Introduction
Monomorphic epitheliotropic intestinal T-cell
lymphoma (MEITL), previously designated as type 2
enteropathy-associated T-cell lymphoma (EATL), is an uncommon
primary intestinal lymphoma originating from intestinal
intraepithelial T lymphocytes (1,2).
Unlike classical EATL, MEITL is not associated with celiac disease
and is more frequently seen in East Asian populations.
Histologically, MEITL is characterized by medium-sized monomorphic
lymphocytes with a cytotoxic immunophenotype, typically
CD3+, CD4-, CD8+ and
TIA-1+ (2,3). MEITL is characterized by rapid
progression and poor prognosis, attributed to therapeutic
resistance and complications such as intestinal perforation or
obstruction amid treatment, with a 5-year survival rate of
approximately 20% and a 5-year relapse-free survival rate of only
4% (1). Treatment is challenging
as chemotherapy alone is rarely curative, although autologous
transplantation combined with high-dose chemotherapy has been shown
to be effective (2,3).
MEITL lesions are predominantly located in the
jejunum and ileum and typically manifest as gastrointestinal
perforation or obstruction (1-3).
Prior studies have documented metastatic lesions in the central
nervous system, liver, and spleen, whereas no cases of gallbladder
involvement have been reported (4). Herein, we present a case of MEITL
with concurrent intestinal and gallbladder involvement, and
highlights the diagnostic and therapeutic relevance of surgical
resection.
Case report
A 57-year-old woman with a 2-week history of
abdominal distention was admitted to Otsu Red Cross Hospital (Otsu,
Japan) on January 2023, owing to abrupt-onset abdominal pain and
vomiting. On physical examination, she had notable abdominal
distension and pronounced tenderness. She was bedridden, and her
Eastern Cooperative Oncology Group (ECOG) performance status was 3.
Laboratory tests revealed leukocytopenia (white blood cell count,
2,600/µl; neutrophils, 85%; lymphocytes, 10%; monocytes, 5.0%;
eosinophils, 0%; basophils. 0%), but no abnormal lymphocytes were
detected on the peripheral blood smear. Thrombocytosis
(47.4x104/µl), low total protein (6.1 g/dl),
hypoalbuminemia (1.9 g/dl), elevated levels of C-reactive protein
(9.2 mg/dl), and soluble interleukin-2 receptor (3,274 U/ml) were
noted; lactate dehydrogenase levels were within the normal range
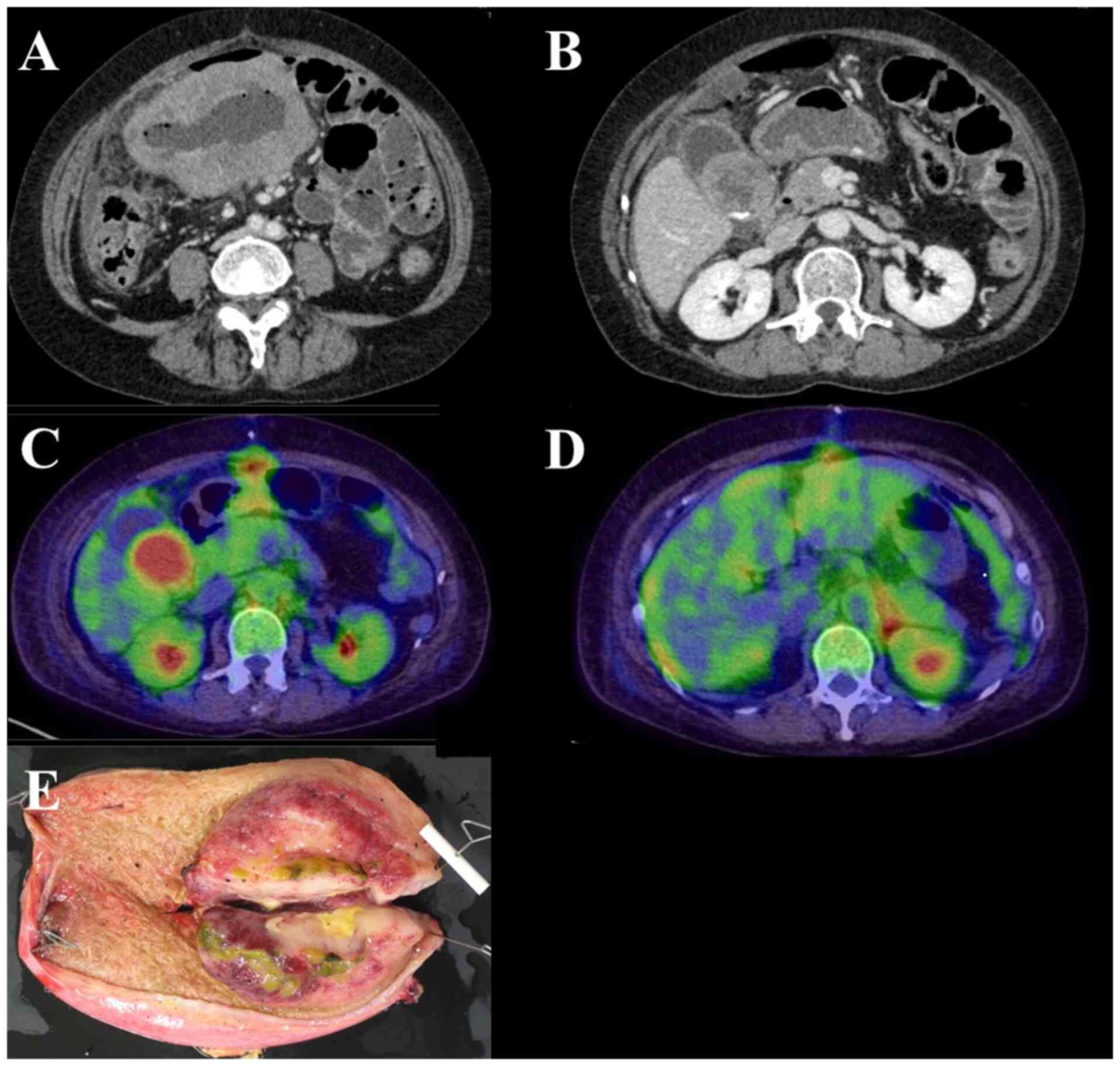
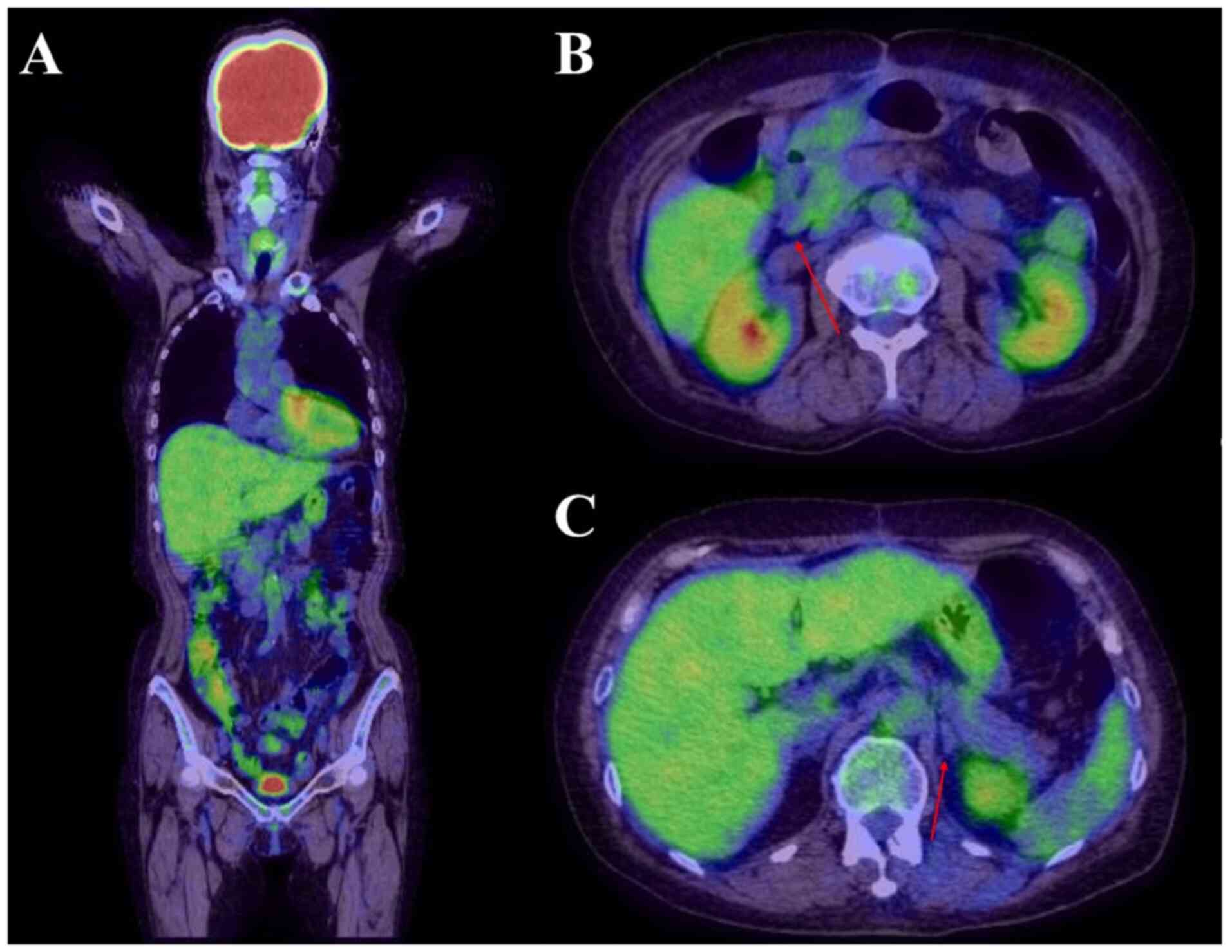
(Table I). Contrast-enhanced
computed tomography (CT) showed circumferential thickening and
perforation of the jejunum wall. In addition, a gallbladder mass
and wall thickening were observed, with enlargement of the
surrounding lymph nodes (Fig. 1A
and B).
