (5). However, the
majority of these are low-grade cases of well-differentiated
adenocarcinoma (WDC) and moderately differentiated adenocarcinoma
(MDC). Poorly differentiated adenocarcinoma (PDC), corresponding to
the high grade of NEC, is also a rare histological type of CRC,
with an incidence rate of 3.3-18% in Japan (6,7).
Considering the morphological similarities between
these two rare, poorly differentiated cancers of the colon and
rectum (i.e., NEC and PDC), it is plausible that certain colorectal
adenocarcinomas with poor prognoses that contain PDC components may
have morphological or biomarker-related similarities to NEC. The
retinoblastoma 1 (Rb) protein is a tumor suppressor that is
frequently dysfunctional across numerous cancer types. Loss of Rb,
which is detected in approximately half of pancreatic NECs, is
considered a hallmark of NEC. To elucidate the clinicopathological
features and clinical outcomes of colorectal NECs, as well as
enhance the current understanding of this disease, cases of CRC
diagnosed as PDC at our institution were investigated and those
that exhibited NEC-like characteristics, such as the expression of
certain NEMs and the loss of Rb, were analyzed.
Materials and methods
Patients and clinical data
collection
Between January 2009 and December 2019, a total of
816 patients underwent CRC resection surgery at the Department of
Gastroenterological Surgery of Yokohama City University Hospital
(Yokohama, Japan). Cases of CRC that exhibited PDC, either wholly
or in part, were selected based on pathological diagnoses that were
confirmed by two independent pathologists. This study
retrospectively analyzed clinical data from a total of 74 diagnosed
PDC cases. The reviewed data included variables, such as age at
diagnosis, sex, histology, lymph node metastasis, clinical stage
and curability. Tumor locations were classified into right-sided
colon (cecum, ascending colon, transverse colon and appendix) and
left-sided colon (descending colon, sigmoid colon, rectum and
anus). All patients underwent clinical evaluation at the hospital
and received appropriate management. Follow-up information was
secured for all 74 cases. The present study was approved by the
Ethical Review Board of Yokohama City University (Yokohama, Japan;
approval no.: B200700086).
Immunohistochemistry (IHC)
staining
Tumor tissues from the 74 PDC cases were
formalin-fixed and paraffin-embedded. The resultant paraffin blocks
were sectioned to a thickness of 4 µm for IHC staining. The
sections were stained with antibodies against CgA (1:400 dilution;
cat. no. ab15160; Abcam), Syn (1:200 dilution; cat. no. ab14692;
Abcam), INSM1 (1:400 dilution; clone A-8; cat. no. sc-271408; Santa
Cruz Biotechnology, Inc.), Rb (1:800 dilution; cat. no. ab181616;
Abcam) and Ki-67 (1:50 dilution; clone MIB-1; cat. no. m7240; Dako;
Agilent Technologies, Inc.). All sections were incubated overnight
at 4˚C with diluted primary antibodies in PBS, and PBS was used to
replace the primary antibody as a negative control. Anti-mouse IgG
or anti-rabbit IgG [ready to use; Histofine SAB-PO (M) or (R) kit;
Nichirei Biosciences Inc.] were used as secondary antibodies and
were incubated at room temperature (20-25˚C). Diaminobenzidine was
used as the chromogen. The sections were examined and photographed
using a microscope (BX41; Olympus Corp.). For each case, three
representative regions were randomly selected. Within each, three
high-power fields (magnification, x400) were then randomly selected
before the staining was evaluated by ImageJ (version. 1.53k;
National Institutes of Health). In the present study, cases were
classified as NEM-positive if they were positive for at least one
NEM. Any PDC cases that expressed NEMs were re-evaluated by a
pathologist (IK) with >17 years of experience in terms of their
morphological features, to determine whether NEC was indeed
present. All slices were deparaffinized and stained with
hematoxylin and eosin (H&E) in advance according to the
established protocol (8).
Statistical analysis
Statistical analyses were performed using the IBM
SPSS Statistics software version 29.0 (IBM Corp.). Clinical and
pathological characteristics were compared using Mann-Whitney U,
Pearson's Chi-squared and Fisher's exact tests, as appropriate.
Univariate and multivariate analyses were performed to identify
prognostic factors. A Cox proportional hazards model was utilized
to calculate hazard ratios, assessing the risk of mortality between
groups. Statistical significance was set at P<0.05.
Results
Clinicopathological patient
characteristics
Of 816 total CRC cases, 74 (9.1%) were identified as
PDC. These were further divided into 13 that were positive for NEMs
and 61 that were negative, based on the IHC staining results. The
details of these 74 cases are presented in Table I. The median age of the patients
with PDC was 68 years (range, 28-89 years). NEM-negative cases were
more frequently observed among older patients (P=0.007). No
significant differences were observed in terms of sex distribution
among the patients. Primary tumors in the cecum, ascending colon,
transverse colon and appendix were classified as right-sided colon
(35.1%), whereas those in the descending colon, sigmoid colon,
rectum and anus were classified as left-sided colon (64.9%). No
significant differences were observed in terms of tumor location.
In only eight cases (10.8%), the majority of the tumors consisted
of PDC. In the remaining 66 cases (89.2%), WDC or MDC was
predominant, with only portions of tumors exhibiting PDC. Lymph
node metastasis was observed in 23 patients, including 10 that were
NEM-positive. A statistically significant difference in lymph node
metastasis was noted between NEM-positive and NEM-negative patients
(P<0.001). Staging distribution was as follows: One patient
(1.4%) was classified as stage I, 19 (25.7%) as stage II, 39
(52.7%) as stage III and 15 (20.2%) as stage IV. Regarding
curability, 63 patients (85.1%) underwent R0 and R1 resections,
while 11 patients (14.9%) underwent R2 resections. No statistically
significant differences were observed in terms of resection
rates.
 | Table IClinicopathological characteristics of
the cases classified as PDC (n=74). |
Table I
Clinicopathological characteristics of
the cases classified as PDC (n=74).
