Introduction
Pancreatic cancer is one of the solid cancers with
the poorest prognoses, and its incidence has more than doubled in
the past 25 years (1). Currently,
the incidence and mortality of patients with pancreatic cancer are
increasing worldwide (2). Although
much of this increase is due to aging, there are other risk factors
for pancreatic cancer, including smoking, obesity, diabetes, and
alcohol consumption (3).
Considering that pancreatic cancer is often diagnosed at an
advanced stage, its 5-year survival rate is low, ranging from 2 to
9% (4). Only approximately 20% of
patients are diagnosed at a stage where surgical resection is
possible, and the 5-year survival rate of patients who undergo
surgical resection is approximately 15-25% (3). CA19-9 is a valuable tumor marker for
evaluating pancreatic cancer (5).
However, because it may be affected by obstructive jaundice and
cholangitis, CA19-9 levels alone may be insufficient and further
biomarkers are needed.
Preoperative nutritional status has been shown to
affect the survival of patients with pancreatic cancer (4,6,7).
Prealbumin, a 55 kDa homotetrameric protein, is primarily
synthesized in the liver, the primary site of its production, and
is found in the blood. It is also known as transthyretin because of
its role in transporting thyroid hormones, including thyroxine (T4)
and triiodothyronine (T3), as well as holo-retinol-binding protein,
a complex of retinol-binding protein and vitamin A. Prealbumin has
a circulating half-life of approximately 2 days, which is shorter
than that of albumin, which is approximately 20 days. Therefore,
prealbumin is superior for assessing short-term changes in the
body's nutritional status and may be a more sensitive nutritional
indicator than albumin (8,9). Low prealbumin levels may be a risk
factor for survival in patients with gastric cancer (10) and hepatocellular carcinoma
(11). The C-reactive
protein-to-prealbumin ratio (12),
fibrinogen-to-prealbumin ratio (13), and prealbumin as a factor in
prognostic scoring systems (14)
have been reported in pancreatic cancer. However, reports
evaluating the prognostic significance of prealbumin itself in
pancreatic cancer are lacking.
Therefore, in the present study, we aimed to assess
the clinicopathologic and prognostic significance of preoperative
prealbumin level in patients with pancreatic cancer.
Materials and methods
Patients
This retrospective study included 95 patients (49
male and 46 female patients) with a median age of 73 years (range:
33-87 years) who were diagnosed with pancreatic cancer and
underwent radical resection between 2011 and 2021 in Toho
University Omori Medical Center. The present study was approved by
the Ethics Committee of Toho University Omori Medical Center
(approval nos. M23174, 21320, 21039, 20200, 20196, 19056 and 18002)
and conducted following the guidelines stipulated in the
Declaration of Helsinki. Information about the study was disclosed
on the institutional website, and potential participants were free
to opt out; those who did not opt out were included and those who
did opt out were excluded. We accessed the medical records of the
patients for the purpose of this specific study in August 2024. The
following clinicopathologic factors were included to evaluate their
association with preoperative prealbumin levels: sex, age, body
mass index (BMI), tumor depth, lymph node status, white blood cell
counts, platelet counts, and C-reactive protein (CRP) levels.
Pathological findings were determined using the Japanese
Classification of Pancreatic Cancer, 8th edition, based on the
tumor-node-metastasis classification (15). This study examined cutoff values
based on median, mean, and quartiles. We chose the median as the
cutoff value because it is more stable and easier to interpret. The
median preoperative prealbumin level of 21.1 mg/dl was considered
the cutoff value for all patients. Based on the cutoff value, the
patients were categorized into low and high prealbumin groups to
evaluate the association of preoperative prealbumin levels with
clinicopathologic factors, overall survival (OS), and
recurrence-free survival (RFS). OS was defined as the interval from
the date of surgery to the date of death or last follow-up, and RFS
was defined as the interval from the date of surgery to the date of
known recurrence.
Statistical analysis
Unpaired Student's t-test and Fisher's exact
probability test were used for two-group comparisons. Pearson's
correlation coefficient was used to evaluate the correlation
between the two groups. OS and RFS were calculated using the
Kaplan-Meier method, and differences between groups were evaluated
using the log-rank test. Multivariate analyses were performed using
Cox proportional hazards regression. All statistical analyses were
performed using EZR version 1.68(16). Two-sided P<0.05 was considered
to indicate a statistically significant difference.
