Introduction
Hormone receptor-positive (HR+)/human epidermal
growth factor receptor 2-negative (HER2-) breast cancer is the most
common subtype of metastatic breast cancer (MBC), accounting for
~65% of all cases (1). The
development of cyclin-dependent kinase 4/6 (CDK4/6) is crucial in
HR+ breast cancer onset, survival and progression, facilitating the
G1-to-S phase cell cycle transition (2,3).
CDK4/6 inhibitors (CDK4/6i) block this pathway by inhibiting
phosphorylation of tumor suppressor retinoblastoma protein,
preventing tumor cell proliferation and inducing G1 phase arrest
(4,5). In combination with endocrine therapy
(ET), CDK4/6i have demonstrated benefits in terms of overall
survival (OS) and progression-free survival (PFS) in several
clinical trials, both as the first- and second-line therapies
(6-14).
As a result, CDK4/6i combined with ET has become the standard
treatment for HR+/HER2-advanced breast cancer (ABC) (15-17).
However, chemotherapy remains the preferred option
for patients with HR+/HER2-MBC experiencing rapid tumor progression
or a high tumor burden, such as visceral crisis (15-18).
Visceral crisis is defined as severe organ dysfunction
characterized by rapid symptom progression, complaints and
laboratory abnormalities. Chemotherapy is often required to achieve
a quick response and provide relief within a limited timeframe.
However, the side effects of chemotherapy can be significant.
Meanwhile, the time-to-response (TTR) for CDK4/6i combined with ET
has become much shorter than for hormone monotherapy, presenting a
treatment dilemma for cases of metastatic HR+/HER2-breast cancer
with visceral crisis (8-14).
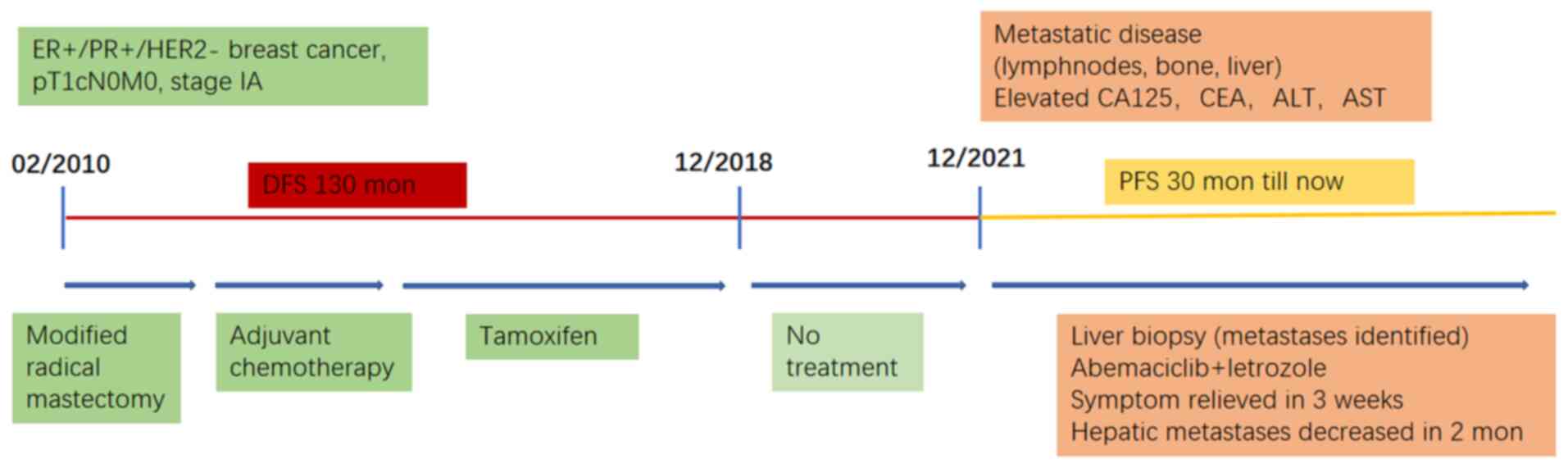
The present study reported the case of a patient
with HR+/HER2-MBC diagnosed in 2021 who presented with multiple
lymph node, liver and bone metastases. The patient had a
disease-free survival (DFS) period of 11 years before relapse
occurred 3 years after the discontinuation of adjuvant ET. Due to
severe liver function and a poor general condition, chemotherapy
was deemed to likely worsen the patient's condition. Given the
patient's prior positive response to ET, CDK4/6i combined with ET
was chosen as the first-line treatment, even in the presence of
visceral crisis. Considering drug availability, abemaciclib plus
letrozole were administered. The patient's condition improved
rapidly and remained stable for >30 months. At the same time,
side effects were well-tolerated and the patient's quality of life
was enhanced.