| Item | Total | NEMs+ (n=13) | NEMs- (n=61) | P-value |
|---|
| Age, years | 68 (28-89) | 60 (28-84) | 70 (39-89) | 0.007 |
| Sex | | | | 0.602 |
|
Male | 35 (47.3) | 7 (53.8) | 28 (45.9) | |
|
Female | 39 (52.7) | 6 (46.2) | 33 (54.1) | |
| Tumor location | | | | >0.999 |
|
Right-sided
colon | 26 (35.1) | 5 (38.5) | 21 (34.4) | |
|
Cecum | 7 (9.5) | 0 (0) | 7 (11.5) | |
|
Ascending
colon | 11 (14.9) | 3 (23.1) | 8 (13.1) | |
|
Transverse
colon | 7 (9.5) | 2 (15.4) | 5 (8.2) | |
|
Appendix | 1 (1.4) | 0 (0) | 1 (1.6) | |
|
Left-sided
colon | 48 (64.9) | 8 (61.5) | 40 (65.6) | |
|
Descending
colon | 3 (4.1) | 0 (0) | 3 (4.9) | |
|
Sigmoid
colon | 14 (18.9) | 1 (7.7) | 13 (21.3) | |
|
Rectum | 25 (33.8) | 5 (38.5) | 20 (32.8) | |
|
Anus | 6 (8.1) | 2 (15.4) | 4 (6.6) | |
| Histology | | | | |
|
PDC | 8 (10.8) | 1 (7.7) | 7 (11.5) | >0.999 |
|
WDC>PDC | 4 (5.4) | 0 (0) | 4 (6.6) | |
|
MDC>PDC | 44 (59.5) | 9 (69.2) | 35 (57.4) | |
|
WDC+MDC>PDC | 18 (24.3) | 3 (23.1) | 15 (24.6) | |
| Lymph node
metastases | | | | <0.001 |
|
+ | 23 (31.1) | 10 (76.9) | 13 (21.3) | |
|
- | 51 (68.9) | 3 (23.1) | 48 (78.7) | |
| Stage | | | | 0.761 |
|
I | 1 (1.4) | 0 (0) | 1 (1.6) | |
|
II | 19 (25.7) | 2 (15.4) | 17 (27.9) | |
|
III | 39 (52.7) | 8 (61.5) | 31 (50.8) | |
|
IV | 15 (20.3) | 3 (23.1) | 12 (19.7) | |
| Curability | | | | >0.999 |
|
R0, 1 | 63 (85.1) | 11 (84.6) | 52 (85.2) | |
|
R2 | 11 (14.9) | 2 (15.4) | 9 (14.8) | |
IHC of NEMs
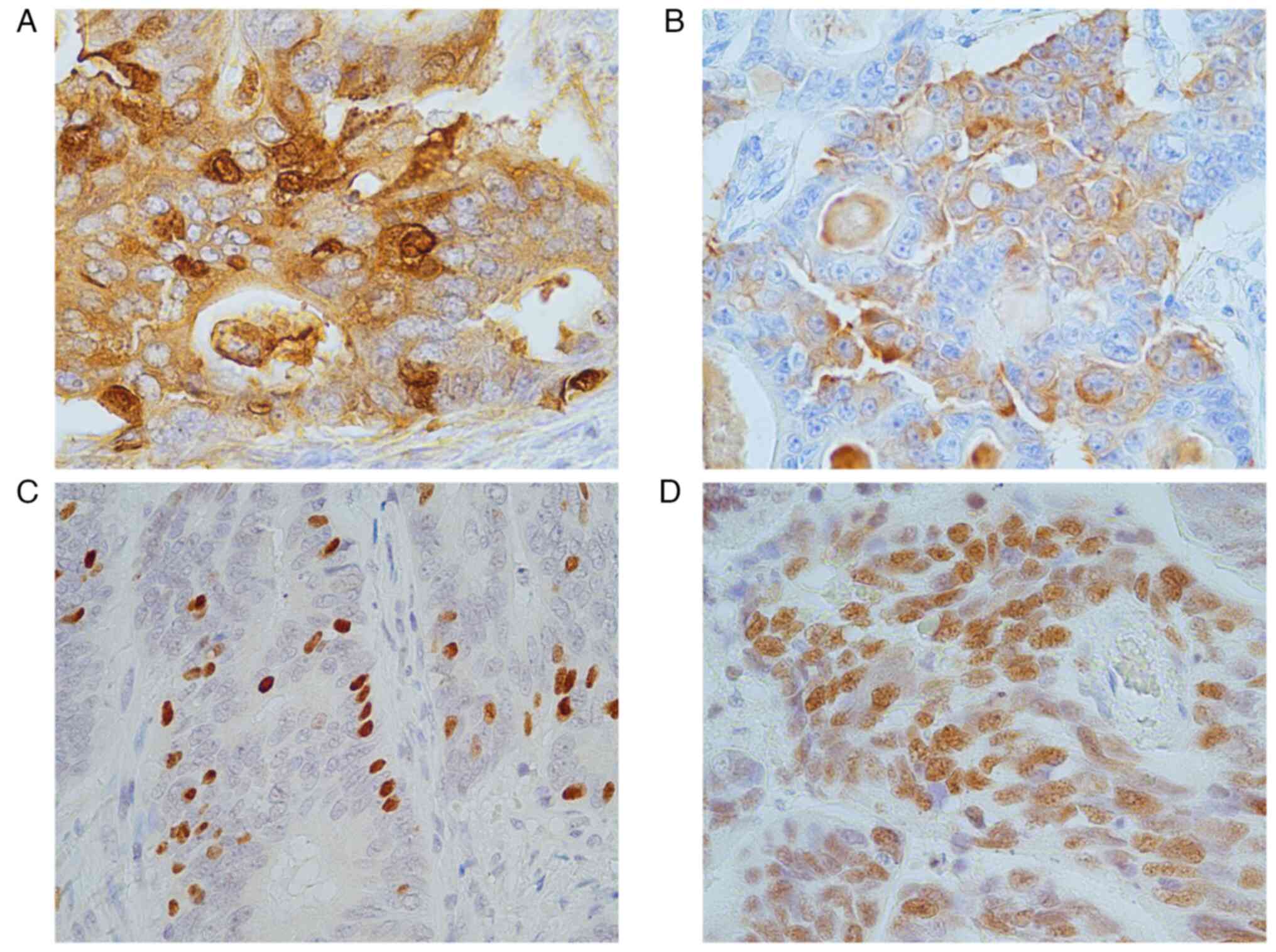
Among the 74 cases, 13 (17.5%) were NEM-positive,
including four cases with diffuse staining and nine cases with
focal staining. Representative images of the immunostaining for
each are provided in Fig. 1. The
summary of clinicopathological characteristics for the 13 cases
classified as NEM-positive is presented in Table II. Among the 13 NEM-positive
cases, PDC was primarily identified, accounting for 84.6% of these
cases. The majority of patients (76.9%) were aged <68 years and
69.2% cases exhibited high proliferation rates (Ki-67 index
>55%). Of note, two cases showed a loss of Rb. The detailed
histopathological characteristics and IHC findings of these 13
cases are summarized in Table SI.