 | Table ILaboratory findings at the first
visit. |
Table I
Laboratory findings at the first
visit.
| Test | Result | Reference range |
|---|
| White blood cell,
/µl | 2,600 | 3,900-9,800 |
| Neutrophil, % | 85 | 40-75 |
| Monocyte, % | 5.0 | 2.0-10 |
| Lymphocyte, % | 10 | 18-49 |
| Basophil, % | 0.0 | 0.0-2.0 |
| Eosinophil, % | 0.0 | 0.0-8.0 |
| Hemoglobin,
g/dl | 12.1 | 11.1-15.1 |
| Platelet count,
x104/µl | 47.4 | 13.0-37.0 |
| Total protein,
g/dl | 6.1 | 6.5-8.5 |
| Albumin, g/dl | 1.9 | 3.9-4.9 |
| Total bilirubin,
mg/dl | 0.76 | 0.2-1.2 |
| Aspartate
aminotransferase, U/l | 17 | 8-40 |
| Alanine
aminotransferase, U/l | 15 | 8-40 |
| Lactate
dehydrogenase, U/l | 148 | 124-222 |
| Urea, mg/dl | 17.2 | 8.0-20.0 |
| Creatinine,
mg/dl | 0.61 | 0.40-0.80 |
| C-reactive protein,
mg/dl | 9.2 | 0.00-0.50 |
| Prothrombin
time-international normalized ratio | 0.98 | |
| Activated partial
thromboplastin time, sec | 25.6 | 24.3-38.9 |
| Soluble
interleukin-2 receptor, U/ml | 3,274 | 157-474 |
| Human
T-lymphotropic virus type 1 antigen | Negative | Negative |
Based on all findings, emergency surgery was
performed on the day of admission. Intraoperatively, we observed
swelling of the jejunum with a 2-cm perforation and a mass located
at the gallbladder neck with adhesions to the liver. The affected
site of the small intestine was fused to the large mesentery and
transverse colon, and the perforated area was resected. The
gallbladder had severe adhesions owing to inflammation, and there
were concerns that its removal could damage the bile duct. To avoid
complications, we decided to preserve the gallbladder in the first
surgery. Although the raw sample of the jejunum mass was submitted
for flow cytometry, it was assessed to be unsuitable for the
analysis and could not be analyzed.
For the histological analysis, the biopsied
specimens were fixed in 10% buffered formalin for 24 h at room
temperature (RT) and embedded in paraffin. Sections with a
thickness of 4 µm were prepared from the paraffin block and stained
with hematoxylin for 5 min and eosin for 1 min at RT.
Immunohistochemistry (IHC) for CD3, CD8, CD4, CD5, CD20, CD56,
TIA-1 and EBER-ISH was performed on 4 µm-thick sections obtained
from the paraffin block using primary antibodies (Table II). IHC staining was conducted
using automated immunostaining devices, VENTANA BenchMark ULTRA
(Roche) and Histostainer 48A (Nichirei). Briefly, before staining,
to block endogenous peroxidases, the IHC device-dedicated reagent
(ultra View DAB universal; Roche Tissue Diagnostics) was used in
CD3, CD4, CD8, CD5, CD20, CD56, TIA-1 staining; their
temperature/duration were 36˚C/4 min and RT/5 min, respectively. In
the deparaffinization process, EZ prep (Roche Tissue Diagnostics)
was used. For EBER-ISH, in situ hybridization was carried
out using a digoxigenin-labeled probe specific for Epstein-Barr
virus-encoded RNA, with signal detection via anti-digoxigenin
antibodies and subsequent chromogenic substrate application. The
stained sections were then observed under a light microscope (BX53;
Olympus Corporation).
 | Table IIAntibodies used for
immunohistochemistry staining. |
Table II
Antibodies used for
immunohistochemistry staining.
| Antibody | Catalog number | Manufacturer |
|---|
| CD3 | M7254 | Dako |
| CD4 | M7310 | Dako |
| CD5 | M3641 | Dako |
| CD8 | M7103 | Dako |
| CD20 | M0755 | Dako |
| CD56 | M7304 | Dako |
| TIA-1 | IM2550 | Beckman |
| EBER-ISH | 780-2842 | Ventana |
Flow cytometry and Southern blotting were outsourced
to SRL, Inc. (https://www.srl-group.co.jp/english/), a commercial
testing company. Flow cytometry analysis was conducted on
surgically removed samples. Immediately after excision, surgically
removed samples were collected and maintained on ice (4˚C). The
specimens were initially incubated in the dark at 4˚C for 5 min to
stabilize the cell surface antigens. Following red blood cell
lysis, the cells were resuspended in a cell preservation medium
composed of 500 ml RPMI 1640, 25 ml 5% fetal bovine serum (FBS;
cat. no. 164210-500; Procell Life Science & Technology Co.,
Ltd.), and 5 ml Penicillin-Streptomycin solution (cat. no.
DXT-0503; ScienCell Research Laboratories, Inc.). After cell
counting, the suspension was adjusted to a concentration of
3.0x106 cells/ml. A total of 100 µl of the cell
suspension was dispensed into each tube, followed by the addition
of fluorochrome-conjugated monoclonal antibodies according to the
antibody panel detailed in Table
III. The samples were then incubated at 4˚C for 30 min in the
dark to ensure optimal antigen-antibody binding. During the washing
procedure, the cells were centrifuged at 400 x g for 5 min at 4˚C,
the supernatant was carefully removed, and the cells were
resuspended in cold PBS. This washing step was repeated three
times. Appropriate staining controls (e.g., isotype and unstained
controls) were included to validate the specificity of the staining
and to guide the gating strategy. Finally, the cells were
maintained at 4˚C in the dark and analyzed as soon as possible.
