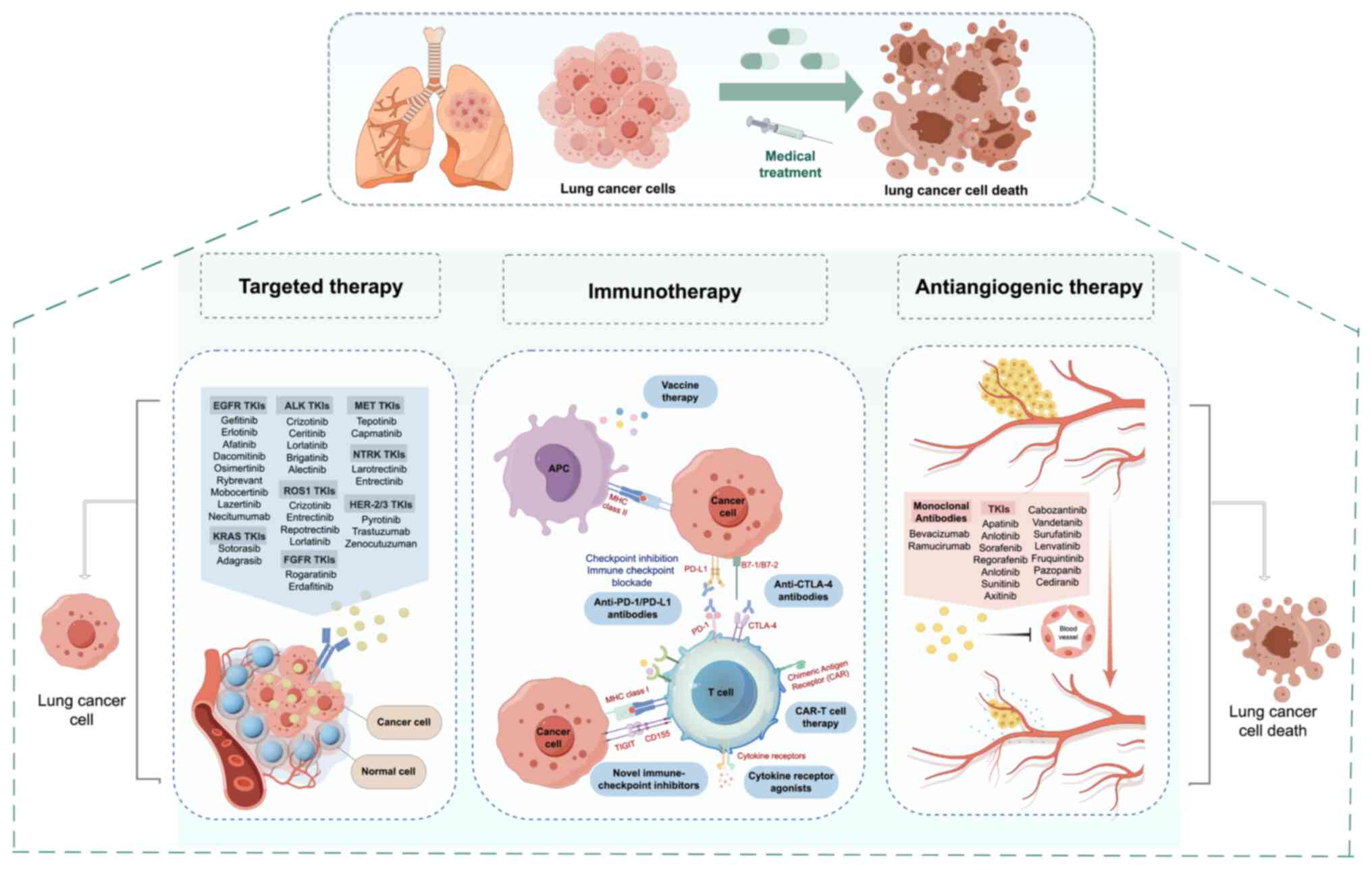
|
1
|
Asamura H, Nishimura KK, Giroux DJ,
Chansky K, Hoering A, Rusch V and Rami-Porta R: Members of the
IASLC Staging and Prognostic Factors Committee and of the Advisory
Boards, Participating Institutions. IASLC lung cancer staging
project: The new database to inform revisions in the ninth edition
of the TNM classification of lung cancer. J Thorac Oncol.