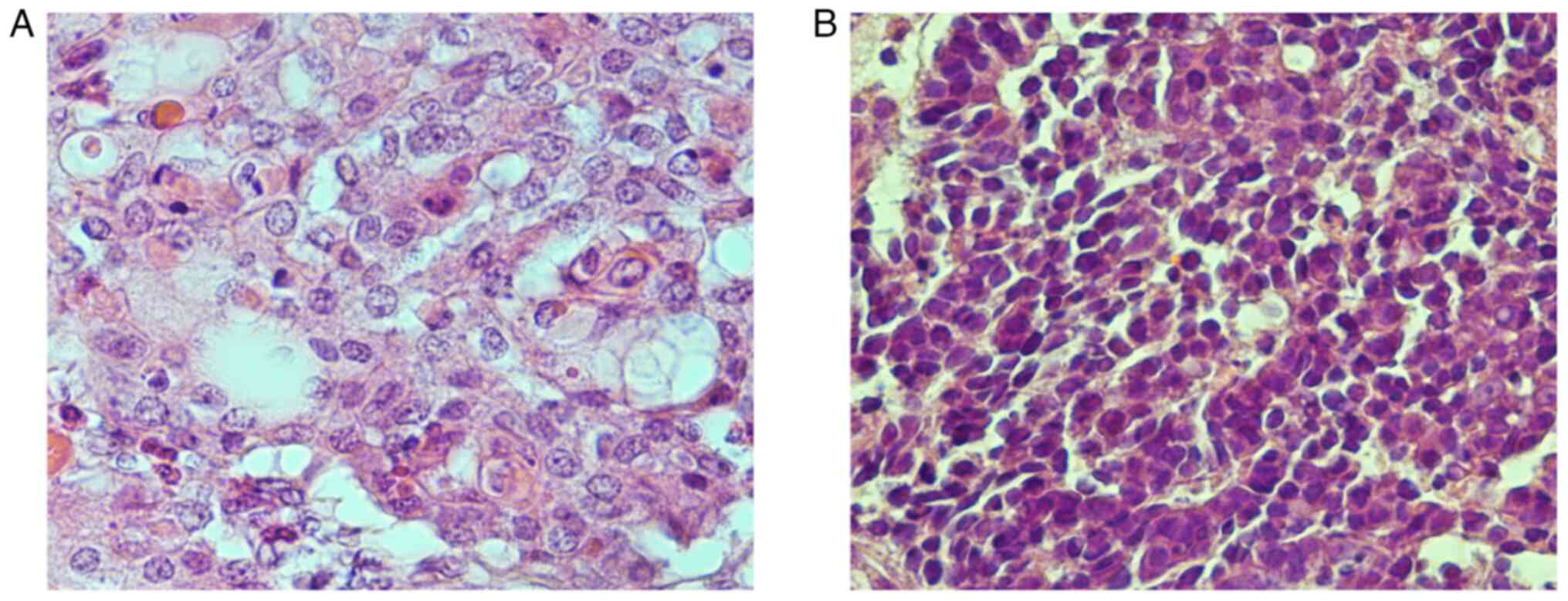
Following re-examination of the 13 NEM-positive PDC sites, two
cases were morphologically identified as NEC, including one large
cell NEC (LCNEC) and one small cell NEC (SCNEC). H&E staining
for these cases is shown in Fig.
2. The expression rates of CgA and Syn were 69.2% (9/13) each,
while that of INSM1 reached 100% (13/13). All patients exhibited a
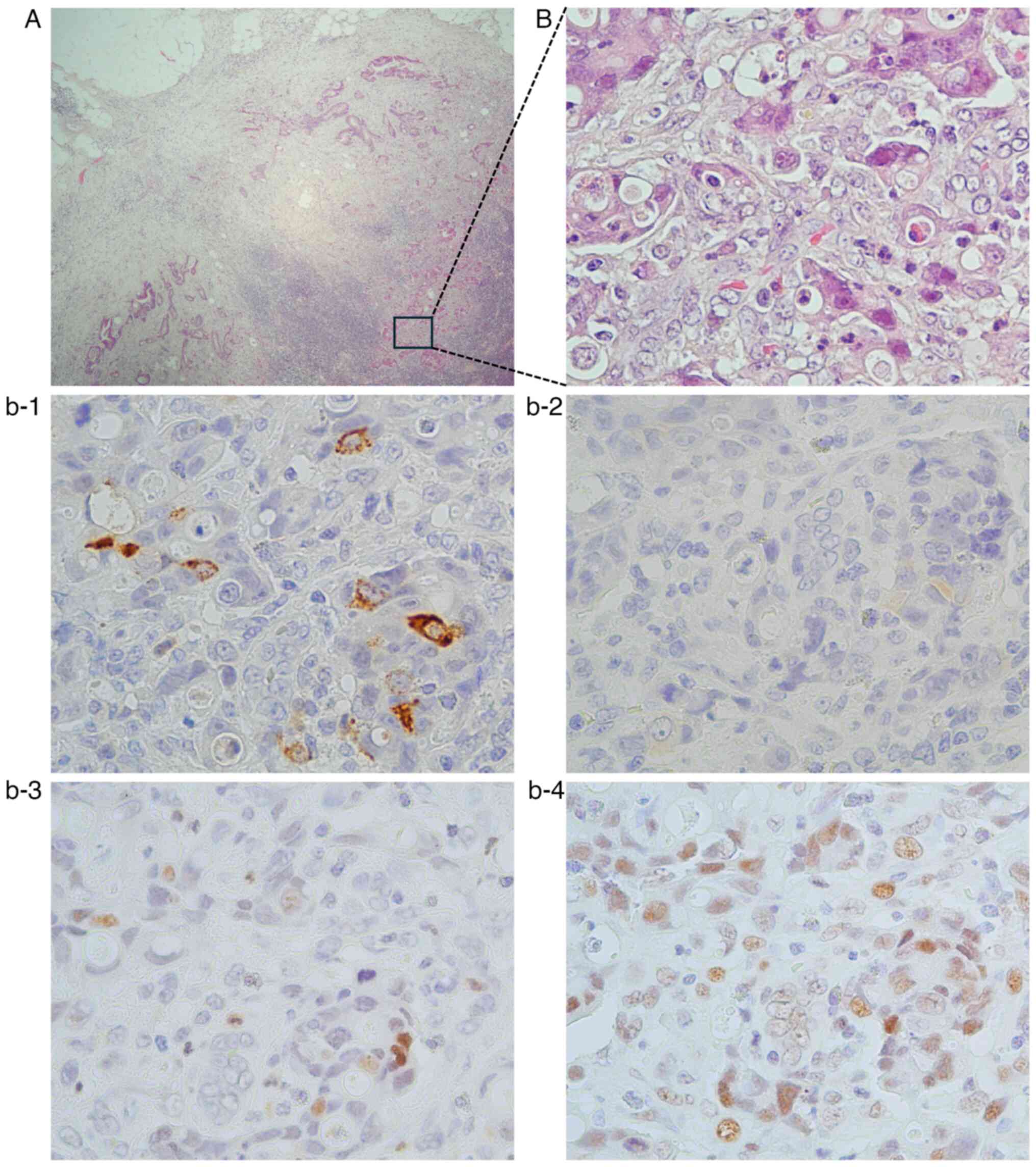
Ki-67 index of >20%. Of the 13 cases, 10 had lymph node
metastases, of which only one case was positive for NEMs within the
lymph node metastases (Fig. 3).