Flow cytometry was performed using the FACSLyric system (BD
Biosciences). Data acquisition and analysis were conducted
following the prescribed protocol, with data analysis performed BD
FACSDiva.
 | Table IIIFluorochrome-conjugated monoclonal
antibodies used for flow cytometry analysis. |
Table III
Fluorochrome-conjugated monoclonal
antibodies used for flow cytometry analysis.
| Reagent name | Catalog number | Manufacturer |
|---|
| MsIgG (FITC) | 340755 | BD Biosciences |
| MsIgG (PE) | 349043 | BD Biosciences |
| CD2 (FITC) | 555326 | BD Biosciences |
| CD3 (PE) | 555333 | BD Biosciences |
| CD4 (APC-H7) | 560158 | BD Biosciences |
| CD5 (FITC) | 347303 | BD Biosciences |
| CD7 (APC) | 561604 | BD Biosciences |
| CD8 (BV510) | 563256 | BD Biosciences |
| CD10 (PE) | 555375 | BD Biosciences |
| CD11c (BV510) | 563026 | BD Biosciences |
| CD16 (BV510) | 740203 | BD Biosciences |
| CD19 (BV421) | 562440 | BD Biosciences |
| CD20 (APC-H7) | 560853 | BD Biosciences |
| CD23 (BV421) | 562707 | BD Biosciences |
| CD25 (BV421) | 564033 | BD Biosciences |
| CD30 (FITC) | 555829 | BD Biosciences |
| CD34 (APC) | 555824 | BD Biosciences |
| CD38 (APC) | 555462 | BD Biosciences |
| CD56 (APC) | 555518 | BD Biosciences |
| CD45 (PerCP) | 347464 | BD Biosciences |
| Kappa light chains
(PE) | 562052 | BD Biosciences |
| Lambda light chains
(APC-H7) | 561325 | BD Biosciences |
The Southern blot process was used to analyze
specific DNA sequences from lymphoma tissue. Immediately after
surgical removal, the tissue was frozen at -80˚C to preserve DNA
integrity. Lymphoma cells were isolated from the frozen tissue, and
genomic DNA was extracted using an automated system (WPC-1,
Malcolm) and quantified for quality and yield. The DNA was then
digested with restriction enzymes (e.g., HindIII or
BamHI, Roche Diagnostics) at 37˚C for about 2 h. The
resulting fragments were separated on a 1% agarose gel (Agarose S,
Fujifilm Wako) in TAE or TBE buffer at ~100 V for approximately 1
h. After electrophoresis, the gel was incubated in an alkaline
solution (0.5 M NaOH with 1.5 M NaCl) at room temperature for 30
min to denature the DNA, which was then transferred onto a nylon
membrane by capillary action. The DNA was immobilized by UV
crosslinking with a spectrolinker (XL-1500, Nippon Genetics) at 120
mJ/cm². The membrane was pre-hybridized in a blocking solution
(Blocking Reagent, Roche Diagnostics) at 42˚C for 1 h before being
hybridized overnight at 42˚C in the MI 100 (Clabo) oven with a
digoxigenin-labeled probe specific for lymphoma-related gene
rearrangements. Following hybridization, the membrane was
washed-first in 2X SSC with 0.1% SDS at room temperature and then
in 0.1X SSC with 0.1% SDS at 65˚C for 15 min- to remove unbound
probe. Specific signals were detected using anti-digoxigenin
antibodies conjugated to alkaline phosphatase, followed by
treatment with the chemiluminescent substrate CDP-Star. The emitted
light was captured on X-ray film, which was developed using an
automatic processor (MXP-2000, Kodak). Finally, the band sizes and
intensities were compared with a molecular weight marker to
determine the presence and structure of gene rearrangements
characteristic of lymphoma.
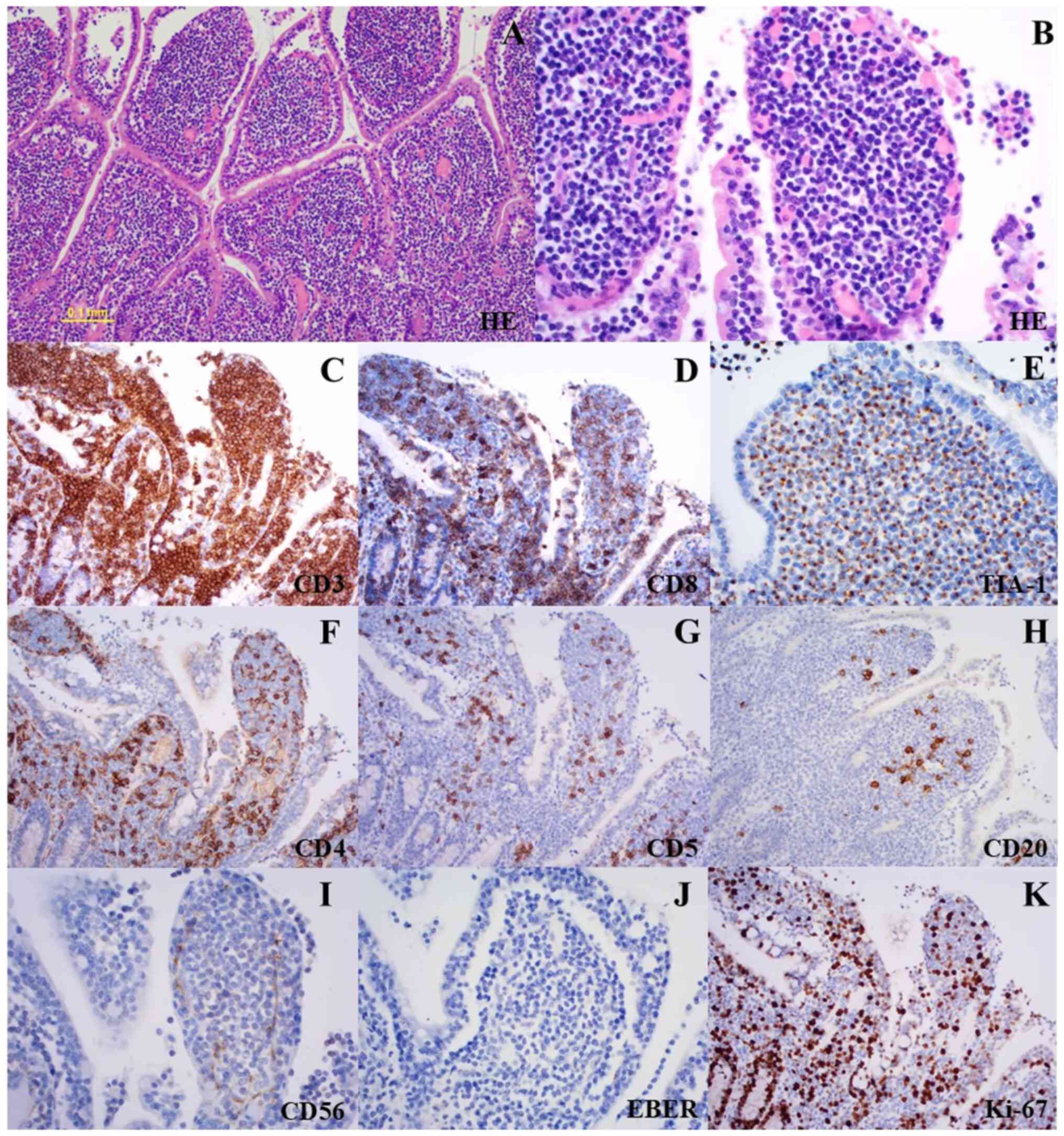
Histopathological analysis of the resected jejunum
revealed a dense infiltrate of medium-sized monomorphic lymphocytes
with round nuclei and dispersed chromatin in all layers of the
intestinal wall (Fig. 2A and
B). Widespread infiltration was
also seen in the mucosal layer near the jejunum mass, with
intraepithelial infiltration and mucosal flattening. The unaffected
area of the specimen showed no mucosal flattening, plasma cell
infiltration, or other findings indicative of celiac disease. Upon
immunostaining, the abnormal lymphocytes were positive for CD3,
CD8, and TIA-1, and negative for CD4, CD5, CD20, CD56, and
EBER-ISH. The Ki-67 proliferation index was approximately 40%
(Fig. 2C-K). Background
inflammatory cells, including normal T and B cells, were also
present. CD4- and CD5-positive cells were interpreted as normal T
cells, whereas CD20 positivity was indicative of B cells. However,
distinguishing between normal T-cell and the T-cell tumor can be
difficult. Immunostaining of TCR using immunohistochemistry (IHC)
is challenging to perform under the Japanese insurance system.