18:564–575. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rami-Porta R, Nishimura KK, Giroux DJ,
Detterbeck F, Cardillo G, Edwards JG, Fong KM, Giuliani M, Huang J,
Kernstine KH Sr, et al: The international association for the study
of lung cancer lung cancer staging project: Proposals for revision
Of the TNM stage groups in the forthcoming (Ninth) edition of the
TNM classification for lung cancer. J Thorac Oncol. 19:1007–1027.
2024.
|
|
3
|
Lu S, Lu C, Xiao Y, Zhu W, He Q, Xie B,
Zhou J, Tao Y, Liu S and Xiao D: Comparison of EML4-ALK fusion gene
positive rate in different detection methods and samples of
non-small cell lung cancer. J Cancer. 11:1525–1531. 2020.
|
|
4
|
Imyanitov EN, Iyevleva AG and Levchenko
EV: Molecular testing and targeted therapy for non-small cell lung
cancer: Current status and perspectives. Crit Rev Oncol Hematol.
157(103194)2021.
|
|
5
|
Shields MD, Chen K, Dutcher G, Patel I and
Pellini B: Making the rounds: Exploring the role of circulating
tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci.
23(9006)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W,
Tang XM, Sun F, Lu HM, Deng J, et al: Liquid biopsy in lung cancer:
Significance in diagnostics, prediction, and treatment monitoring.
Mol Cancer. 21(25)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hussain MS, Gupta G, Ghaboura N, Moglad E,
Almalki WH, Alzarea SI, Kazmi I, Ali H, MacLoughlin R, Loebenberg
R, et al: Exosomal ncRNAs in liquid biopsies for lung cancer. Clin
Chim Acta. 565(119983)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pezzuto A, Terzo F, Graziani ML, Ricci A,
Bruno P and Mariotta S: Lung cancer requires multidisciplinary
treatment to improve patient survival: A case report. Oncol Lett.
14:3035–3038. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hardavella G, Chorostowska-Wynimko J and
Blum TG: Lung cancer: An update on the multidisciplinary approach
from screening to palliative care. Breathe (Sheff).
20(240117)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McCurdy M, McAleer MF, Wei W, Ezhil M,
Johnson V, Khan M, Baker J, Luo D, Ajani J and Guerrero T:
Induction and concurrent taxanes enhance both the pulmonary
metabolic radiation response and the radiation pneumonitis response
in patients with esophagus cancer. Int J Radiat Oncol Biol Phys.
76:816–823. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Duan Y, Wang Y, Lu S, Zeng M, Liu L, Dai Q
and Yin R: Adverse event profile of albumin-bound paclitaxel: A
real-world pharmacovigilance analysis. Front Pharmacol.
15(1448144)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Minguet J, Smith KH and Bramlage P:
Targeted therapies for treatment of non-small cell lung
cancer-recent advances and future perspectives. Int J Cancer.
138:2549–2561. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wagner SA, Szczesniak PP, Voigt A, Gräf JF
and Beli P: Proteomic analysis of tyrosine phosphorylation induced
by exogenous expression of oncogenic kinase fusions identified in
lung adenocarcinoma. Proteomics. 21(e2000283)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang T, Wang G, Liu Y, Feng L, Wang M,
Liu J, Chen Y and Ouyang L: Development of small-molecule
tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion
cancers. Acta Pharm Sin B. 11:355–372. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schoenfeld AJ and Hellmann MD: Acquired
resistance to immune checkpoint inhibitors. Cancer Cell.