Case presentation
A 37-year-old woman underwent a left breast-modified
radical mastectomy for breast cancer in February 2010 at the
Affiliated Hospital of Qingdao University (Qingdao, China).
Pathological examination revealed invasive ductal carcinoma
(histological grade II; tumor size, 1.2x1.0x1.0 cm) with no
axillary lymph node metastasis. The pathological stage was
pT1cN0M0, stage IA, based on the eighth edition of the Cancer
Staging Manual for Breast Cancer by the American Joint Committee on
Cancer (19). The pathological
assessments were performed in the Affiliated Hospital of Qingdao
University (Qingdao, China). Immunohistochemical analysis was
performed on formalin-fixed, paraffin-embedded tissue sections
using standard procedures for tumor specimens. ER, PR and HER2
status and the Ki-67 index were evaluated by two experienced
pathologists from the Department of Pathology independently.
Immunohistochemical (IHC) results showed estrogen receptor (ER)
(+), progesterone receptor (PR) (+), HER2 (0) and Ki67 (25%) (IHC
antibody information and images are not available due to the
interval of more than 13 years). The patient received four cycles
of adjuvant chemotherapy with docetaxel and epirubicin and ET with
tamoxifen (from March 2010 to December 2018). Radiotherapy was not
administered postoperatively and ET had been discontinued for >3
years before relapse occurred.
In December 2021, the patient presented to the
emergency department of the Affiliated Hospital of Qingdao
University (Qingdao, China) with abdominal pain, distension, mild
dyspnea and joint pain, accompanied by significant physical
weakness, with an Eastern Cooperative Oncology Group performance
score of 2(20). Physical
examination revealed hepatomegaly. Computed tomography (CT) scans
showed pleural effusion, lymphangitis carcinomatosis, bone
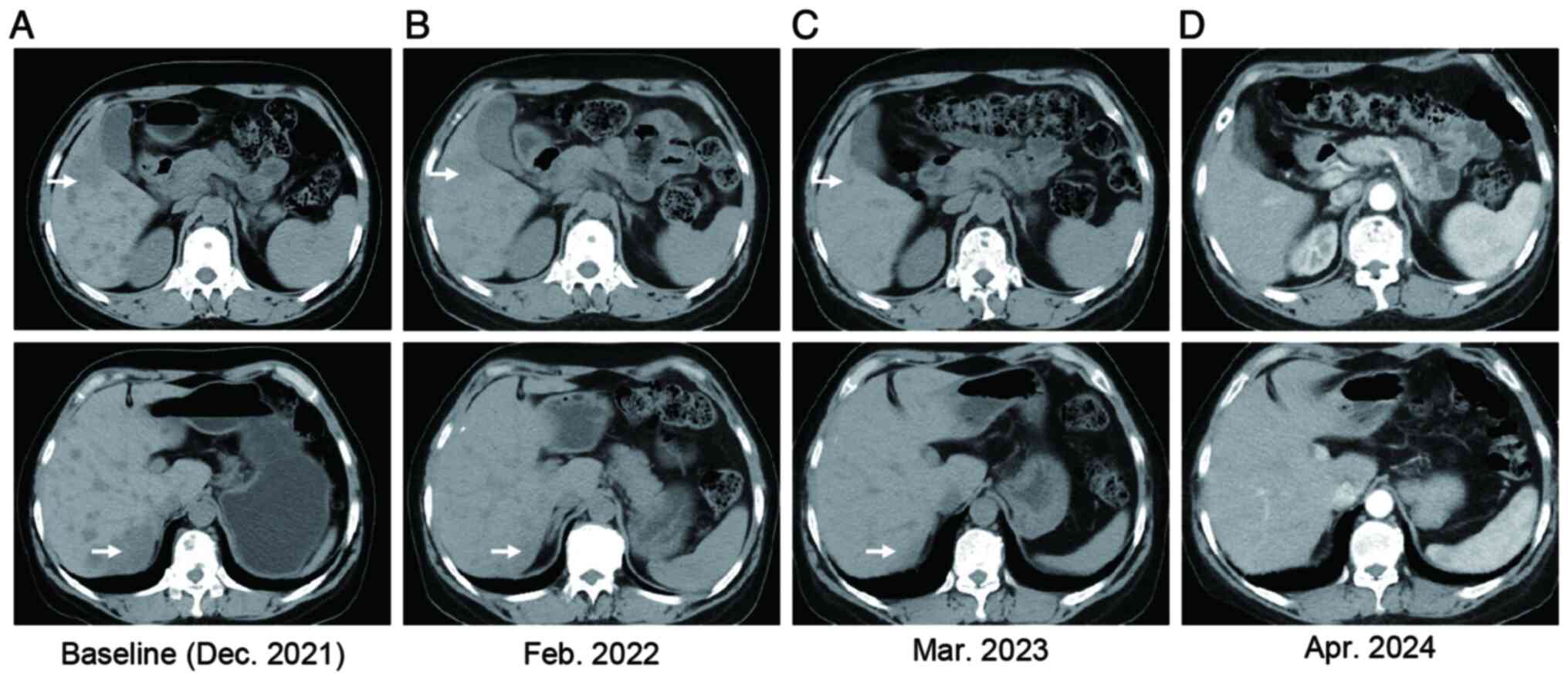
metastases and liver metastases (Fig.
1A). Multiple suspected enlarged lymph nodes were detected in
the mediastinum, as well as supraclavicular and infraclavicular
fossae. Laboratory tests indicated elevated serum levels of