Therefore, it was not evaluated in this case. Southern blot
analysis showed that T-cell receptor β-chain Jβ2, β-chain Jβ1,
β-chain Cβ1, and γ-chain Jγ were rearranged, whereas T-cell
receptor δ-chain Jδ1 was not clonally rearranged (Fig. 3). These findings typically indicate
clonal expansion of mature T cells, which are generally considered
to have a TCR-αβ–positive immunophenotype.
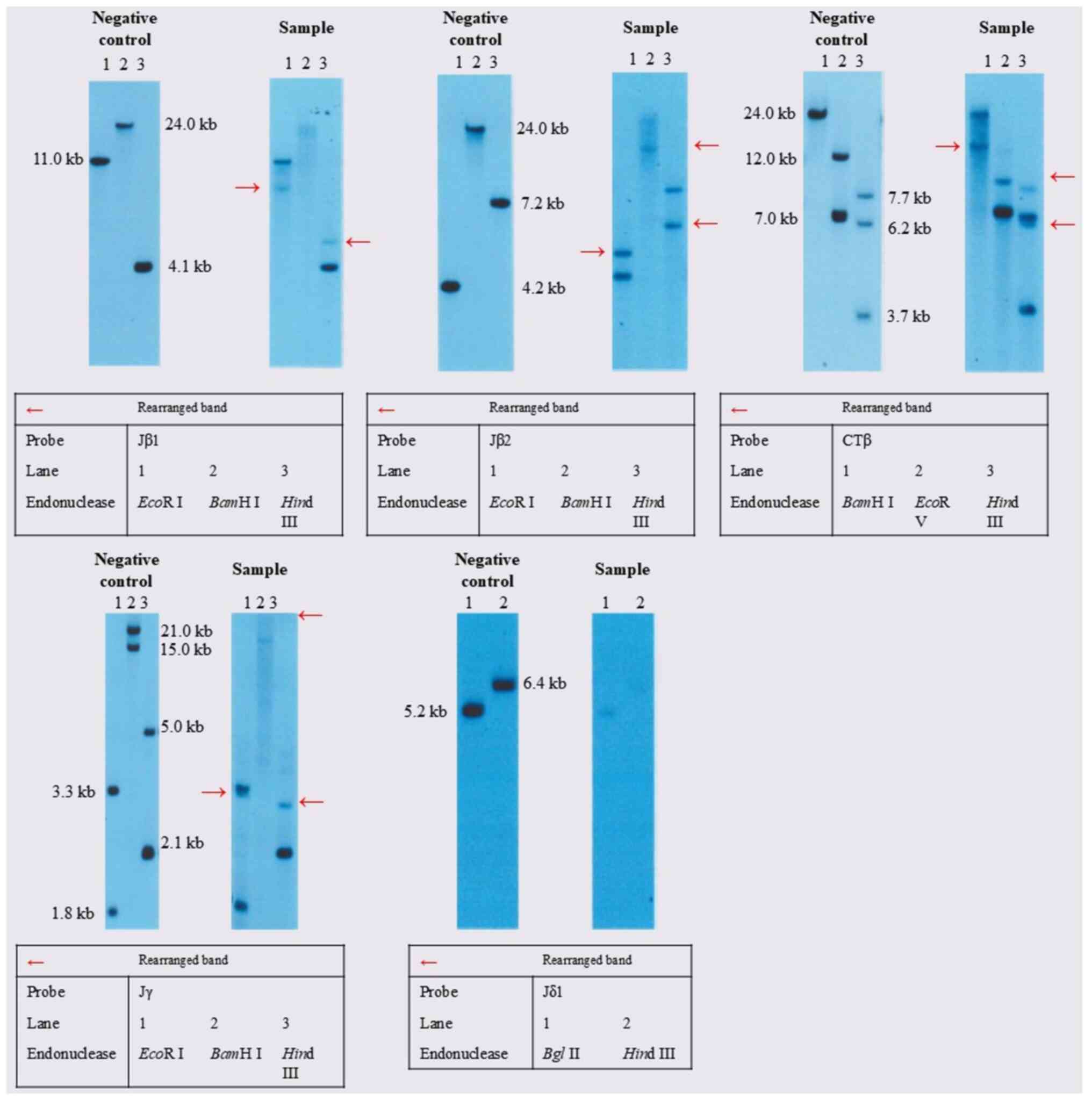 | Figure 3TCR rearrangement findings. Southern
blot was performed as follows. i) DNA Extraction; ii) Restriction
Enzyme Digestion. The common recognition sites for the restriction
endonucleases used are as follows: EcoRI: 5'-GAATTC-3',
BamHI: 5'-GGGATCC-3', HindIII: 5'-AAGCTT-3'. iii)
Electrophoresis; iv) Southern Transfer; v) Hybridization; vi)
Chemiluminescent Detection; vii) Interpretation. In the negative
control, bands corresponding to the nucleotides affected by the
restriction enzymes are observed in each lane. Bands at a specific
molecular weight correspond to the un-rearranged configuration.
Other hand, bands that appear at alternative molecular weights
indicate that rearrangement has occurred. The appearance of a
distinct band (marked with a red arrow) in the sample, which is
absent in the negative control, reflects a clonal rearrangement in
the TCR gene. This rearranged band is evidence that the affected
T-cells have undergone the rearrangement process, leading to the
unique configuration associated with clonal expansion, as seen in
T-cell lymphomas. T-cell receptor β-chain Jβ1, β-chain Jβ2, β-chain
Cβ1, and γ-chain Jγ were rearranged. T-cell receptor δ-chain Jδ1
was not clonally rearranged. TCR, T-cell receptor. |
We considered MEITL for her diagnosis, and
differential diagnosis included enteropathy-associated T-cell
lymphoma (EATL), indolent T-cell lymphoproliferative disorder of
the gastrointestinal tract, and other natural killer (NK)-cell
lymphomas, such as extranodal NK/T-cell lymphoma (ENKL). EATL is
commonly associated with celiac disease, and histopathology often
reveals pleomorphic lymphoma cells composed of medium to
large-sized cells, which are different from those observed in the
present case. Moreover, an indolent T-cell lymphoproliferative
disorder of the gastrointestinal tract follows a gradual course by
definition, which was not consistent with her very aggressive
presentation. In other NK-cell lymphomas, including ENKL, lymphoma
cells demonstrate the presence of Epstein-Barr virus, which was not
proven in her pathological analysis. Based on the absence of celiac
disease, aggressive clinical course, and characteristic
histopathological and immunophenotypic features, we could rule out
enteropathy-associated T-cell lymphoma, indolent T-cell
lymphoproliferative disorder of the gastrointestinal tract, and
other NK-/T-cell lymphomas, and established a diagnosis of MEITL
(5).