37:443–455. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lopez-Chavez A, Young T, Fages S, Leon L,
Schiller JH, Dowlati A, Brahmer JR, Johnson DH and Sandler A:
Bevacizumab maintenance in patients with advanced non-small-cell
lung cancer, clinical patterns, and outcomes in the Eastern
cooperative oncology group 4599 study: Results of an exploratory
analysis. J Thorac Oncol. 7:1707–1712. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
first-line carboplatin/paclitaxel plus bevacizumab or placebo in
Chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chu T, Zhang W, Zhang B, Zhong R, Zhang X,
Gu A, Shi C, Wang H, Xiong L, Lu J, et al: Efficacy and safety of
first-line anlotinib-based combinations for advanced non-small cell
lung cancer: A three-armed prospective study. Transl Lung Cancer
Res. 11:1394–1404. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Camerini A, Del Conte A, Pezzuto A, Scotti
V, Facchinetti F, Ciccone LP, Perna M, Sartori G, Puccetti C, Ricci
A, et al: Selection criteria and treatment outcome for advanced
non-small cell lung cancer (NSCLC) patients unfit for
platinum-based first-line therapy: Results of the MOON-OSS
observational trial. Cancers (Basel). 14(6074)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chan BA and Hughes BG: Targeted therapy
for non-small cell lung cancer: Current standards and the promise
of the future. Transl Lung Cancer Res. 4:36–54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marmarelis ME and Langer CJ: Treatment of
patients with non-small-cell lung cancer harboring rare oncogenic
mutations. Clin Lung Cancer. 21:395–406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cho BC, Lu S, Felip E, Spira AI, Girard N,
Lee JS, Lee SH, Ostapenko Y, Danchaivijitr P, Liu B, et al:
Amivantamab plus lazertinib in previously untreated EGFR-mutated
advanced NSCLC. N Engl J Med. 391:1486–1498. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou C, Tang KJ, Cho BC, Liu B, Paz-Ares
L, Cheng S, Kitazono S, Thiagarajan M, Goldman JW, Sabari JK, et
al: Amivantamab plus chemotherapy in NSCLC with EGFR Exon 20
insertions. N Engl J Med. 389:2039–2051. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang M, Yang JC, Mitchell PL, Fang J,
Camidge DR, Nian W, Chiu CH, Zhou J, Zhao Y, Su WC, et al:
Sunvozertinib, a selective EGFR inhibitor for previously treated
non-small cell lung cancer with EGFR exon 20 insertion mutations.
Cancer Discov. 12:1676–1689. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto
T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, et al: Phase II study
of crizotinib in east asian patients with ROS1-positive advanced
non-small-cell lung cancer. J Clin Oncol. 36:1405–1411.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dziadziuszko R, Krebs MG, De Braud F,
Siena S, Drilon A, Doebele RC, Patel MR, Cho BC, Liu SV, Ahn MJ, et
al: Updated integrated analysis of the efficacy and safety of
entrectinib in locally advanced or metastatic ROS1 fusion-positive
non-small-cell lung cancer. J Clin Oncol. 39:1253–1263.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Drilon A, Camidge DR, Lin JJ, Kim SW,
Solomon BJ, Dziadziuszko R, Besse B, Goto K, de Langen AJ, Wolf J,
et al: Repotrectinib in ROS1 fusion-positive non-small-cell lung
cancer. N Engl J Med. 390:118–131. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Drilon A, Subbiah V, Gautschi O, Tomasini
P, de Braud F, Solomon BJ, Tan DSW, Alonso G, Wolf J, Park K, et
al: Selpercatinib in patients With RET fusion-positive
non-small-cell lung cancer: Updated safety and efficacy from the
registrational LIBRETTO-001 phase I/II trial. J Clin Oncol.
41:385–394. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Griesinger F, Curigliano G, Thomas M,
Subbiah V, Baik CS, Tan DSW, Lee DH, Misch D, Garralda E, Kim DW,
et al: Safety and efficacy of pralsetinib in RET fusion-positive
non-small-cell lung cancer including as first-line therapy: Update
from the ARROW trial. Ann Oncol. 33:1168–1178. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li C, Nguyen V, Clark KN, Zahed T, Sharkas
S, Filipp FV and Boiko AD: Down-regulation of FZD3 receptor
suppresses growth and metastasis of human melanoma independently of
canonical WNT signaling. Proc Natl Acad Sci USA. 116:4548–4557.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Riely GJ, Smit EF, Ahn MJ, Felip E,
Ramalingam SS, Tsao A, Johnson M, Gelsomino F, Esper R, Nadal E, et
al: Phase II, open-label study of encorafenib plus binimetinib in
patients with BRAF(V600)-Mutant metastatic non-small-cell lung
cancer. J Clin Oncol. 41:3700–3711. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rocco D, Gravara LD, Palazzolo G and
Gridelli C: The treatment of a new entity in advanced non-small
cell lung cancer: MET exon 14 skipping mutation. Curr Med Chem.