carbohydrate antigen 125 (CA125; 151.00 U/ml; normal range, 0-35
U/ml), carcinoembryonic antigen (CEA; 216.00 U/ml; normal range,
0-3.4 U/ml), aminotransferase and bilirubin. Glutamic oxaloacetic
transaminase was 477.00 U/l (normal range, 14-36 U/l) and alanine
aminotransferase was 433.00 U/l (normal range, 9-52 U/l). Bilirubin
was 119.73 µmol/l (normal range, 3-22 µmol/l), with no biliary
obstruction evident on CT. No metastasis was detected in other
organs. A CT-guided liver biopsy was performed in December 2021,
confirming metastatic breast invasive ductal carcinoma. IHC results
showed ER (+) (cat. no. 790-4325, sp1), PR (+) (cat. no. 790-4296,
IE2), HER2 (1+) (cat. no. 790-4493, 4B5) and Ki67 (20%) (cat. no.
790-4286, 30-9; all from Roche Diagnostics).
Given the patient's DFS of >10 years and poor
liver function precluding chemotherapy, abemaciclib plus letrozole
was set as the first-line treatment, despite the patient's visceral
crisis. Abemaciclib 100 mg was administered twice daily and
letrozole was added ~2 weeks after a slight improvement in liver
function. Zoladex (a goserelin implant) was administered for
ovarian function suppression and denosumab for bone protection.
After 21 days of treatment, the patient's chest
tightness and shortness of breath improved, and CT scans showed
decreased pleural effusion and volume-reduced lymph nodes (Fig. S1). From the second treatment
cycle, the abemaciclib dose was increased to 150 mg twice daily.
After two treatment cycles, the number and volume of multiple
hepatic metastases decreased significantly (Fig. 1B). The patient's serum glutamic
oxaloacetic transaminase and alanine aminotransferase levels
normalized within 10 days, and elevated bilirubin levels returned
to normal within 40 days (Table
SI). Serum CA-125 levels normalized after 2 months of treatment
and have since then remained within the normal range. The patient's
serum CEA levels continued to decrease slightly throughout the
treatment (Fig. 2), indicating
ongoing therapeutic efficacy. Regular enhanced CT examination of
the neck, chest, upper abdomen, lower abdomen and pelvic cavity was
performed every 2-3 months (Fig.