To determine the nature of the gallbladder mass, the
patient underwent additional diagnostic tests. Positron emission
tomography revealed radiotracer-avid lesions on the hepatic and
intestinal surfaces (Fig. 1C). The
mesentery and left adrenal gland were also involved, and the
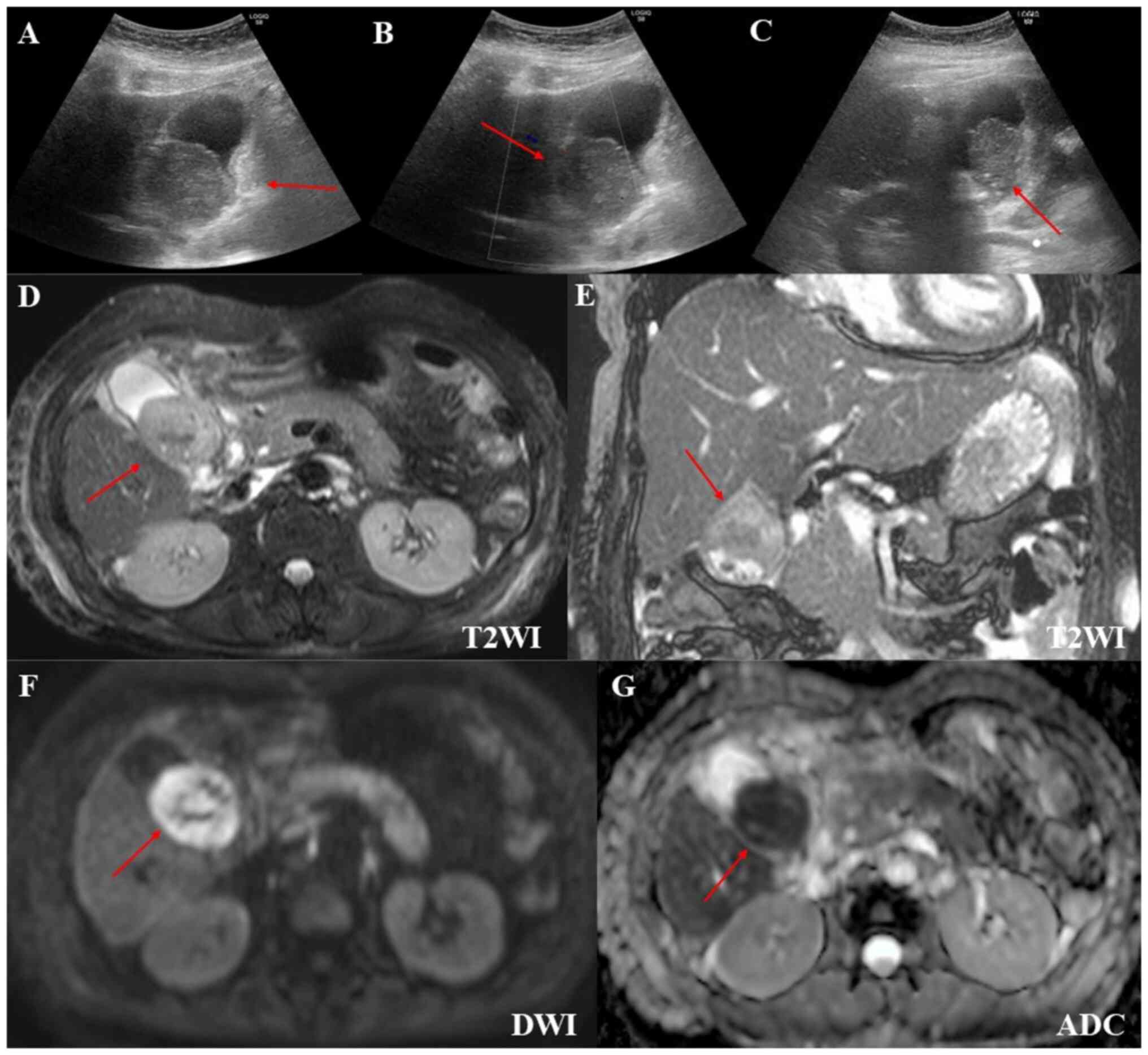
gallbladder mass was suspected to be infiltrated by MEITL (Fig. 1D). Abdominal ultrasound showed a
hypoechoic gallbladder mass that was not deformed by body movement
and had poor blood flow. Gallbladder wall thickening was observed
in some areas, but the lamina structure was preserved (Fig. 4A-C). On magnetic resonance imaging
(MRI), the gallbladder mass was iso-to-hypointense on T2-weighted
images, hyperintense on diffusion-weighted images, and had a low
apparent diffusion coefficient (ADC) value. Multiple gallstones
were also observed (Fig.
4D-G).
These imaging findings raised suspicion of lymphoma;
however, distinguishing it from gallbladder cancer based on imaging
only was impossible. Nonetheless, a diagnosis of gallbladder
lymphoma would have meant a significant risk of gallbladder
perforation owing to chemotherapy. Therefore, following the
reduction in drainage from the surgical site wound and the
subsidence of the patient's fever and abdominal pain symptoms, 25
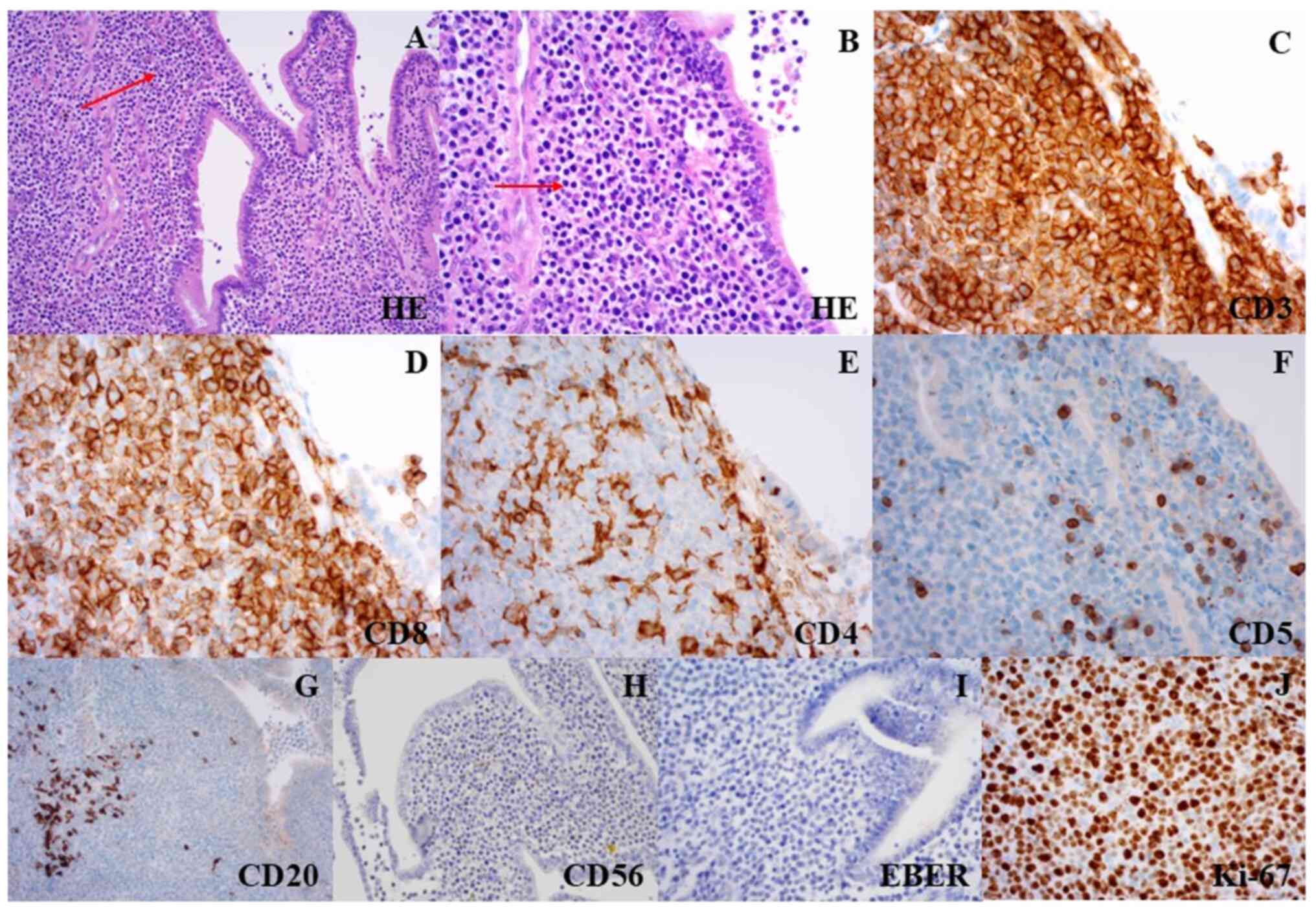
days post-admission, we performed a cholecystectomy (Fig. 1E). Histopathological analysis
showed an infiltrate of monomorphic lymphocyte-like cells, similar
to that in the jejunum lesion. Upon immunostaining, the abnormal
lymphocytes were positive for CD3, CD8, and negative for CD4, CD5,
CD20, CD56, and EBER-ISH (Fig.