31:3043–3056. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bourhis A, Remoué A and Uguen A: KRAS and
BRAF double mutations and functional classes of BRAF mutations in
non-small-cell lung cancers. Clin Lung Cancer. 21:e240–e242.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang G, Xu H, Yang Y, Zhang S, Xu F, Hao
X, Li J, Xing P, Hu X, Liu Y, et al: Pyrotinib combined with
apatinib for targeting metastatic non-small cell lung cancer with
HER2 alterations: A prospective, open-label, single-arm phase 2
study (PATHER2). BMC Med. 20(277)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo
H, Shi B, Liu J, He Z, Yu G, et al: Open-label, multicenter, phase
II study of RC48-ADC, a HER2-Targeting antibody-drug conjugate, in
patients with locally advanced or metastatic urothelial carcinoma.
Clin Cancer Res. 27:43–51. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dy GK, Govindan R, Velcheti V, Falchook
GS, Italiano A, Wolf J, Sacher AG, Takahashi T, Ramalingam SS,
Dooms C, et al: Long-Term outcomes and molecular correlates of
sotorasib efficacy in patients with pretreated KRAS G12C-mutated
non-small-cell lung cancer: 2-Year analysis of CodeBreaK 100. J
Clin Oncol. 41:3311–3317. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
de Langen AJ, Johnson ML, Mazieres J,
Dingemans AC, Mountzios G, Pless M, Wolf J, Schuler M, Lena H,
Skoulidis F, et al: Sotorasib versus docetaxel for previously
treated non-small-cell lung cancer with KRAS(G12C) mutation: A
randomised, open-label, phase 3 trial. Lancet. 401:733–746.
2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jänne PA, Riely GJ, Gadgeel SM, Heist RS,
Ou SI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E, et
al: Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C)
mutation. N Engl J Med. 387:120–131. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lorthiois E, Gerspacher M, Beyer KS,
Vaupel A, Leblanc C, Stringer R, Weiss A, Wilcken R, Guthy DA,
Lingel A, et al: JDQ443, a structurally novel, pyrazole-based,
covalent inhibitor of KRAS(G12C) for the treatment of solid tumors.
J Med Chem. 65:16173–16203. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shi Z, Weng J, Niu H, Yang H, Liu R, Weng
Y, Zhu Q, Zhang Y, Tao L, Wang Z, et al: D-1553: A novel KRAS(G12C)
inhibitor with potent and selective cellular and in vivo antitumor
activity. Cancer Sci. 114:2951–2960. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lassen U, Bokemeyer C, Garcia-Foncillas J,
Italiano A, Vassal G, Paracha N, Marian M, Chen Y, Linsell L and
Abrams K: prognostic value of neurotrophic tyrosine receptor kinase
gene fusions in solid tumors for overall survival: A systematic
review and meta-analysis. JCO Precis Oncol.
7(e2200651)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Doz F, van Tilburg CM, Geoerger B,
Højgaard M, Øra I, Boni V, Capra M, Chisholm J, Chung HC, DuBois
SG, et al: Efficacy and safety of larotrectinib in TRK
fusion-positive primary central nervous system tumors. Neuro Oncol.
24:997–1007. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Paz-Ares L, Barlesi F, Siena S, Ahn MJ,
Drilon A, Conley A, Rolfo C, Wolf J, Seto T, Doebele R, et al:
Patient-reported outcomes from STARTRK-2: A global phase II basket
study of entrectinib for ROS1 fusion-positive non-small-cell lung
cancer and NTRK fusion-positive solid tumours. ESMO Open.
6(100113)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu L, Wang F and Luo F: MET-targeted
therapies for the treatment of non-small-cell lung cancer: A
systematic review and meta-analysis. Front Oncol.