Results
Association between preoperative
prealbumin levels and clinicopathologic factors
The prealbumin levels according to clinicopathologic
factors are shown in Fig. 1. Among
the clinicopathologic factors, patients with high CRP levels had
significantly lower prealbumin levels than those with low CRP
levels (P=0.007).
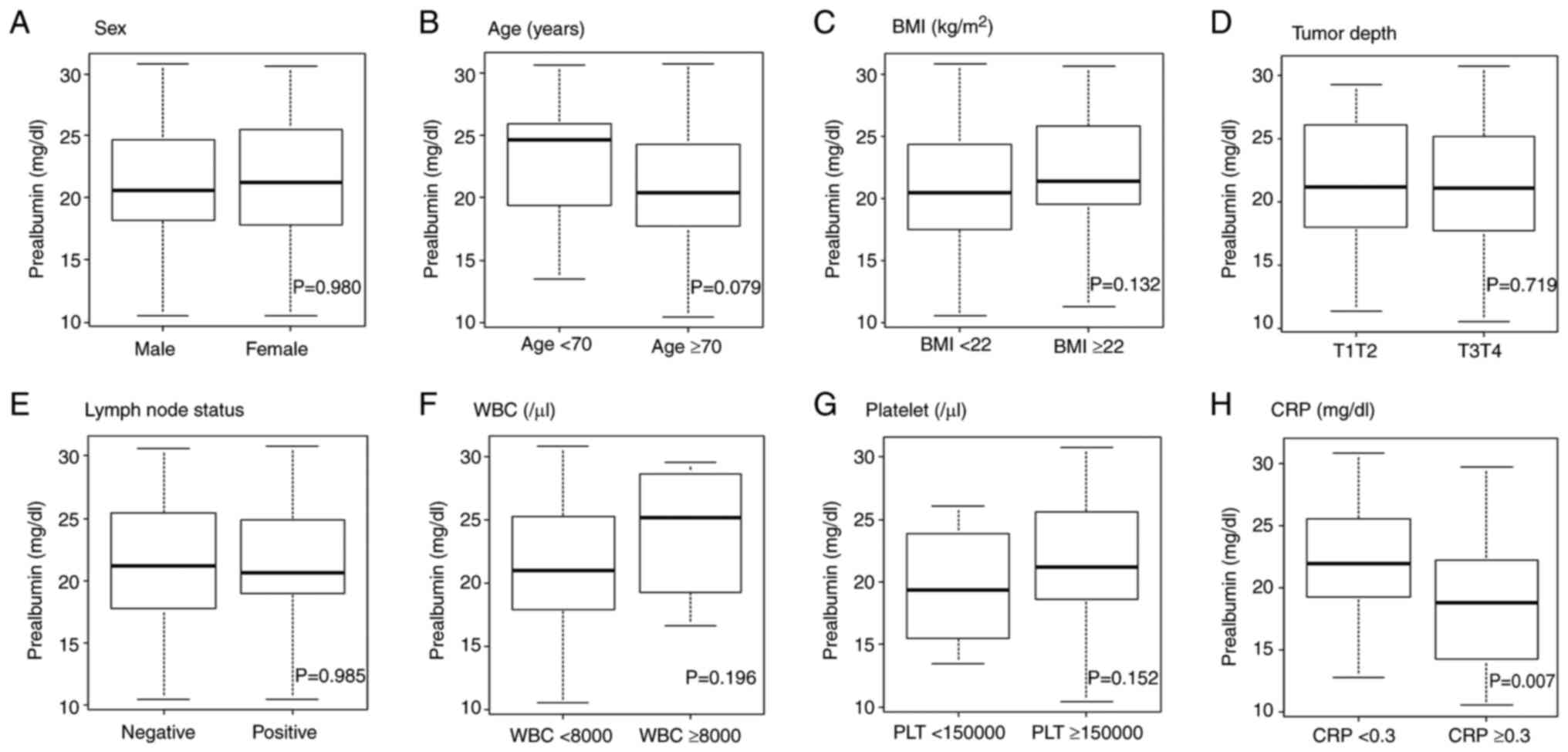 | Figure 1Comparison of prealbumin levels in
patients split according to clinicopathological factors. (A) Sex,
(B) age, (C) BMI, (D) tumor depth, (E) lymph node status, (F) WBC,
(G) platelet, (H) CRP. Data were analyzed using unpaired Student's
t-test. BMI, body mass index; CRP, C-reactive protein; WBC, white
blood cell. |
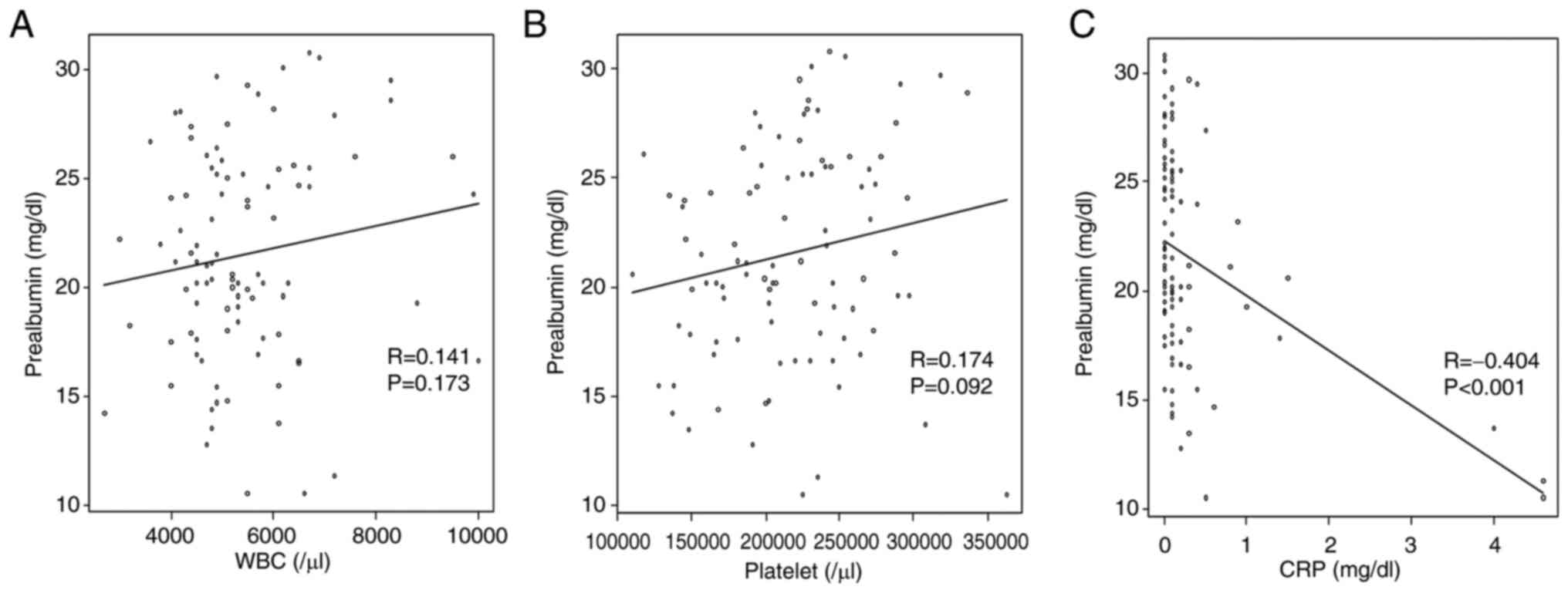
Association between preoperative
prealbumin levels and blood collection items
The prealbumin levels based on white blood cell
counts, platelet counts, and CRP levels are shown in Fig. 2. There was a moderate negative
correlation between preoperative prealbumin and CRP levels
(R=-0.404, P<0.001).
Association of clinicopathologic
factors between low and high prealbumin groups
The patients were divided into the following two
groups based on the median preoperative prealbumin levels: low
(<21.1 mg/dl, n=47) and high (≥21.1 mg/dl, n=48) groups.
Table I shows the association
between clinicopathologic factors and prealbumin levels. No
clinicopathologic factors were associated with low prealbumin
levels.
 | Table IClinicopathologic factors of patients
with pancreatic cancer. |
Table I
Clinicopathologic factors of patients
with pancreatic cancer.