1C). Pleural effusion decreased and resolved within 3 months,
and multiple enlarged lymph nodes shrunk significantly. No signs of
lymphangitis carcinomatosis were seen on a recent CT scan (June
2024). Regular whole-body scans every 12 months showed that bone
metastasis remained stable and no signs of brain metastasis were
observed on craniocerebral MRI. In addition, the patient's general
condition improved significantly shortly after the start of
treatment, and the patient's self-described quality of life (as
determined from patient self-statements at the average monthly
outpatient visit) was maintained. The toxicity of abemaciclib plus
letrozole was tolerable and mild neutropenia occurred. Mild
diarrhea occurred in February 2022 and was controlled with drugs.
No dose reduction of abemaciclib was required throughout the
treatment. Recent examinations (April 2024) indicated stable liver
metastases (Fig. 1D) and normal
glutamic oxaloacetic transaminase, alanine aminotransferase and
bilirubin. The patient's PFS has reached 30 months (Fig. 3).
Discussion
ET is generally recommended for patients with
HR+/HER2-MBC unless hormonal resistance is suspected or visceral
crisis is present. Visceral crisis is characterized by rapid
disease progression and severe organ dysfunction due to multiple
metastases, such as in the liver, bone marrow or lungs. Almost all
guidelines for the diagnosis and treatment of breast cancer
recommend chemotherapy as the preferred choice for ABC in the
presence of visceral crisis (17,18,21).
Chemotherapy is expected to provide more timely disease relief and
higher response rates (RR) than ET. However, higher toxicity and
other adverse effects are unavoidable.
CDK4/6i have achieved promising therapeutic outcomes
in patients with HR+/HER2-ABC, offering new potential treatment
strategies (22). Furthermore,
CDK4/6i-based therapy elicits an objective response within 3 months
of treatment initiation in most responders (23,24).
However, the lack of head-to-head studies comparing first-line
CDK4/6i plus ET with combined chemotherapy in patients with
HR+/HER2-breast cancer with visceral crisis hinders its application
in this context.
Three CDK4/6i have been approved by the Food and
Drug Administration for treating MBC: Palbociclib, abemaciclib and
ribociclib (15). Abemaciclib is
also approved for adjuvant therapy in HR+/HER2-early breast cancer
with a high risk of recurrence (25). In addition to halting cell-cycle
progression of cancer cells in the G1 phase, which is expected for
all three CDK4/6i, abemaciclib can also induce G2-phase arrest by
suppressing CDK1 and CDK2, which are critical for cell-cycle
progression through the S-phase and mitosis (26).
In the MONARCH 3 trial, a double-blinded, randomized
phase III study, abemaciclib combined with an aromatase inhibitor
showed significant clinical benefits in postmenopausal patients
with HR+/HER2-ABC. After a median follow-up of ~8 years, the median
OS was 66.8 months for abemaciclib vs. 53.7 months for
placebo, which is clinically meaningful but without statistical
significance. In a subgroup analysis of patients with visceral
disease, the median OS was 63.7 months for abemaciclib vs.
48.8 months for placebo (27). The
addition of abemaciclib also prolonged PFS and chemotherapy-free
survival with no new safety concerns. The PFS was 29 months for
abemaciclib vs. 14.8 months for placebo. The proportion of
patients who achieved PFS of >6 years was significantly higher
in the abemaciclib group (23.3 vs. 4.3%). The addition of
abemaciclib resulted in a higher objective response rate in all
subgroups, particularly in patients with liver metastases,
PR-tumors and high-grade tumors (28).
An exploratory analysis of the MONALEESA series III
studies (Monaleesa-2, -3 and -7) in patients with visceral
metastases was presented at the 2022 European Society of Medical
Oncology meeting (Yardley DA, et al, abs. 205P). It showed that
CDK4/6i plus ET as a first-line treatment for patients with
visceral metastases (including liver and multiple metastases)
extended the median PFS (mPFS) by nearly 15 months compared with
placebo (29.6 vs. 14.7 months; hazard ratio, 0.56; 95% CI,
0.47 to 0.67), and extended the median OS by nearly 12 months (63.4
vs. 51.8 months; hazard ratio, 0.78; 95% CI, 0.64-0.96).