12(1013299)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang SR, Schultheis AM, Yu H, Mandelker D,
Ladanyi M and Büttner R: Precision medicine in non-small cell lung
cancer: Current applications and future directions. Semin Cancer
Biol. 84:184–198. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Izumi H, Matsumoto S, Liu J, Tanaka K,
Mori S, Hayashi K, Kumagai S, Shibata Y, Hayashida T, Watanabe K,
et al: The CLIP1-LTK fusion is an oncogenic driver in
non-small-cell lung cancer. Nature. 600:319–323. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Poole A, Karuppiah V, Hartt A, Haidar JN,
Moureau S, Dobrzycki T, Hayes C, Rowley C, Dias J, Harper S, et al:
Therapeutic high affinity T cell receptor targeting a KRAS(G12D)
cancer neoantigen. Nat Commun. 13(5333)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang H, Zhang Y, Zhu Y, Dong T and Liu Z:
Understanding the treatment response and resistance to targeted
therapies in non-small cell lung cancer: Clinical insights and
perspectives. Front Oncol. 14(1387345)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sini C, Tuzi A, Rossi G, Russo A and
Pezzuto A: Acquired resistance in oncogene-addicted non-small-cell
lung cancer. Future Oncol. 14:29–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wu J and Lin Z: Non-Small cell lung cancer
targeted therapy: Drugs and mechanisms of drug resistance. Int J
Mol Sci. 23(15056)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22(40)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hendriks LE, Kerr KM, Menis J, Mok TS,
Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, et
al: Non-oncogene-addicted metastatic non-small-cell lung cancer:
ESMO Clinical practice guideline for diagnosis, treatment and
follow-up. Ann Oncol. 34:358–376. 2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Levra MG, Cotté FE, Corre R, Calvet C,
Gaudin AF, Penrod JR, Grumberg V, Jouaneton B, Jolivel R, Assié JB
and Chouaïd C: Immunotherapy rechallenge after nivolumab treatment
in advanced non-small cell lung cancer in the real-world setting: A
national data base analysis. Lung Cancer. 140:99–106.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Brahmer JR, Lee JS, Ciuleanu TE, Caro RB,
Nishio M, Urban L, Audigier-Valette C, Lupinacci L, Sangha R,
Pluzanski A, et al: Five-Year survival outcomes with nivolumab plus
ipilimumab versus chemotherapy as first-line treatment for
metastatic non-small-cell lung cancer in CheckMate 227. J Clin
Oncol. 41:1200–1212. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Boyer M, Şendur MAN, Rodríguez-Abreu D,
Park K, Lee DH, Çiçin I, Yumuk PF, Orlandi FJ, Leal TA, Molinier O,
et al: Pembrolizumab plus ipilimumab or placebo for metastatic
non-small-cell lung cancer with PD-L1 tumor proportion score ≥50%:
Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol.
39:2327–2338. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cho BC, Abreu DR, Hussein M, Cobo M, Patel
AJ, Secen N, Lee KH, Massuti B, Hiret S, Yang JCH, et al:
Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a
first-line treatment for PD-L1-selected non-small-cell lung cancer
(CITYSCAPE): Primary and follow-up analyses of a randomised,
double-blind, phase 2 study. Lancet Oncol. 23:781–792.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Genova C, Dellepiane C, Carrega P,
Sommariva S, Ferlazzo G, Pronzato P, Gangemi R, Filaci G, Coco S
and Croce M: Therapeutic implications of tumor microenvironment in
lung cancer: Focus on immune checkpoint blockade. Front Immunol.
12(799455)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Datar I, Sanmamed MF, Wang J, Henick BS,
Choi J, Badri T, Dong W, Mani N, Toki M, Mejías LD, et al:
Expression analysis and significance of PD-1, LAG-3, and TIM-3 in
Human non-small cell lung cancer using spatially resolved and
multiparametric single-cell analysis. Clin Cancer Res.