| Variable | Number of patients
(n=95) | Low prealbumin group
<21.1 mg/dl (n=47) | High prealbumin group
≥21.1 mg/dl (n=48) | P-valuea |
|---|
| Sex | | | | 0.838 |
|
Male | 49 | 25 | 24 | |
|
Female | 46 | 22 | 24 | |
| Age, years | | | | 0.079 |
|
<70 | 31 | 11 | 20 | |
|
≥70 | 64 | 36 | 28 | |
| BMI,
kg/m2 | | | | 0.307 |
|
<22 | 49 | 27 | 22 | |
|
≥22 | 46 | 20 | 26 | |
| Tumor depth | | | | >0.999 |
|
T1T2 | 21 | 10 | 11 | |
|
T3T4 | 74 | 37 | 37 | |
| Lymph node
status | | | | 0.516 |
|
Negative | 64 | 30 | 34 | |
|
Positive | 31 | 17 | 14 | |
| White blood cell,
/µl | | | | 0.677 |
|
<8,000 | 89 | 45 | 44 | |
|
≥8,000 | 6 | 2 | 4 | |
| Platelet, /µl | | | | 0.552 |
|
<150,000 | 12 | 7 | 5 | |
|
≥150,000 | 83 | 40 | 43 | |
| CRP, mg/dl | | | | 0.137 |
|
<0.3 | 75 | 34 | 41 | |
|
≥0.3 | 20 | 13 | 7 | |
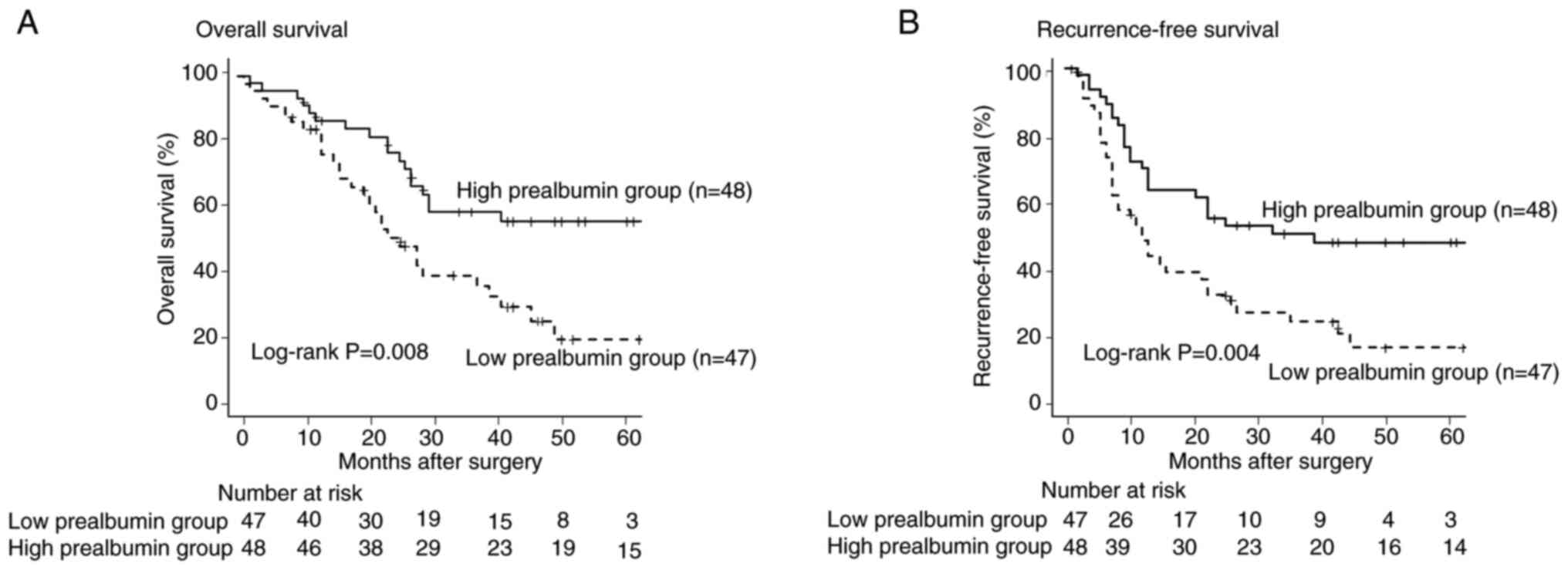
Comparison of overall survival between
low and high prealbumin groups
The OS of the low prealbumin group was significantly
lower than that of the high prealbumin group (P=0.008, Fig. 3A). Univariate analysis revealed
that OS was significantly lower in patients with low prealbumin
levels, positive lymph node status, and high CRP levels than that
in patients with high prealbumin levels, negative lymph node
status, and low CRP levels (P<0.05, Table II left panel). Multivariate
analysis revealed that low prealbumin levels, positive lymph node
status, and high CRP levels were independent poor prognostic
factors for OS (P<0.05, Table
II right panel).