Liver metastasis was obtained from only one case and the sample
tested negative for NEMs.
 | Table IIClinicopathological characteristics
of 13 cases classified as NEM-positive. |
Table II
Clinicopathological characteristics
of 13 cases classified as NEM-positive.
| Clinicopathological
characteristics | Total (n=13) |
|---|
| Age, years | |
|
>68 | 3 (23.1) |
|
<68 | 10 (76.9) |
| Morphological
findings after re-examination | |
|
PDC | 11 (84.6) |
|
LCNEC | 1 (7.7) |
|
SCNEC | 1 (7.7) |
| NEMs | |
|
CgA | |
|
+ | 9 (69.2) |
|
- | 4 (30.8) |
|
Syn | |
|
+ | 9 (69.2) |
|
- | 4 (30.8) |
|
INSM1 | |
|
+ | 13(100) |
|
- | 0 (0) |
| Staining
pattern | |
|
Diffuse | 4 (30.8) |
|
Focal | 9 (69.2) |
| Rb | |
|
+ | 11 (84.6) |
|
- | 2 (15.4) |
| Ki-67, % | |
|
>55 | 9 (69.2) |
|
20-55 | 4 (30.8) |
|
<20 | 0 (0) |
| NEMs of L/N
meta | |
|
CgA | |
|
+ | 1 (7.7) |
|
- | 9 (69.2) |
|
N/A | 3 (23.1) |
|
Syn | |
|
+ | 0 (0) |
|
- | 10 (76.9) |
|
N/A | 3 (23.1) |
|
INSM1 | |
|
+ | 1 (7.7) |
|
- | 9 (69.2) |
|
N/A | 3 (23.1) |
IHC of Rb
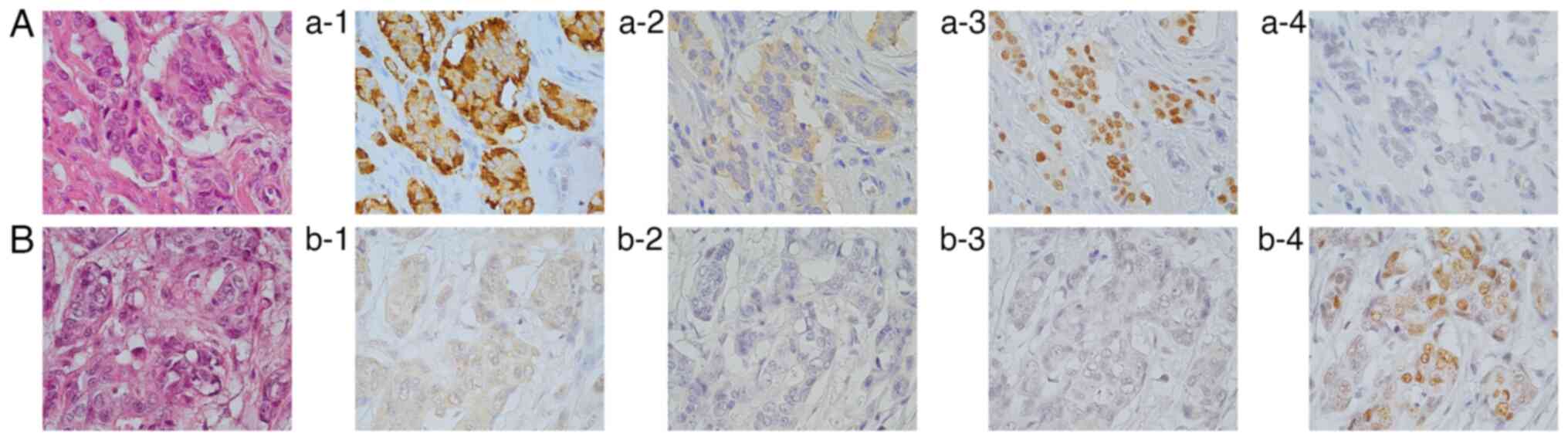
A total of two cases showed loss of Rb in PDC
lesions (cases 1 and 2 in Table
SI). Case 1 was a pure PDC with both NEM-positive and
NEM-negative areas (Fig. 4). Of
note, there was loss of Rb in the NEM-positive areas, whereas it
remained positive in the NEM-negative ones. Case 2 had PDC with a
predominant MDC area. Upon re-examination by a pathologist, the PDC
area was reclassified as LCNEC. All three NEMs tested negative in
the MDC area. Conversely, CgA expression was negative in the PDC
area, whereas Syn and INSM1 were strongly positive (Fig. 5). Loss of Rb was detected in the
PDC area, while Rb remained positive in the MDC area.