5A-I). Flow cytometry results showed that the tumor cells were
positive for CD3, CD7, CD8, CD38, CD56, and TCRαβ and negative for
CD4, CD5, CD20, CD30, and TCRγδ (Fig.
6A). T-cell receptor β-chain Jβ2, β-chain Jβ1, β-chain Cβ1, and
γ-chain Jγ were rearranged, whereas T-cell receptor δ-chain Jδ1 was
not clonally rearranged (Fig. 6B).
The rearranged band in the sample was consistent with that observed
in the jejunum, suggesting that the tumor originated from the same
clone. Karyotyping analysis was unavailable. We confirmed
consistent histopathological findings in the jejunum and
gallbladder tumors, leading to a diagnosis of MEITL with
gallbladder involvement. Bone marrow aspirates and biopsy revealed
no lymphoma cell involvement. Head CT and MRI showed no evidence of
central nervous system (CNS) involvement. The patient's
International Prognostic Index was 3, based on poor ECOG
performance status, advanced stage, and extranodal lesions
(6). Her Prognostic Index for
T-cell lymphoma was 1, based on poor ECOG performance status
(7).
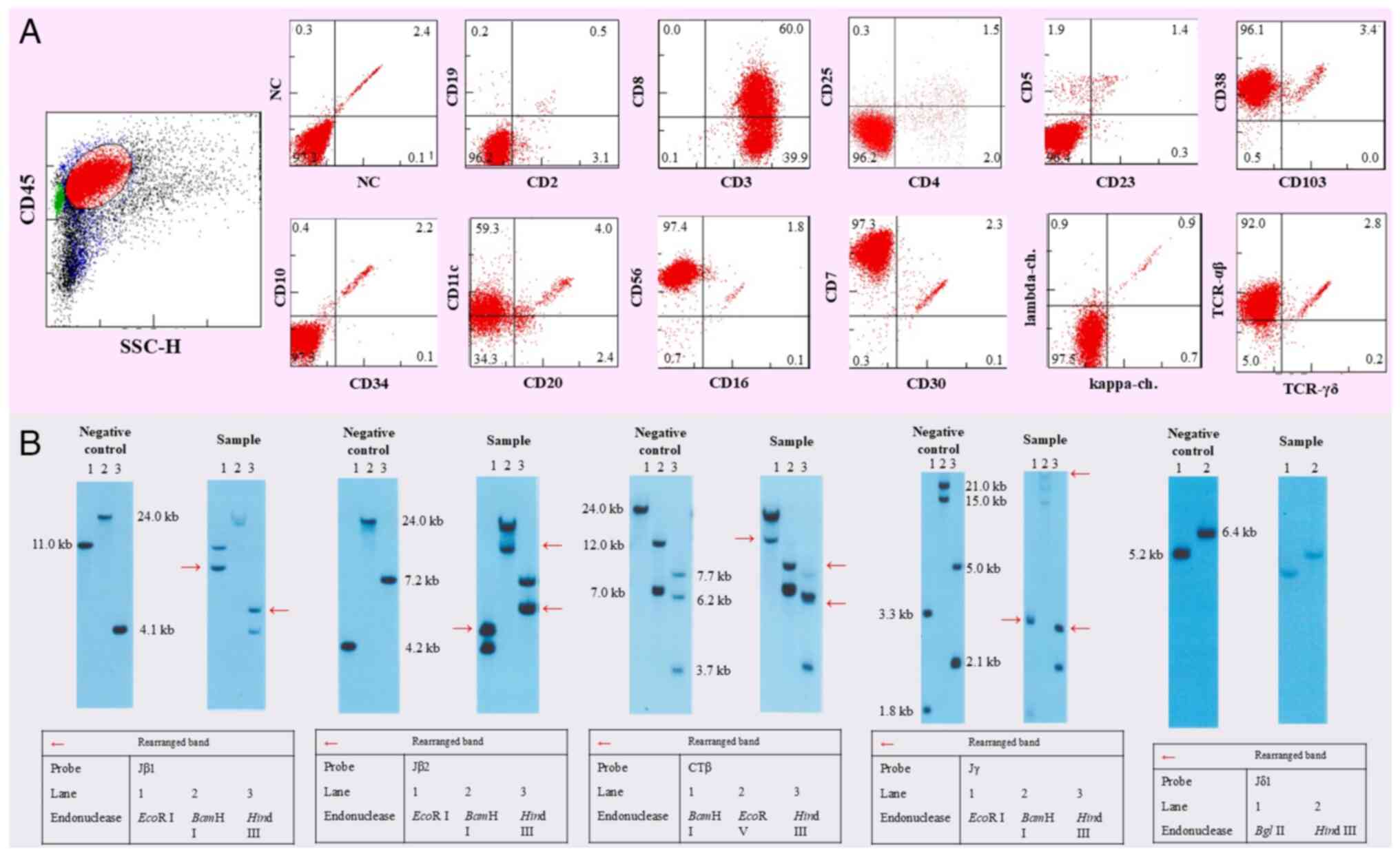 | Figure 6Flow cytometry and TCR rearrangement
findings. (A) On flow cytometry, the tumor cells were positive for
CD3, CD7, CD8, CD38, CD56, TCRαβ, and negative for CD4, CD5, CD20,
CD30, and TCRγδ. (B) The appearance of a distinct band (marked with
a red arrow) in the sample, which is absent in the negative
control, reflects a clonal rearrangement in the TCR gene. This
rearranged band is evidence that the affected T-cells have
undergone the rearrangement process, leading to the unique
configuration associated with clonal expansion, as seen in T-cell
lymphomas. T-cell receptor β-chain Jβ1, β-chain Jβ2, β-chain Cβ1,
and γ-chain Jγ were rearranged. T-cell receptor δ-chain Jδ1 was not
clonally rearranged. TCR, T-cell receptor. |
Despite the surgical repair of the jejunum
perforation on the day of admission, cholecystectomy 25 days after
admission, and supportive care, her general condition continued to
deteriorate; however, although her poor clinical condition was
concerning, we considered that controlling the lymphoma was
necessary. Therefore, 46 days after admission, chemotherapy was
started according to the ifosfamide-cisplatin-etoposide (ICE)
protocol, which resulted in marked tumor size reduction and
improved her ECOG performance status. Considering the reported
response rate of CHOP for MEITL is only about 40% (8) and the previous promising report of a
Newcastle regimen for enteropathy-associated T-cell lymphoma
(including MEITL) consisting of ifosfamide, etoposide, and
epirubicin combined with autologous transplantation (9), we opted for ICE therapy instead. This
therapy is routinely used at our institution, substituting a
platinum agent for epirubicin because epirubicin is not typically
used for lymphomas in Japan. Following three cycles of ICE therapy
(10) (etoposide 100
mg/m2 on days 1-3, carboplatin 650 mg/body, equivalent
to the area under the curve=5 on day 2, and ifosfamide 5,000
mg/m2 on day 2) and one cycle of
cyclophosphamide-cytarabine (high-dose
AraC)-dexamethasone-steroid-etoposide (CHASE) therapy
(cyclophosphamide 1,200 mg/m2 on day 1, Ara-C 2,000
mg/m2 on days 2-3, etoposide 100 mg/m2 on
days 1-3, and dexamethasone 40 mg/body on days 1-3), a regimen
developed in Japan specifically for stem cell harvesting (11). ICE and CHASE therapies are fully
covered by insurance in Japan and are approved for this condition.