 | Table IIUnivariate and multivariate analysis
of clinicopathological factors for predicting overall survival of
patients with pancreatic cancer. |
Table II
Univariate and multivariate analysis
of clinicopathological factors for predicting overall survival of
patients with pancreatic cancer.
| Variable | Number of patients
(n=95) |
P-valuea | Multivariate
analysis Hazard ratio (95% confidence interval) |
P-valueb |
|---|
| Sex | | 0.424 | | |
|
Male | 49 | | | |
|
Female | 46 | | | |
| Age, years | | 0.059 | | |
|
≥70 | 64 | | | |
|
<70 | 31 | | | |
| BMI,
kg/m2 | | 0.668 | | |
|
≥22 | 46 | | | |
|
<22 | 49 | | | |
| Tumor depth | | 0.632 | | |
|
T3T4 | 74 | | | |
|
T1T2 | 21 | | | |
| Lymph node
status | | 0.026 | 1.800
(1.011-3.205) | 0.046 |
|
Positive | 31 | | | |
|
Negative | 64 | | | |
| White blood cell,
/µl | | 0.789 | | |
|
≥8,000 | 6 | | | |
|
<8,000 | 89 | | | |
| Platelet, /µl | | 0.308 | | |
|
<150,000 | 12 | | | |
|
≥150,000 | 83 | | | |
| CRP, mg/dl | | 0.001 | 2.588
(1.377-4.864) | 0.003 |
|
≥0.3 | 20 | | | |
|
<0.3 | 75 | | | |
| Prealbumin,
mg/dl | | 0.008 | 1.974
(1.095-3.559) | 0.024 |
|
<21.1 | 47 | | | |
|
≥21.1 | 48 | | | |
Comparison of recurrence-free survival
between low and high prealbumin groups
The RFS of the low prealbumin group was
significantly lower than that of the high prealbumin group
(P=0.004, Fig. 3B). Univariate
analysis revealed that RFS was significantly lower in patients with
low preoperative prealbumin levels, positive lymph node status, and
high CRP levels than that in patients with high prealbumin levels,
negative lymph node status, and low CRP levels (P<0.05, Table III left panel). Multivariate
analysis revealed that low preoperative prealbumin levels, positive
lymph node status, and high CRP levels were independent poor
prognostic factors for RFS (P<0.05, Table III right panel).
 | Table IIIUnivariate and multivariate analysis
of clinicopathological factors for predicting recurrence-free
survival of patients with pancreatic cancer. |
Table III
Univariate and multivariate analysis
of clinicopathological factors for predicting recurrence-free
survival of patients with pancreatic cancer.
| Variable | Number of patients
(n=95) |
P-valuea | Multivariate
analysis Hazard ratio (95% confidence interval) |
P-valueb |
|---|
| Sex | | 0.412 | | |
|
Male | 49 | | | |
|
Female | 46 | | | |
| Age, years | | 0.203 | | |
|
≥70 | 64 | | | |
|
<70 | 31 | | | |
| BMI,
kg/m2 | | 0.169 | | |
|
≥22 | 46 | | | |
|
<22 | 49 | | | |
| Tumor depth | | 0.091 | | |
|
T3T4 | 74 | | | |
|
T1T2 | 21 | | | |
| Lymph node
status | | 0.028 | 1.922
(1.144-3.231) | 0.014 |
|
Positive | 31 | | | |
|
Negative | 64 | | | |
| White blood cell,
/µl | | 0.618 | | |
|
≥8,000 | 6 | | | |
|
<8,000 | 89 | | | |
| Platelet, /µl | | 0.686 | | |
|
<150,000 | 12 | | | |
|
≥150,000 | 83 | | | |
| CRP, mg/dl | | 0.003 | 2.251
(1.254-4.041) | 0.007 |
|
≥0.3 | 20 | | | |
|
<0.3 | 75 | | | |
| Prealbumin,
mg/dl | | 0.004 | 1.931
(1.146-3.255) | 0.013 |
|
<21.1 | 47 | | | |
|
≥21.1 | 48 | | | |
Comparison of clinicopathologic
factors between patients with and without recurrence
The comparison of clinicopathologic factors between
patients with and without recurrence during the postoperative
observation period showed that those with recurrence had
significantly lower prealbumin levels (P=0.033) and were more
likely to have a positive lymph node status (P=0.022) (Table IV).
 | Table IVComparison of the clinicopathological
factors between the patients with and without recurrence. |
Table IV
Comparison of the clinicopathological
factors between the patients with and without recurrence.