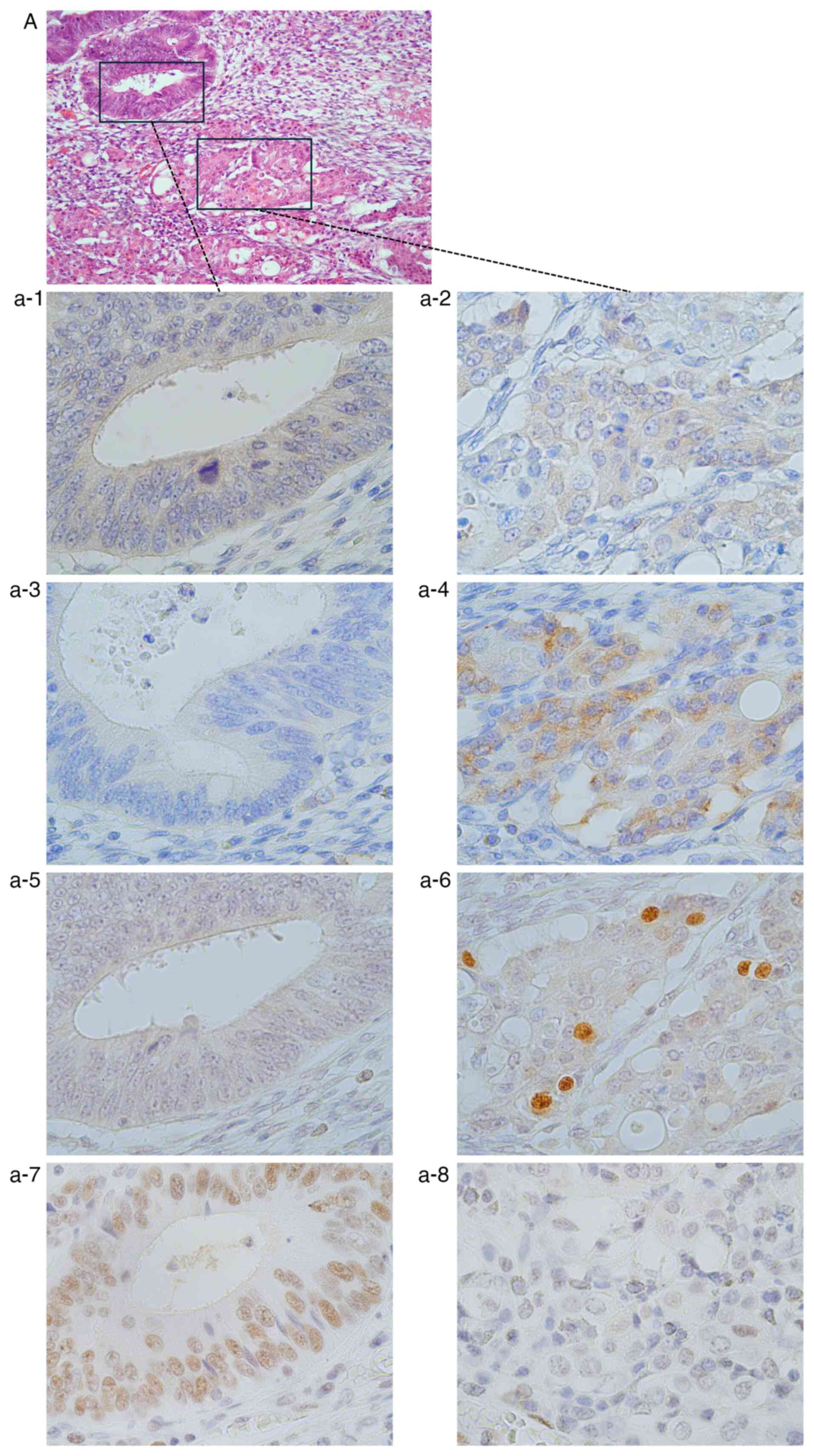 | Figure 5Case of Rb loss (case 2 in Table SI). (A) H&E staining
(magnification, x200). Immunohistochemistry of (a-1, a-3, a-5 and
a-7) the MDC areas and (a-2, a-4, a-6 and a-8) the PDC areas. In
the MDC areas, (a-1) CgA, (a-3) Syn and (a-5) INSM1 were clearly
negative. (a-7) Rb was positive. In the PDC areas, (a-2) CgA was
negative, while (a-4) Syn and (a-6) INSM1 were positive. (a-8) Rb
was negative (magnification, x400). PDC, poorly differentiated
adenocarcinoma; MDC, moderately differentiated adenocarcinoma; CgA,
chromogranin A; Syn, synaptophysin; INSM1, insulinoma-associated 1;
Rb, retinoblastoma 1. |
Prognostic factor analysis
Table III
presents the results of uni- and multivariate analyses for the 74
CRC cases using clinical factors. In the univariate analysis, stage
and curability emerged as potentially significant prognostic
markers. NEM-positivity did not reach statistical significance
(P=0.075). The multivariate analysis, incorporating significant
markers from the univariate one, as well as NEM-positivity status,
identified curability (P<0.0001) and NEM-positivity (P=0.017) as
significant independent prognostic markers.
 | Table IIIPrognostic factor analysis of 74 PDC
cases. |
Table III
Prognostic factor analysis of 74 PDC
cases.
| | Univariate | Multivariate |
|---|
| Factor | P-value | P-value | HR (95% CI) |
|---|
| Sex (male vs.
female) | 0.253 | | |
| Age (<68 vs.
>68 years) | 0.408 | | |
| Predominant
histology (PDC vs. MDC and/or WDC) | 0.360 | | |
| Stage (I/II vs.
III/IV) | 0.005 | 0.109 | 1.604
(0.895-2.996) |
| Curability (R0, 1
vs. R2) | <0.0001 | <0.0001 | 7.072
(2.667-18.762) |
| CEA (<6 vs.
>6 ng/ml) | 0.053 | 0.693 | 0.999
(0.992-1.005) |
| NEMs+ (yes vs.
no) | 0.075 | 0.017 | 3.135
(1.231-7.981) |
Discussion
In the present study, it was found that 17.6% of CRC
tumors classified as PDC exhibited NEM expression, which represents
a necessary condition for diagnosing NEC. In addition, 15.4% of
these cases also showed Rb loss, which is an important feature of
NEC. This suggests that, among CRC tumors that are morphologically
classified as PDC, there may be cases that exhibit NEC
characteristics as well.