The RIGHT Choice study was the first prospective,
randomized, controlled, head-to-head clinical study to compare
CDK4/6i plus endocrine regimens with combination chemotherapy
regimens in pre/perimenopausal patients with highly invasive
HR+/HER2-ABC. The study included 222 patients, with 112 in the
first-line ribociclib plus ET group and 110 in the combination
chemotherapy group, including 47.7% of patients with
investigator-assessed visceral crisis (29). After a median follow-up of 37.0
months, the median PFS was 21.8 months for ribociclib plus ET
(17.4-26.7 months) and 12.8 months for combination chemotherapy
(10.1-18.4 months) with statistical significance. The overall RR
was 66.1 and 61.8% in the ribociclib and the chemotherapy group,
respectively. The median TTR was 4.9 months in the ribociclib group
vs. 3.2 months in the chemotherapy group. The study
demonstrates that first-line treatment with ribociclib plus ET
offers significant PFS benefits, similar RRs and better
tolerability compared to combination chemotherapy in patients with
HR+/HER2-ABC (30). The subgroup
PFS benefit was in consistency with the overall analysis, with the
benefit being diminished in patients with visceral crisis and
recurrent disease. CDK4/6i is highly effective in treating
HR+/HER2-ABC, and the addition of CDK4/6i may result in rapid and
deep remission (29,30). Although the TTR in the ribociclib
group was prolonged by ~1.7 months, there was no statistically
significant difference. In addition, the incidence of adverse
effects decreased significantly in the ribociclib-treated group. No
noticeable difference was observed in OS between the two groups,
suggesting no meaningful difference in survival benefit. CDK4/6i
plus endocrine regimens provide a more tolerated treatment option
and are also effective for patients with HR+HER2-breast cancer who
could only receive chemotherapy in the past.
The present study reported on a patient with
HR+/HER2-MBC with visceral crisis. The patient showed a good
response to Abemaciclib and letrozole and the PFS of this patient
reached >30 months. Overall, clinical studies comparing
combination chemotherapy and targeted therapy plus ET in the
first-line treatment of HR+/HER2-ABC with visceral crisis have
supported a shift in clinical practice, possibly establishing the
dominant position of CDK4/6i and changing the status of
chemotherapy in the treatment of patients with visceral crisis.
While the contribution of chemotherapy regimens in managing
HR+/HER2-ABC cannot be overstated, multiple factors should be
considered to make treatment decisions in clinical practice.
Individualized precision therapy that considers tumor biological
characteristics is the key determinant of palliative
pharmacological treatment. However, for patients with relatively
high ER expression and expected endocrine sensitivity, CDK4/6i plus
ET may be the better option, as supported by the findings of the
RIGHT Choice study (30).
Supplementary Material
Decreased pleural effusion and
volume-reduced lymph nodes in CT scans. (A) The baseline of pleural
effusion at the beginning of abemaciclib and letrozole (December
2021). (B) Decreased pleural effusion in CT scan (January 2022).
(C) The baseline of enlarged lymph nodes at the beginning of
abemaciclib and letrozole (December 2021). (D) Volume-reduced lymph
nodes in CT scan (January 2022). (E) The baseline of enlarged lymph
nodes around hilar region at the beginning of abemaciclib and
letrozole (December 2021). (F) Volume-reduced lymph nodes around
hilar region in CT scan (January 2022).
Changes of the patient's serum AST,
ALT and bilirubin levels.
Acknowledgements
We acknowledge and appreciate Dr Xia Li from the
Department of Pathology of the Affiliated Hospital of Qingdao
University for her valuable efforts collecting pathological
information related to this case.
Funding
Funding: This study was supported by the National Science
Foundation of China (grant no. 82303815).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW contributed to acquiring data and writing of the
manuscript. XZ and YM were engaged in formal analysis and prepared
the figures. ML interpreted the radiological images. WL treated the
patient and contributed to the conceptualization of the study. All
authors have read and approved the final version of the manuscript.