| Variable | Number of patients
(n=95) | Recurrence group
(n=60) | Non-recurrence
group (n=35) |
P-valuea |
|---|
| Sex | | | | 0.209 |
|
Male | 49 | 34 | 15 | |
|
Female | 46 | 26 | 20 | |
| Age, years | | | | 0.264 |
|
≥70 | 64 | 43 | 21 | |
|
<70 | 31 | 17 | 14 | |
| BMI,
kg/m2 | | | | 0.209 |
|
≥22 | 46 | 26 | 20 | |
|
<22 | 49 | 34 | 15 | |
| Tumor depth | | | | 0.125 |
|
T3T4 | 74 | 50 | 24 | |
|
T1T2 | 21 | 10 | 11 | |
| Lymph node
status | | | | 0.022 |
|
Positive | 31 | 25 | 6 | |
|
Negative | 64 | 35 | 29 | |
| White blood cell,
/µl | | | | >0.999 |
|
≥8,000 | 6 | 4 | 2 | |
|
<8,000 | 89 | 56 | 33 | |
| Platelet, /µl | | | | >0.999 |
|
<150,000 | 12 | 8 | 4 | |
|
≥150,000 | 83 | 52 | 31 | |
| CRP, mg/dl | | | | 0.117 |
|
≥0.3 | 20 | 16 | 4 | |
|
<0.3 | 75 | 44 | 31 | |
| Prealbumin,
mg/dl | | | | 0.033 |
|
<21.1 | 47 | 35 | 12 | |
|
≥21.1 | 48 | 25 | 23 | |
Comparison of recurrence sites between
low and high prealbumin groups
Recurrence rates in the liver (P=0.038) and
peritoneum (P=0.012) were higher in the low prealbumin group than
those in the high prealbumin group (Table V).
 | Table VComparison of the recurrence site
between the low and high prealbumin groups; all cases (n=95). |
Table V
Comparison of the recurrence site
between the low and high prealbumin groups; all cases (n=95).
| Recurrence
site | Number | Low prealbumin
group (<21.1 mg/dl) (n=47) | High prealbumin
group (≥21.1 mg/dl) (n=48) |
P-valuea |
|---|
| Lung | 12 | 6 | 6 | >0.999 |
| Liver | 25 | 17 | 8 | 0.038 |
| Local site | 24 | 16 | 8 | 0.062 |
| Lymph node | 10 | 7 | 3 | 0.199 |
| Peritoneal | 20 | 15 | 5 | 0.012 |
| Bone | 5 | 2 | 3 | >0.999 |
Discussion
The OS and RFS were significantly lower in the low
prealbumin group than in the high prealbumin group. Low prealbumin
levels were an independent risk factor for poor OS and RFS.
There is no consensus on the cutoff value of
prealbumin levels in patients with pancreatic cancer. In the
present study, because the median (21.1 mg/dl) and mean (21.4
mg/dl) values were highly similar, we chose the median as the
cutoff value. The normal prealbumin levels range from 22.0 to 40.0
mg/dl. The cutoff values for gastric cancer and hepatocellular
carcinoma are 20(10) and
17(11) mg/dl, respectively, and
the cutoff value in the prognostic scoring system for pancreatic
cancer is 23(14) mg/dl.
Therefore, the cutoff value used in the present study was
reasonable.
No clinicopathologic factors were associated with
low prealbumin levels. However, low prealbumin levels were weakly
associated with old age and high CRP levels. Prealbumin is a marker
of nutritional status, and malnutrition is closely associated with
old age (17). A low prealbumin
level is also a marker reflecting acute inflammation (9). Park et al (18) reported that acute inflammation
increases CRP levels and decreases prealbumin synthesis in the
liver. Consequently, low prealbumin levels may correlate with high
CRP levels.
In this study, we minimized the effects of
preoperative inflammation, liver dysfunction, and malnutrition by
waiting until the patient was ready to undergo surgery. In
addition, only a few patients had diseases that could potentially
affect prealbumin levels, such as amyloid transthyretin
amyloidosis, viral hepatitis, and autoimmune diseases. However,
since prealbumin is a negative acute-phase protein, the possibility
of a potential bias due to a decrease in its concentration due to
inflammatory changes cannot be denied. In past reports, including
other cancers, the effects of these conditions have not been
examined, so further research is needed.