According to the WHO classification of tumors, 5th
edition, epithelial malignancies of the colon and rectum may be
broadly classified into three types: Adenocarcinoma, neuroendocrine
neoplasm (NEN) and mixed tumors that contain both (9). NEN can be further classified into
NETs and NECs. The histological macro-classifications of epithelial
malignancies of the colon and rectum are, therefore,
adenocarcinoma, NET and NEC.
Adenocarcinomas represent the majority of CRC
tumors, which may be divided into several distinct morphologic
variants, >90% of which are WDCs or MDCs. According to the
Multi-Institutional Registry of Large Bowel Cancer in Japan
(10), ~95% of CRCs are
adenocarcinomas. Among these, 93.5% are WDCs or MDCs. PDCs account
for only 3.3% of all CRCs in Japan. In the present study, pure PDC
was found in only eight cases (~1%). Ueno et al (11) indicated that PDC components can at
times be found even within WDCs or MDCs and that even a small
amount of PDC can impact the prognosis of CRC. Therefore, the
present study included cases wherein only portions of the tumors
exhibited PDC in order to analyze all sites with morphological PDC
presentation.
According to the WHO classification of NENs from
2022(3), NENs can be divided into
two categories: Well-differentiated and poorly differentiated.
Well-differentiated NENs are NETs including G1, G2 and G3 grades,
while poorly differentiated NENs are NECs. Originally, in the 2010
WHO classification (12), NETs
were classified into three categories (G1, G2 and NEC) based on
cell proliferation. The main issue with this classification was
that when the Ki-67 labeling index exceeds 20%, it becomes
difficult to distinguish between NET-G3s and NECs (13). In the 2017 WHO classification
(14), a solution to this issue
was proposed for pancreatic NENs (pNENs) specifically by
categorizing NET-G3s as well-differentiated NENs and NECs as poorly
differentiated NENs. In the 2019 WHO classification, this
categorization was expanded from pNENs to gastroenteropancreatic
(GEP) NENs (15). Currently, NEC
is positioned as a poorly differentiated cancer within the NEN
category and serves as the counterpart to PDC in
adenocarcinomas.
NECs are malignant tumors that can occur throughout
the body. According to data from the SEER study (16), ~90% occur in the lungs and GEP-NECs
account for ~4.2%. Among GEP-NECs, the colon represents the most
common site, accounting for 29%. However, NECs of the colon and
rectum are rare. NECs are typically classified as SCNECs or LCNECs.
SCNECs are considered sufficiently distinctive for histological
diagnosis, whereas it is often difficult to distinguish LCNECs from
PDCs based solely on morphology (17,18).
Furthermore, in the lungs, where the majority of cases occur,
distinguishing SCNECs from LCNECs may at times be difficult,
leading to misdiagnosis (19).
However, distinguishing between PDCs and NECs based solely on
morphology can be challenging. NECs may be present in certain
patients with CRC who are diagnosed as PDC. In the present study,
two cases of NEM-positive PDC were considered morphologically
likely to be NECs after re-examination. In one other case, NEC was
suspected based on morphology; however, because it was
NEM-negative, the diagnosis remained PDC (data not shown). In the
present study, three cases in which morphological distinction
between PDC and NEC was difficult were also observed; however, this
was a low percentage (4%).
The simplest method to differentiate NENs is to
confirm NEM expression. According to the 2022 WHO classification,
Syn, CgA and INSM1 are considered appropriate antibodies for NEMs.
Syn has high sensitivity but low specificity, whereas CgA has high
specificity but low sensitivity. INSM1, however, has high
sensitivity as well as specificity (3). In the present study, out of the 13
patients who tested positive for NEMs, seven (53.8%) tested
positive for all three antibodies. Furthermore, two patients
(15.4%) tested positive for only one antibody and only INSM1 was
positive in both instances. INSM1 was the only antibody that was
positive in all 13 cases. The present results also suggest that
INSM1 has the highest sensitivity for detecting NEC features.
Ki-67 and Rb are also important factors in the
characterization of NENs. Ki-67 is an important factor in NET
grading. A Ki-67 labeling index of ≥20% serves as the diagnostic
criterion for NET-G3 or NEC. According to the 2022 WHO
classification (3), Ki-67 is often
≥55% in NEC, whereas it is typically lower in NET-G3. A Ki-67 level
of 55% as a cut-off was proposed in the Nordic NEC study, which
focused on NECs with Ki-67 labeling indices of >20%. It has been
shown that NECs with Ki-67 indexes of ≥55% have poor prognoses but
are highly sensitive to platinum-based chemotherapy. On the other
hand, NECs with Ki-67 indexes of <55% do not respond to
platinum-based chemotherapy, but have much better prognoses
(20). In typical CRCs, the median
Ki-67 labeling index is ~40% (21), with ~40% having Ki-67 indexes of
≤50% (22). In the present study,
the Ki-67 labeling index of NEM-positive areas was >55% in 9
cases, many of which met the criteria for NEC.
The tumor suppressor gene Rb is known to cause
cancer when inactivated. Inactivation of Rb occurs at a high rate
in small-cell lung cancer, with reports of 60% (23) and 89% (24). Similarly, inactivation also occurs
in ~50% of GEP-NECs (25). Loss of
Rb is an important feature of NEC that can be used to distinguish
it from NET-G3 (26,27). On the other hand, in CRC, the rate
of inactivation has been reported to be low, at 0.21% (28). In the present study, Rb loss was
observed in two cases. Of note, it was only observed in
NEM-positive areas, whereas Rb expression was maintained in
NEM-negative areas in the same cases (cases 1 and 2 in Table SI). Even in the other 11 cases
where Rb expression was maintained, there was almost no NEM
expression in the predominant areas, such as the WDCs and MDCs.
NEM-positive and NEM-negative areas were confirmed in the same
specimen. In case 1 (Table SI),
despite being morphologically the same PDC tissue, there were areas
that were NEM-negative and Rb-positive, as well as some that were
NEM-positive and showed Rb loss. Colorectal NEC is typically
associated with overlying adenomas or adenocarcinomas rather than
NETs (29). Ogimi et al
(30) analyzed the distribution of
NEMs in CRC and normal mucosal tissues and suggested that NECs may
originate from preexisting adenocarcinomas. Iijima et al
(31) explored the histogenesis of
combined pulmonary NECs by examining EGFR and p53 mutations and
found that some combined NECs arose from non-NEC components. In the
present study, particularly in cases 1 and 2, a similar situation
was suggested, wherein NECs may have arisen from
adenocarcinomas.