25:4663–4673. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Qu J, Mei Q, Chen L and Zhou J: Chimeric
antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer
(NSCLC): Current status and future perspectives. Cancer Immunol
Immunother. 70:619–631. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pockley AG, Vaupel P and Multhoff G: NK
cell-based therapeutics for lung cancer. Expert Opin Biol Ther.
20:23–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Paijens ST, Vledder A, de Bruyn M and
Nijman HW: Tumor-infiltrating lymphocytes in the immunotherapy era.
Cell Mol Immunol. 18:842–859. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sadagopan A, Michelakos T, Boyiadzis G,
Ferrone C and Ferrone S: Human leukocyte antigen class I
antigen-processing machinery upregulation by anticancer therapies
in the era of checkpoint inhibitors: A review. JAMA Oncol.
8:462–473. 2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chowell D, Morris LGT, Grigg CM, Weber JK,
Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, et
al: Patient HLA class I genotype influences cancer response to
checkpoint blockade immunotherapy. Science. 359:582–587.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106(dju124)2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lee KW, Van Cutsem E, Bang YJ, Fuchs CS,
Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Chao J, et al:
Association of tumor mutational burden with efficacy of
pembrolizumab±chemotherapy as first-line therapy for gastric cancer
in the phase III KEYNOTE-062 study. Clin Cancer Res. 28:3489–3498.
2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Marei HE, Hasan A, Pozzoli G and
Cenciarelli C: Cancer immunotherapy with immune checkpoint
inhibitors (ICIs): Potential, mechanisms of resistance, and
strategies for reinvigorating T cell responsiveness when resistance
is acquired. Cancer Cell Int. 23(64)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Dotsu Y, Muraoka D, Ogo N, Sonoda Y, Yasui
K, Yamaguchi H, Yagita H, Mukae H, Asai A and Ikeda H: Chemical
augmentation of mitochondrial electron transport chains tunes T
cell activation threshold in tumors. J Immunother Cancer.
10(e003958)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hong Y, Robbins Y, Yang X, Mydlarz WK,
Sowers A, Mitchell JB, Gulley JL, Schlom J, Gameiro SR, Sievers C
and Allen CT: Cure of syngeneic carcinomas with targeted IL-12
through obligate reprogramming of lymphoid and myeloid immunity.
JCI Insight. 7(e157448)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Liu Y, Feng C, Zhou Y, Shao X and Chen M:
Simulating the dynamic intra-tumor heterogeneity and therapeutic
responses. Cancers (Basel). 14(1645)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sharma P, Hu-Lieskovan S, Wargo JA and
Ribas A: Primary, adaptive, and acquired resistance to cancer
immunotherapy. Cell. 168:707–723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhao C, Zhang R, Yang H, Gao Y, Zou Y and
Zhang X: Antibody-drug conjugates for non-small cell lung cancer:
Advantages and challenges in clinical translation. Biochem
Pharmacol. 226(116378)2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Meric-Bernstam F, Makker V, Oaknin A, Oh
DY, Banerjee S, González-Martín A, Jung KH, Ługowska I, Manso L,
Manzano A, et al: Efficacy and safety of trastuzumab deruxtecan in
patients With HER2-expressing solid tumors: Primary results from
the DESTINY-PanTumor02 phase II Trial. J Clin Oncol. 42:47–58.
2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Shimizu T, Sands J, Yoh K, Spira A, Garon
EB, Kitazono S, Johnson ML, Meric-Bernstam F, Tolcher AW, Yamamoto
N, et al: First-in-human, phase I dose-escalation and
dose-expansion study of trophoblast cell-surface antigen 2-directed
antibody-drug conjugate datopotamab deruxtecan in non-small-cell
lung cancer: TROPION-PanTumor01. J Clin Oncol. 41:4678–4687.
2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Smit EF, Felip E, Uprety D, Nagasaka M,
Nakagawa K, Rodríguez LPA, Pacheco JM, Li BT, Planchard D, Baik C,
et al: Trastuzumab deruxtecan in patients with metastatic
non-small-cell lung cancer (DESTINY-Lung01): Primary results of the
HER2-overexpressing cohorts from a single-arm, phase 2 trial.