Low prealbumin level was an independent risk factor
for poor OS and RFS. There are two main reasons for this
association. First, prealbumin levels are linked to tumor
progression. Cancer cells require more energy than normal cells to
proliferate, and they actively absorb nutrients such as sugar and
amino acids. Thus, nutrients in the body are preferentially
consumed by cancer cells, resulting a deterioration in the
nutritional status and a decrease in prealbumin levels (19). Second, low prealbumin levels are
associated with decreased anti-tumor immunity. A decline in
nutritional status causes a decrease in the function of immune
cells, such as T cells, lymphocytes, macrophages, and natural
killer cells, weakening the immune surveillance mechanism against
cancer cells and making it easier for cancer cells to proliferate
further (9). Therefore, patients
with low prealbumin levels are at risk of deteriorating health
conditions that can lead to tumor recurrence and metastasis after
surgery.
In the present study, the hazard ratios for OS and
RFS were similar, indicating that low prealbumin levels mainly
affect the risk of recurrence and poor treatment response after
recurrence. Chemotherapy in malnourished patients decreases
treatment continuity and efficacy (20). Mękal et al (21) reported the importance of early
nutritional intervention for improving the treatment outcomes in
patients with pancreatic cancer. The short half-life of prealbumin
(2-3 days) facilitates the early assessment of the effects of
nutritional supplementation and changes in nutritional status.
Therefore, monitoring prealbumin levels may help assess patients'
nutritional status and improvement in OS and RFS.
This study excluded CRP, albumin, and CA19-9 from
the multivariate analysis because they were confounding factors
with prealbumin. However, in the report by Liang et al
(14), the multivariate analysis
included prealbumin and clinical pathological factors such as CRP,
albumin, and CA19-9 in creating the prognostic nutritional score
for pancreatic cancer patients. As a result, low prealbumin levels
were an independent poor prognostic factor for OS and RFS (14). The population in this study
comprised 95 patients, while the population in the study by Liang
et al included 621 patients, indicating a significant
difference in sample size. We think this is one reason for the
differences in results. It is possible that the same results could
have been obtained in this study if the population was the same as
in the study by Liang et al. The limitation of this study is
that it involved a small group. Therefore, we aim to increase the
number of cases and re-examine the results in the future through a
multi-center study.
Low prealbumin levels may be associated with liver
metastasis recurrence and peritoneal dissemination. Liver
metastasis and peritoneal dissemination are the most common types
of pancreatic cancer recurrence and are considered poor prognostic
factors (22). Therefore, it is
important to monitor the patients, and attention should be paid to
distant metastases, particularly liver metastases and peritoneal
dissemination, in patients with low prealbumin levels before
surgery.
The present study has several limitations. First,
since it is a new finding that low prealbumin levels are a poor
prognostic factor for pancreatic cancer, there is a lack of
mechanistic insight, a lack of generalizability, and a lack of a
validation cohort. Second, to understand the importance of changes
in prealbumin levels over time, it is essential to consider
postoperative prealbumin levels. Unfortunately, our study only
measured preoperative prealbumin levels and did not include
postoperative prealbumin levels. Finally, the sample size for this
single-center study was relatively small. Other research
institutions need to verify our results, and further large-scale
multicenter prospective studies are required. We aim to resolve
these limitations through future multicenter research.
In conclusion, low prealbumin levels may serve as a
biomarker of poor prognosis in patients with surgically treated
pancreatic cancer. Identifying patients with low prealbumin levels
may help determine cases with poor nutritional status. Enhancing
the nutritional status by monitoring prealbumin levels may improve
OS and RFS in patients with pancreatic cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YM and HS designed this study. YM, YO, HH, YK, RO,
YI, KK, TM and MT were involved in the study conception, design and
data collection. YM and HS wrote the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Toho University Omori Medical Center (approval nos.
M23174, 21320, 21039, 20200, 20196, 19056 and 18002), and we
provided a means of opting out for patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2017 Pancreatic Cancer Collaborators.