A few studies have reported that CRCs with
neuroendocrine differentiation, or NEM-positive CRCs, have poor
prognoses (32,33). In the present study, NEM-positive
PDC was found to be a poor prognostic factor. The rate of lymph
node metastasis was significantly higher in NEM-positive cases vs.
NEM-negative ones. In the present study, among the 10 cases with
lymph node metastasis, only one showed metastasis of NEM-positive
cells in the metastatic lymph nodes. A liver metastasis was
obtained as the distant metastatic tissue of NEM-positive PDC in
one case. However, the cancer cells in this metastatic site were
also NEM-negative. Therefore, it cannot be concluded that
NEM-positive cells are more malignant.
The present study had several limitations. First,
the small sample size, comprising only 13 NEM-positive cases of
PDC, may have limited the generalizability and statistical
significance of the findings. In addition, the study did not
account for all variables that could have influenced prognosis
(e.g., the patients' lifestyle habits and comorbidities), which may
have potentially affected the results. Second, the absence of
comprehensive genetic testing across all of the analyzed cases
precluded a full exploration of the genetic associations between
NEC and other cancer types. Future research with larger patient
populations is necessary. In addition, the development of more
precise diagnostic tools and targeted therapies and a deeper
understanding of the molecular mechanisms underlying NEC and PDC
are imperative to enhance the prognosis for these patients.
The mechanisms underlying the development of PDC and
NEC in CRCs remain largely elusive. It has been demonstrated that
small-cell prostate cancer can emerge during the progression of
prostate adenocarcinoma (34). In
such cases, a distinct treatment approach from that used for
adenocarcinoma is necessary. The current findings indicate that PDC
in CRCs may include components with NEC characteristics. These
results underscore the need to reevaluate existing treatment
protocols for CRC to more effectively address the distinct
challenges presented by NEC and PDC. This could potentially lead to
more personalized and effective treatment strategies. Although the
carcinogenic processes leading to prognostically poor NEC in the
colon remain largely elusive, the present study provides a
preliminary exploration toward their elucidation. Further research
is essential to decipher the molecular mechanisms in CRC cases that
exhibit features of both PDC and NEC.
Supplementary Material
Clinicopathological characteristics
and immunohistochemical findings of 13 cases classified as NE
NEM-positive.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YI, YR and IK designed the study, the main
conceptual ideas and the proof outline. NO, ST, NK, KN, MO, JW and
AI collected the data. YR and ST assembled the data. IK, SY, SF and
IE provided expert advice as pathologists and surgeons, and were
involved in treating some of the patients. YR wrote the manuscript
with support from YI, IK and EK. IE and YI confirm the authenticity
of all the raw data. All of the authors discussed the results,
commented on the manuscript and have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
principles outlined in the Declaration of Helsinki. It was approved
by the Ethics Committee of Yokohama City University (Yokohama,
Japan; approval no. B200700086).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bernick PE, Klimstra DS, Shia J, Minsky B,
Saltz L, Shi W, Thaler H, Guillem J, Paty P, Cohen AM and Wong WD:
Neuroendocrine carcinomas of the colon and rectum. Dis Colon
Rectum. 47:163–169. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Staren ED, Gould VE, Jansson DS, Hyser M,
Gooch GT and Economou SG: Neuroendocrine differentiation in ‘poorly
differentiated’ colon carcinomas. Am Surg. 56:412–419.
1990.PubMed/NCBI
|
|
3
|
Rindi G, Mete O, Uccella S, Basturk O, La
Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M,
et al: Overview of the 2022 WHO classification of neuroendocrine
neoplasms. Endocr Pathol. 33:115–154. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ahadi M, Sokolova A, Brown I, Chou A and
Gill AJ: The 2019 World Health Organization Classification of
appendiceal, colorectal and anal canal tumours: An update and
critical assessment. Pathology. 53:454–461. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hamilton SR, Bosman FT, Boffetta P, Ilyas
M, Morreau H, Nakamura SI, Quirke P, Riboli E and Sobin LH:
Carcinoma of the colon and rectum. In: WHO Classification of
Tumours of the Digestive System. 4th ed edition. Bosman FT,
Carneiro F, Hruban RH and Theise ND (eds). IARC Press, Lyon,
pp134-146, 2010.
|
|
6
|
Takeuchi K, Kuwano H, Tsuzuki Y, Ando T,
Sekihara M, Hara T and Asao T: Clinicopathological characteristics
of poorly differentiated adenocarcinoma of the colon and rectum.
Hepatogastroenterology. 51:1698–1702. 2004.PubMed/NCBI
|
|
7
|
Kazama Y, Watanabe T, Kanazawa T, Tanaka
J, Tanaka T and Nagawa H: Poorly differentiated colorectal
adenocarcinomas show higher rates of microsatellite instability and
promoter methylation of p16 and hMLH1: A study matched for T
classification and tumor location. J Surg Oncol. 97:278–283.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yazawa K, Nakamura F, Masukawa D, Sato S,
Hiroshima Y, Yabushita Y, Mori R, Matsuyama R, Kato I, Taniguchi H,
et al: Low incidence of high-grade pancreatic intraepithelial
neoplasia lesions in a crmp4 gene-deficient mouse model of
pancreatic cancer. Transl Oncol. 13(100746)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
WHO Classification of Tumours Editorial
Board. Digestive system tumours: WHO classification of tumours, 5th
edition, volume 1. World Health Organization, International Agency
for Research on Cancer, Lyon, 2019.
|
|
10
|
Multi-institutional registry of large
bowel cancer in Japan, Japanese Society for Cancer of the Colon and
Rectum, 1992-2015. https://ndlsearch.ndl.go.jp/books/R100000002-I000000155666.
|
|
11
|
Ueno H, Konishi T, Ishikawa Y, Shimazaki
H, Ueno M, Aosasa S, Saiura A, Hase K and Yamamoto J: Prognostic
value of poorly differentiated clusters in the primary tumor in
patients undergoing hepatectomy for colorectal liver metastasis.