YW and WL checked and confirmed the authenticity of the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient consented to the publication of data and
images in the case study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prat A, Pineda E, Adamo B, Galvan P,
Fernandez A, Gaba L, Díez M, Viladot M, Arance A and Muñoz M:
Clinical implications of the intrinsic molecular subtypes of breast
cancer. Breast. 24 (Suppl 2):S26–S35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Braal CL, Jongbloed EM, Wilting SM,
Mathijssen RHJ, Koolen SLW and Jager A: Inhibiting CDK4/6 in breast
cancer with palbociclib, ribociclib, and abemaciclib: Similarities
and differences. Drugs. 81:317–331. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Glaviano A, Wander SA, Baird RD, Yap KC,
Lam HY, Toi M, Carbone D, Geoerger B, Serra V, Jones RH, et al:
Mechanisms of sensitivity and resistance to CDK4/CDK6 inhibitors in
hormone receptor-positive breast cancer treatment. Drug Resist
Updat. 76(101103)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Spring LM, Wander SA, Andre F, Moy B,
Turner NC and Bardia A: Cyclin-dependent kinase 4 and 6 inhibitors
for hormone receptor-positive breast cancer: Past, present, and
future. Lancet. 395:817–827. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Im SA, Lu YS, Bardia A, Harbeck N,
Colleoni M, Franke F, Chow L, Sohn J, Lee KS, Campos-Gomez S, et
al: Overall survival with ribociclib plus endocrine therapy in
breast cancer. N Engl J Med. 381:307–316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Overall survival with ribociclib plus fulvestrant in
advanced breast cancer. N Engl J Med. 382:514–524. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Phase III Randomized study of ribociclib and fulvestrant
in hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin
Oncol. 36:2465–2472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sledge GW, Toi M, Neven P, Sohn J, Inoue
K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al: The
effect of abemaciclib plus fulvestrant on overall survival in
hormone receptor-positive, ERBB2-Negative breast cancer that
progressed on endocrine Therapy-MONARCH 2 A Randomized clinical
trial. JAMA Oncol. 6:116–124. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tripathy D, Im SA, Colleoni M, Franke F,
Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, et al:
Ribociclib plus endocrine therapy for premenopausal women with
hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A
randomised phase 3 trial. Lancet Oncol. 19:904–915. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ribociclib as First-Line Therapy for
HR-Positive, advanced breast cancer. N Engl J Med.
379(2582)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rugo HS, Finn RS, Dieras V, Ettl J,
Lipatov O, Joy AA, Harbeck N, Castrellon A, Iyer S, Lu DR, et al:
Palbociclib plus letrozole as first-line therapy in estrogen
receptor-positive/human epidermal growth factor receptor 2-negative
advanced breast cancer with extended follow-up. Breast Cancer Res
Treat. 174:719–729. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Di Leo A, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for patients with
HR+/HER2-advanced breast cancer. Ann Oncol. 28(1)2017.
|
|
14
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cardoso F, Paluch-Shimon S, Senkus E,
Curigliano G, Aapro MS, Andre F, Barrios CH, Bergh J, Bhattacharyya
GS, Biganzoli L, et al: 5th ESO-ESMO international consensus
guidelines for advanced breast cancer (ABC 5). Ann Oncol.
31:1623–1649. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gradishar WJ, Moran MS, Abraham J, Aft R,
Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et
al: Breast cancer, version 3.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:691–722.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gennari A, André F, Barrios CH, Cortés J,
de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz
SA, et al: ESMO clinical practice guideline for the diagnosis,
staging and treatment of patients with metastatic breast cancer.