Lancet Oncol. 25:439–454. 2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Goto K, Goto Y, Kubo T, Ninomiya K, Kim
SW, Planchard D, Ahn MJ, Smit EF, de Langen AJ, Pérol M, et al:
Trastuzumab deruxtecan in patients with HER2-mutant metastatic
non-small-cell lung cancer: Primary results from the randomized,
phase II DESTINY-lung02 Trial. J Clin Oncol. 41:4852–4863.
2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cheng Y, Wu L, Fang Y, Fan Y, Li X, Zhang
M, Yu Y, Yao Y, Xu R, Guo J, et al: Trastuzumab deruxtecan (T-DXd)
in Chinese patients (pts) with previously treated HER2 mutant
non-small cell lung cancer (NSCLC): Primary analysis from the Phase
2 DESTINY-Lung05 (DL-05) trial. Cancer Res. 84(CT248)2024.
|
|
77
|
Camidge DR, Bar J, Horinouchi H, Goldman
J, Moiseenko F, Filippova E, Cicin I, Ciuleanu T, Daaboul N, Liu C,
et al: Telisotuzumab vedotin monotherapy in patients with
previously treated c-Met Protein-overexpressing advanced
nonsquamous EGFR-wildtype non-small cell lung cancer in the phase
II LUMINOSITY trial. J Clin Oncol. 42:3000–3011. 2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yu HA, Goto Y, Hayashi H, Felip E, Yang
JCH, Reck M, Yoh K, Lee SH, Paz-Ares L, Besse B, et al:
HERTHENA-Lung01, a phase II trial of patritumab deruxtecan
(HER3-DXd) in epidermal growth factor receptor-mutated
non-small-cell lung cancer after epidermal growth factor receptor
tyrosine kinase inhibitor therapy and platinum-based chemotherapy.
J Clin Oncol. 41:5363–5375. 2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ma Y, Huang Y, Zhao Y, Zhao S, Xue J, Yang
Y, Fang W, Guo Y, Han Y, Yang K, et al: BL-B01D1, a first-in-class
EGFR-HER3 bispecific antibody-drug conjugate, in patients with
locally advanced or metastatic solid tumours: A first-in-human,
open-label, multicentre, phase 1 study. Lancet Oncol. 25:901–911.
2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hellmann MD, Nathanson T, Rizvi H, Creelan
BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB,
Abu-Akeel M, et al: Genomic features of response to combination
immunotherapy in patients with advanced non-small-cell lung cancer.
Cancer Cell. 33:843–852.e844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Gordon LG, Merollini KMD, Lowe A and Chan
RJ: A systematic review of financial toxicity among cancer
survivors: We can't pay the co-pay. Patient. 10:295–309.
2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Fukuda A and Okuma Y: From rarity to
reality: Osimertinib's promising horizon in treating uncommon EGFR
mutations in non-small cell lung cancer. Clin Cancer Res.
30:3128–3136. 2024.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Bortolot M, Cortiula F, Fasola G, De
Ruysscher D, Naidoo J and Hendriks LEL: Treatment of unresectable
stage III non-small cell lung cancer for patients who are
under-represented in clinical trials. Cancer Treat Rev.
129(102797)2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Mittal A, Moore S, Navani V, Jiang DM,
Stewart DJ, Liu G and Wheatley-Price P: What is ailing oncology
clinical trials? Can we fix them? Curr Oncol. 31:3738–3751.
2024.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Chua AV Jr, Delmerico J, Sheng H, Huang
XW, Liang E, Yan L, Gandhi S, Puzanov I, Jain P, Sakoda LC, et al:
Under-Representation and under-reporting of minoritized racial and
ethnic groups in clinical trials on immune checkpoint inhibitors.
JCO Oncol Pract. 22(OP2400033)2024.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Waqar SN and Govindan R: Novel therapies
in cancer: Trials and tribulations. Clin Cancer Res. 30:3655–3657.
2024.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Meyer ML, Fitzgerald BG, Paz-Ares L,
Cappuzzo F, Jänne PA, Peters S and Hirsch FR: New promises and
challenges in the treatment of advanced non-small-cell lung cancer.
Lancet. 404:803–822. 2024.PubMed/NCBI View Article : Google Scholar
|