The global, regional, and national burden of pancreatic cancer and
its attributable risk factors in 195 countries and territories,
1990-2017: A systematic analysis for the global burden of disease
study 2017. Lancet Gastroenterol Hepatol. 4:934–947.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu JX, Zhao CF, Chen WB, Liu QC, Li QW,
Lin YY and Gao F: Pancreatic cancer: A review of epidemiology,
trend, and risk factors. World J Gastroenterol. 27:4298–4321.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Klein AP: Pancreatic cancer epidemiology:
Understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Z and Tian YP: Clinical value of
serum tumor markers CA19-9, CA125 and CA72-4 in the diagnosis of
pancreatic carcinoma. Mol Clin Oncol. 2:265–268. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kato Y, Yamada S, Suenaga M, Takami H,
Niwa Y, Hayashi M, Iwata N, Kanda M, Tanaka C, Nakayama G, et al:
Impact of the controlling nutritional status score on the prognosis
after curative resection of pancreatic ductal adenocarcinoma.
Pancreas. 47:823–829. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sakamoto T, Kishino M, Murakami Y,
Miyatani K, Hanaki T, Shishido Y, Kihara K, Matsunaga T, Yamamoto
M, Tokuyasu N and Fujiwara Y: The cachexia index is a prognostic
factor for patients with recurrent pancreatic cancer. Surg Today.
54:1498–1504. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Keller U: Nutritional laboratory markers
in malnutrition. J Clin Med. 8(775)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ranasinghe RN, Biswas M and Vincent RP:
Prealbumin: The clinical utility and analytical methodologies. Ann
Clin Biochem. 59:7–14. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aoyama T, Nakazono M, Segami K, Nagasawa
S, Kano K, Hara K, Maezawa Y, Hashimoto I, Suematsu H, Watanabe H,
et al: Clinical significance of the prealbumin level in gastric
cancer patients who receive curative treatment. J Gastrointest
Cancer. 54:27–34. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li JD, Xu XF, Han J, Wu H, Xing H, Li C,
Yu JJ, Zhou YH, Gu WM, Wang H, et al: Preoperative prealbumin level
as an independent predictor of long-term prognosis after liver
resection for hepatocellular carcinoma: A multi-institutional
study. HPB (Oxford). 21:157–166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kwon CH, Seo HI, Kim DU, Han SY, Kim S,
Lee NK, Hong SB, Ahn JH, Park YM and Noh BG: Clinical significance
of C-reactive protein-to-prealbumin ratio in predicting early
recurrence in resectable pancreatic cancer. Korean J Clin Oncol.
19:11–17. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li C, Fan Z, Guo W, Liang F, Mao X, Wu J,
Wang H, Xu J, Wu D, Liu H, et al: Fibrinogen-to-prealbumin ratio: A
new prognostic marker of resectable pancreatic cancer. Front Oncol.
13(1149942)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liang Y, Guo H, Man Q, Chang S, Wang E and
Gao S: Prognostic nutritional score based on pretreatment
lymphocyte, platelet, and prealbumin predicts prognosis in patients
with pancreatic cancer. J Surg Oncol. 128:831–843. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ishida M, Fujii T, Kishiwada M, Shibuya K,
Satoi S, Ueno M, Nakata K, Takano S, Uchida K, Ohike N, et al:
Japanese classification of pancreatic carcinoma by the Japan
pancreas society: Eighth edition. J Hepatobiliary Pancreat Sci.
31:755–768. 2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Z, Pereira SL, Luo M and Matheson
EM: Evaluation of blood biomarkers associated with risk of
malnutrition in older adults: A systematic review and
meta-analysis. Nutrients. 9(829)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park YM, Seo HI, Noh BG, Kim S, Hong SB,
Lee NK, Kim DU and Han SY: Clinical impact of serum prealbumin in
pancreaticobiliary disease. Korean J Clin Oncol. 18:61–65.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jabłońska B, Pawlicki K and Mrowiec S:
Associations between nutritional and immune status and
clinicopathologic factors in patients with pancreatic cancer: A
comprehensive analysis. Cancers (Basel). 13(5041)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cintoni M, Grassi F, Palombaro M,
Rinninella E, Pulcini G, Di Donato A, Salvatore L, Quero G, Tortora
G, Alfieri S, et al: Nutritional interventions during chemotherapy
for pancreatic cancer: A systematic review of prospective studies.
Nutrients. 15(727)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mękal D, Sobocki J, Badowska-Kozakiewicz
A, Sygit K, Cipora E, Bandurska E, Czerw A and Deptała A:
Evaluation of nutritional status and the impact of nutritional
treatment in patients with pancreatic cancer. Cancers (Basel).
15(3816)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang L, Jin R, Yang X and Ying D: A
population-based study of synchronous distant metastases and
prognosis in patients with PDAC at initial diagnosis. Front Oncol.
13(1087700)2023.PubMed/NCBI View Article : Google Scholar
|