Ann Oncol. 32:1475–1495. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
O'Shaughnessy J: Extending survival with
chemotherapy in metastatic breast cancer. Oncologist. 10 (Suppl
3):S20–S29. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhu H and Doğan BE: American Joint
Committee on Cancer's Staging System for Breast Cancer, Eighth
Edition: Summary for Clinicians. Eur J Breast Health. 17:234–238.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
21
|
Burstein HJ, Somerfield MR, Barton DL,
Dorris A, Fallowfield LJ, Jain D, Johnston SRD, Korde LA, Litton
JK, Macrae ER, et al: Endocrine treatment and targeted therapy for
hormone receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer: ASCO guideline update. J Clin
Oncol. 39:3959–3977. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giuliano M, Schettini F, Rognoni C, Milani
M, Jerusalem G, Bachelot T, De Laurentiis M, Thomas G, De Placido
P, Arpino G, et al: Endocrine treatment versus chemotherapy in
postmenopausal women with hormone receptor-positive, HER2-negative,
metastatic breast cancer: A systematic review and network
meta-analysis. Lancet Oncol. 20:1360–1369. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rugo HS, Finn RS, Gelmon K, Joy AA,
Harbeck N, Castrellon A, Mukai H, Walshe JM, Mori A, Gauthier E, et
al: Progression-free survival outcome is independent of objective
response in patients with estrogen receptor-positive, human
epidermal growth factor receptor 2-negative advanced breast cancer
treated with palbociclib plus letrozole compared with letrozole:
Analysis from PALOMA-2. Clin Breast Cancer. 20:e173–e180.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Janni W, Alba E, Bachelot T, Diab S,
Gil-Gil M, Beck TJ, Ryvo L, Lopez R, Tsai M, Esteva FJ, et al:
First-line ribociclib plus letrozole in postmenopausal women with
HR+, HER2-advanced breast cancer: Tumor response and pain reduction
in the phase 3 MONALEESA-2 trial. Breast Cancer Res Treat.
169:469–479. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Johnston SRD, Harbeck N, Hegg R, Toi M,
Martin M, Shao ZM, Zhang QY, Martinez Rodriguez JL, Campone M,
Hamilton E, et al: Abemaciclib combined with endocrine therapy for
the adjuvant treatment of HR+, HER2-, node-positive, high-risk,
early breast cancer (monarchE). J Clin Oncol. 38:3987–3998.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hafner M, Mills CE, Subramanian K, Chen C,
Chung M, Boswell SA, Everley RA, Liu C, Walmsley CS, Juric D and
Sorger PK: Multiomics profiling establishes the polypharmacology of
FDA-Approved CDK4/6 inhibitors and the potential for differential
clinical activity. Cell Chem Biol. 26:1067–1080.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Goetz MP, Toi M, Huober J, Sohn J, Trédan
O, Park IH, Campone M, Chen SC, Manso LM, Paluch-Shimon S, et al:
Abemaciclib plus a nonsteroidal aromatase inhibitor as initial
therapy for HR+, HER2-advanced breast cancer: Final overall
survival results of MONARCH 3. Ann Oncol. 35:718–727.
2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Johnston S, O'Shaughnessy J, Martin M,
Huober J, Toi M, Sohn J, André VAM, Martin HR, Hardebeck MC and
Goetz MP: Abemaciclib as initial therapy for advanced breast
cancer: MONARCH 3 updated results in prognostic subgroups. NPJ
Breast Cancer. 7(80)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu YS, Mahidin EIBM, Azim H, Eralp Y, Yap
YS, Im SA, Rihani J, Bowles J, Alfaro TD, Wu J, et al: Abstract
GS1-10: Primary Results From the Randomized Phase II RIGHT Choice
Trial of Premenopausal Patients With Aggressive HR+/HER2− Advanced
Breast Cancer Treated With Ribociclib + Endocrine Therapy vs
Physician's Choice Combination Chemotherapy. Cancer Res. 83 (Suppl
5):GS1–GS10. 2023.
|
|
30
|
Lu YS, Mahidin EIBM, Azim H, Eralp Y, Yap
YS, Im SA, Rihani J, Gokmen E, El Bastawisy A, Karadurmus N, et al:
Final results of RIGHT Choice: Ribociclib plus endocrine therapy vs
combination chemotherapy in premenopausal women with clinically
aggressive Hormone Receptor-Positive/Human Epidermal Growth Factor
Receptor 2-Negative advanced breast cancer. J Clin Oncol.
42:2812–2821. 2024.PubMed/NCBI View Article : Google Scholar
|