Surgery. 157:899–908. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND (eds): WHO Classification of tumours of the digestive
system: WHO classification of tumours, 4th edition, volume 3. World
Health Organization, International Agency for Research on Cancer,
Lyon, 2010.
|
|
13
|
Rindi G, Petrone G and Inzani F: The 2010
WHO classification of digestive neuroendocrine neoplasms: A
critical appraisal four years after its introduction. Endocr
Pathol. 25:186–192. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Inzani F, Petrone G and Rindi G: The New
World Health Organization Classification for pancreatic
neuroendocrine neoplasia. Endocrinol Metab Clin North Am.
47:463–470. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Assarzadegan N and Montgomery E: What is
new in the 2019 World Health Organization (WHO) classification of
tumors of the digestive system: Review of selected updates on
neuroendocrine neoplasms, appendiceal tumors, and molecular
testing. Arch Pathol Lab Med. 145:664–677. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dasari A, Mehta K, Byers LA, Sorbye H and
Yao JC: Comparative study of lung and extrapulmonary poorly
differentiated neuroendocrine carcinomas: A SEER database analysis
of 162,983 cases. Cancer. 124:807–815. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Klimstra DS, Beltran H, Lilenbaum R and
Bergsland E: The spectrum of neuroendocrine tumors: Histologic
classification, unique features and areas of overlap. Am Soc Clin
Oncol Educ Book. 92–103. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Scott N, West NP, Cairns A and Rotimi O:
Is medullary carcinoma of the colon underdiagnosed? An audit of
poorly differentiated colorectal carcinomas in a large national
health service teaching hospital. Histopathology. 78:963–969.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Marchevsky AM and Wick MR: Diagnostic
difficulties with the diagnosis of small cell carcinoma of the
lung. Semin Diagn Pathol. 32:480–488. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sorbye H, Welin S, Langer SW, Vestermark
LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K,
et al: Predictive and prognostic factors for treatment and survival
in 305 patients with advanced gastrointestinal neuroendocrine
carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 24:152–160.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fluge Ø, Gravdal K, Carlsen E, Vonen B,
Kjellevold K, Refsum S, Lilleng R, Eide TJ, Halvorsen TB, Tveit KM,
et al: Expression of EZH2 and Ki-67 in colorectal cancer and
associations with treatment response and prognosis. Br J Cancer.
101:1282–1289. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tong G, Zhang G, Liu J, Zheng Z, Chen Y,
Niu P and Xu X: Cutoff of 25% for Ki67 expression is a good
classification tool for prognosis in colorectal cancer in the
AJCC-8 stratification. Oncol Rep. 43:1187–1198. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mori N, Yokota J, Akiyama T, Sameshima Y,
Okamoto A, Mizoguchi H, Toyoshima K, Sugimura T and Terada M:
Variable mutations of the RB gene in small-cell lung carcinoma.
Oncogene. 5:1713–1717. 1990.PubMed/NCBI
|
|
24
|
Febres-Aldana CA, Chang JC, Ptashkin R,
Wang Y, Gedvilaite E, Baine MK, Travis WD, Ventura K, Bodd F, Yu
HA, et al: Rb tumor suppressor in small cell lung cancer: Combined
genomic and IHC analysis with a description of a distinct
Rb-proficient subset. Clin Cancer Res. 28:4702–4713.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Angerilli V, Sabella G, Simbolo M, Lagano
V, Centonze G, Gentili M, Mangogna A, Coppa J, Munari G, Businello
G, et al: Comprehensive genomic and transcriptomic characterization
of high-grade gastro-entero-pancreatic neoplasms. Br J Cancer.
131:159–170. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yachida S, Vakiani E, White CM, Zhong Y,
Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM,
et al: Small cell and large cell neuroendocrine carcinomas of the
pancreas are genetically similar and distinct from
well-differentiated pancreatic neuroendocrine tumors. Am J Surg
Pathol. 36:173–184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hijioka S, Hosoda W, Matsuo K, Ueno M,
Furukawa M, Yoshitomi H, Kobayashi N, Ikeda M, Ito T, Nakamori S,
et al: Rb loss and KRAS mutation are predictors of the response to
platinum-based chemotherapy in pancreatic neuroendocrine neoplasm
with grade 3: A Japanese multicenter pancreatic NEN-G3 study. Clin
Cancer Res. 23:4625–4632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
AACR Project GENIE Consortium. AACR
project GENIE: Powering precision medicine through an international
consortium. Cancer Discov. 7:818–831. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shia J, Tang LH, Weiser MR, Brenner B,
Adsay NV, Stelow EB, Saltz LB, Qin J, Landmann R, Leonard GD, et
al: Is nonsmall cell type high-grade neuroendocrine carcinoma of
the tubular gastrointestinal tract a distinct disease entity? Am J
Surg Pathol. 32:719–731. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ogimi T, Sadahiro S, Kamei Y, Chan LF,
Miyakita H, Saito G, Okada K, Suzuki T and Kajiwara H: Distribution
of Neuroendocrine marker-positive cells in colorectal cancer tissue
and normal mucosal tissue: Consideration of histogenesis of
neuroendocrine cancer. Oncology. 97:294–300. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Iijima M, Yokobori T, Mogi A, Shimizu K,
Yajima T, Kosaka T, Ohtaki Y, Obayashi K, Nakazawa S, Gombodorj N,
et al: Genetic and immunohistochemical studies investigating the
histogenesis of neuroendocrine and carcinomatous components of
combined neuroendocrine carcinoma. Ann Surg Oncol. 26:1744–1750.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cho YB, Yang SS, Lee WY, Song SY, Kim SH,
Shin HJ, Yun SH and Chun HK: The clinical significance of
neuroendocrine differentiation in T3-T4 node-negative colorectal
cancer. Int J Surg Pathol. 18:201–206. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Grabowski P, Schönfelder J, Ahnert-Hilger
G, Foss HD, Heine B, Schindler I, Stein H, Berger G, Zeitz M and
Scherubl H: Expression of neuroendocrine markers: A signature of
human undifferentiated carcinoma of the colon and rectum. Virchows
Arch. 441:256–263. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Conteduca V, Oromendia C, Eng KW, Bareja
R, Sigouros M, Molina A, Faltas BM, Sboner A, Mosquera JM, Elemento
O, et al: Clinical features of neuroendocrine prostate cancer. Eur
J Cancer. 121:7–18. 2019.PubMed/NCBI View Article : Google Scholar
|