We also referred to case reports from Japan (12,13)
where autologous transplantation was performed after achieving
complete remission (CR) with CHASE or ICE therapy. The patient
underwent autologous peripheral blood stem cell transplantation and
achieved complete remission as confirmed by positron emission
tomography (Fig. 7) in 233 days
after admission. Unfortunately, she passed away 2 months after the
transplantation owing to central nervous system recurrence.
Discussion
In this study, we presented a rare case of
cooccurrence of two uncommon conditions in the same patient: a rare
type of intestinal lymphoma and a previously unreported gallbladder
invasion by MEITL. Cholecystectomy was beneficial both for
establishing the diagnosis and completing chemotherapy safely,
without chemotherapy-induced perforation.
Although gallbladder malignancies encompass a wide
variety of malignant tumors, carcinomas constitute the majority of
cases. Gallbladder lymphomas are uncommon, accounting for only
0.1-0.2% of all gallbladder malignancies because this organ does
not usually contain lymphoid tissue (14). Two leading hypotheses have been
introduced regarding the causes of lymphomagenesis in the
gallbladder (15,16): i) lymph follicles formed owing to
chronic inflammation, such as from gallstones, and ii) lymphoma
from outside the gallbladder homing to the gallbladder wall through
certain adhesive factors. In this case, identical cytomorphology
was observed, suggesting that the lymphoma originating in the
intestinal tract had infiltrated the gallbladder.
Most gallbladder lymphomas are of B-cell lineage,
with mucosa-associated lymphoid tissue lymphoma and diffuse large
B-cell lymphoma being the most commonly reported types. Although
sporadic cases of other B-cell lymphomas arising from or involving
the gallbladder have been reported (17-19),
to the best of our knowledge, cases of gallbladder T-cell
lymphomas, including MEITL, have not been documented.
Histopathological analysis of surgical specimens is
the most accurate method for determining the nature of gallbladder
masses. Imaging techniques such as ultrasound and MRI are less
invasive and can also help differentiate between gallbladder
carcinoma and lymphoma, including identifying lymphoma subtypes.
However, the radiographic features of gallbladder MEITL remain
unknown. On ultrasound, lymphoma lesions are typically confined to
the submucosa, presenting with wall thickening and generally
maintained lamina structure, as observed in this case. In contrast,
gallbladder carcinoma often destroys the inner mucosal lamina
(14,20-23).
On MRI, malignant lymphomas and carcinoma demonstrate hyperintense
signals on T2-weighted images and low ADC (15,17,20,24-28).
In our case, the gallbladder mass demonstrated a low ADC but
iso-to-hypointense T2-weighted signal. Among different lymphoma
types, high-grade lymphomas, like diffuse large B-cell lymphoma,
often form solid masses and cause irregular gallbladder wall
thickening on CT. Conversely, low-grade lymphomas, such as
mucosa-associated lymphoid tissue lymphoma, follicular lymphoma,
and small lymphocytic lymphoma, typically show mild wall thickening
(14). In the present case, the
gallbladder mass exhibited irregular wall thickening, resembling
aggressive B-cell lymphomas.
In cases of suspected gallbladder lymphoma,
cholecystectomy is vital not only for the diagnosis but also for
the safe administration of chemotherapy and avoiding gallbladder
perforation during treatment. Gastrointestinal lymphomas are also
at high risk for perforation owing to progression and chemotherapy
(29). Additionally, gallbladder
perforation may occur. Thus, surgical resection is preferred for
safe treatment (30), as
illustrated in the present case.
There is no established treatment for MEITL. The
National Comprehensive Cancer Network guidelines recommend a
CHOP-like regimen as the first-line treatment for peripheral T-cell
lymphomas. However, the reported response rate of CHOP for MEITL is
only about 40% (8). Additionally,
the efficacy of adding etoposide to CHOP therapy for T-cell
lymphomas is still under debate (31). A 2010 study reported the successful
use of a Newcastle regimen for enteropathy-associated T-cell
lymphoma (including MEITL) consisting of ifosfamide, etoposide, and
epirubicin combined with autologous transplantation (9). Since epirubicin is uncommonly used in
Japan, we opted for ICE therapy, which is commonly employed at our
institution. There have been reports from Japan of cases in which
ICE or CHASE therapy, followed by autologous transplantation,
resulted in complete remission (12,13).
The patient responded well to the first course of ICE, prompting
the administration of three courses. No CNS involvement was
detected on head imaging, and CNS prophylaxis was not administered
owing to concerns regarding treatment toxicity. Although CNS
involvement in MEITL is rare (4),
there have been cases where patients relapsed early
post-transplantation, similar to our patient (32,33).
Therefore, further investigations into optimal induction
chemotherapy regimens and the necessity for CNS prophylaxis are
warranted.
A limitation of this study is that TCR expression
could not be assessed. Although we submitted a raw sample of the
jejunal mass for flow cytometry, it was unsuitable for analysis,
and results were unavailable.
In conclusion, we reported the first case of MEITL
with gallbladder involvement. Despite the advances in imaging
techniques, considering the low incidence of gallbladder lymphoma
and previously reported cases of gallbladder lymphomas mimicking or
coexisting with gallbladder carcinoma, cholecystectomy is still
recommended as a diagnostic and therapeutic intervention. Following
a definitive diagnosis, initiating prompt and appropriate treatment
is imperative.
Acknowledgements
The authors would like to thank SRL, Inc. for
performing the flow cytometry and Southern blotting.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TO collected the data and wrote the first draft of
the manuscript. TS performed pathological analysis. MN, YK, TT and
MT provided hematological clinical information, including the
disease course, therapeutic strategy selection and interpretation
of hematological findings. MN and MT supervised the manuscript
writing, editing, and review. JT and TI provided clinical
information on surgical treatment and perioperative findings,
including details of jejunum resection and the cholecystectomy. MT
coordinated the project and edited the manuscript. MN and MT
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient's family provided written informed
consent for the publication of their data and any related
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Delabie J, Holte H, Vose JM, Ullrich F,
Jaffe ES, Savage KJ, Connors JM, Rimsza L, Harris NL,
Müller-Hermelink K, et al: Enteropathy-associated T-cell lymphoma:
clinical and histological findings from the international
peripheral T-cell lymphoma project. Blood. 118:148–155.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jantunen E, Boumendil A, Finel H, Luan JJ,
Johnson P, Rambaldi A, Haynes A, Duchosal MA, Bethge W, Biron P, et
al: Autologous stem cell transplantation for enteropathy-associated
T-cell lymphoma: A retrospective study by the EBMT. Blood.
121:2529–2532. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stuver R, Epstein-Peterson ZD and Horwitz
SM: Few and far between: clinical management of rare extranodal
subtypes of mature T-cell and NK-cell lymphomas. Haematologica.
108:3244–3260. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yi JH, Lee GW, Do YR, Jung HR, Hong JY,
Yoon DH, Suh C, Choi YS, Yi SY, Sohn BS, et al: Multicenter
retrospective analysis of the clinicopathologic features of
monomorphic epitheliotropic intestinal T-cell lymphoma. Ann
Hematol. 98:2541–2550. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hang JF, Yuan CT, Chang KC, Wang RC, Chen
BJ, Hsieh PP, Huang WT, Chuang WY, Chen TW, Yeh YC, et al: Targeted
next-generation sequencing reveals a wide morphologic and
immunophenotypic spectrum of monomorphic epitheliotropic intestinal
T-cell lymphoma. Am J Surg Pathol. 46:1207–1218. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gallamini A, Stelitano C, Calvi R, Bellei
M, Mattei D, Vitolo U, Morabito F, Martelli M, Brusamolino E,
Iannitto E, et al: Peripheral T-cell lymphoma unspecified (PTCL-U):
A new prognostic model from a retrospective multicentric clinical
study. Blood. 103:2474–2479. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Daum S, Ullrich R, Heise W, Dederke B,
Foss HD, Stein H, Thiel E, Zeitz M and Riecken EO: Intestinal
non-Hodgkin's lymphoma: a multicenter prospective clinical study
from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J
Clin Oncol. 21:2740–2746. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sieniawski M, Angamuthu N, Boyd K, Chasty
R, Davies J, Forsyth P, Jack F, Lyons S, Mounter P, Revell P, et
al: Evaluation of enteropathy-associated T-cell lymphoma comparing
standard therapies with a novel regimen including autologous stem
cell transplantation. Blood. 115:3664–3670. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gisselbrecht C, Glass B, Mounier N, Singh
Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O,
Hagberg H, et al: Salvage regimens with autologous transplantation
for relapsed large B-cell lymphoma in the rituximab era. J Clin
Oncol. 28:4184–4190. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ogura M, Yamamoto K, Morishima Y,
Wakabayashi M, Tobinai K, Ando K, Uike N, Kurosawa M, Gomyo H,
Taniwaki M, et al: R-high-CHOP/CHASER/LEED with autologous stem
cell transplantation in newly diagnosed mantle cell lymphoma:
JCOG0406 STUDY. Cancer Sci. 109:2830–2840. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ishibashi H, Nimura S, Kayashima Y,
Takamatsu Y, Aoyagi K, Harada N, Kadowaki M, Kamio T, Sakisaka S
and Takeshita M: Multiple lesions of gastrointestinal tract
invasion by monomorphic epitheliotropic intestinal T-cell lymphoma,
accompanied by duodenal and intestinal enteropathy-like lesions and
microscopic lymphocytic proctocolitis: A case series. Diagn Pathol.
11(66)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Umino A, Kubota Y, Honda-Yoshigai M,
Okazaki T, Yoshihara Y, Wakayama K, Kawasaki S, Kusaba K, Kimura S
and Sueoka E: Monitoring of tumor cells by flow cytometry permits
rapid evaluation of disease progression in monomorphic
epitheliotropic intestinal T-cell lymphoma. Cytometry B Clin Cytom.
100:454–456. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ono A, Tanoue S, Yamada Y, Takaji Y, Okada
F, Matsumoto S and Mori H: Primary malignant lymphoma of the
gallbladder: A case report and literature review. Br J Radiol.
82:e15–e19. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mittal PK, Moreno CC, Kalb B, Mittal A,
Camacho JC, Maddu K, Kitajima HD, Quigley BC, Kokabi N and Small
WC: Primary biliary tract malignancies: MRI spectrum and mimics
with histopathological correlation. Abdom Imaging. 40:1520–1557.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dierlamm J, Baens M, Wlodarska I,
Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, De Wolf-Peeters
C, Hagemeijer A, Van den Berghe H and Marynen P: The apoptosis
inhibitor gene API2 and a novel 18q gene, MLT, are recurrently
rearranged in the t(11;18)(q21;q21) associated with
mucosa-associated lymphoid tissue lymphomas. Blood. 93:3601–3609.
1999.PubMed/NCBI
|
|
17
|
Hosoda K, Shimizu A, Kubota K, Notake T,
Hayashi H, Yasukawa K, Umemura K, Kamachi A, Goto T, Tomida H, et
al: Gallbladder Burkitt's lymphoma mimicking gallbladder cancer: A
case report. World J Gastroenterol. 28:675–682. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Manesh M, Henry R, Gallagher S, Greas M,
Sheikh MR and Zielsdorf S: Hodgkin lymphoma masquerading as
perforated gallbladder adenocarcinoma: A case report. World J
Gastrointest Surg. 13:1279–1284. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kusunoki R, Fujishiro H, Yoshimura M,
Sawada K, Suemitsu S, Kataoka M, Fujiwara A, Tsukano K, Kotani S,
Yamanouchi S, et al: Intravascular large B-cell lymphoma mimicking
hepatobiliary infection: A case report and literature review.
Intern Med. 58:1885–1889. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cocco G, Delli Pizzi A, Basilico R,
Fabiani S, Taraschi AL, Pascucci L, Boccatonda A, Catalano O and
Schiavone C: Imaging of gallbladder metastasis. Insights Imaging.
12(100)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ayub A, Rehmani S, Al-Ayoubi AM, Lewis E,
Santana-Rodríguez N, Raad W, Bhora F and Kim G: Primary
non-Hodgkin's lymphoma of the gallbladder: A population-based
analysis. Anticancer Res. 37:2581–2586. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kalra N, Gupta P, Singhal M, Gupta R,
Gupta V, Srinivasan R, Mittal BR, Dhiman RK and Khandelwal N:
Cross-sectional imaging of gallbladder carcinoma: An update. J Clin
Exp Hepatol. 9:334–344. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Honda M, Furuta Y, Naoe H and Sasaki Y:
Primary mucosa-associated lymphoid tissue (MALT) lymphoma of the
gallbladder and review of the literature. BMJ Case Rep.
2017(bcr2017220161)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Psarras K, Symeonidis N, Vlachaki E,
Baltatzis M, Papatolios G, Pavlidis E, Mouratidou C, Venizelos I,
Pavlidis T, Sakantamis A and Nikolaidou C: Primary gallbladder
small lymphocytic lymphoma as a rare postcholecystectomy finding.
Case Rep Hematol. 2014(716071)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rahim S, Ahmad Z, Chundriger Q, Ahmed A,
Ali N and Abdul-Ghafar J: Secondary involvement of gallbladder by
acute lymphoblastic leukemia presenting clinically as cholecystitis
in a young patient: A case report. World J Surg Oncol.
21(63)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mani H, Climent F, Colomo L, Pittaluga S,
Raffeld M and Jaffe ES: Gall bladder and extrahepatic bile duct
lymphomas: Clinicopathological observations and biological
implications. Am J Surg Pathol. 34:1277–1286. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Furlan A, Ferris JV, Hosseinzadeh K and
Borhani AA: Gallbladder carcinoma update: Multimodality imaging
evaluation, staging, and treatment options. AJR Am J Roentgenol.
191:1440–1447. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zemour J, Marty M, Lapuyade B, Collet D
and Chiche L: Gallbladder tumor and pseudotumor: Diagnosis and
management. J Visc Surg. 151:289–300. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vaidya R, Habermann TM, Donohue JH, Ristow
KM, Maurer MJ, Macon WR, Colgan JP, Inwards DJ, Ansell SM, Porrata
LF, et al: Bowel perforation in intestinal lymphoma: Incidence and
clinical features. Ann Oncol. 24:2439–2443. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kato H, Naganuma T, Iizawa Y, Kitagawa M,
Tanaka M and Isaji S: Primary non-Hodgkin's lymphoma of the
gallbladder diagnosed by laparoscopic cholecystectomy. J
Hepatobiliary Pancreat Surg. 15:659–663. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wöhrer S, Chott A, Drach J, Püspök A,
Hejna M, Hoffmann M and Raderer M: Chemotherapy with
cyclophosphamide, doxorubicin, etoposide, vincristine and
prednisone (CHOEP) is not effective in patients with
enteropathy-type intestinal T-cell lymphoma. Ann Oncol.
15:1680–1683. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nato Y, Miyazaki K, Imai H, Nakano E,
Kageyama Y, Ino K, Fujieda A, Matsumoto T, Tawara I, Tanaka K, et
al: Early central nervous system relapse of monomorphic
epitheliotropic intestinal T-cell lymphoma after cord blood
transplantation. Int J Hematol. 114:129–135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kubota Y and Kusaba K: Monomorphic
epitheliotropic intestinal T-cell lymphoma involving the central
nervous system. Blood. 131(1765)2018.PubMed/NCBI View Article : Google Scholar
|