Introduction
Angiomatous nasal polyps (ANPs) are an uncommon
subtype of inflammatory sinonasal polyp, accounting ~4-5% of all
nasal polyps (1). They are
characterized by significant vascular proliferation and
angiectasis, rendering them susceptible to vascular compromise,
which may lead to venous stasis, thrombosis and infarction
(2,3).
The clinical and radiological characteristics of
ANPs frequently resemble those of other sinonasal masses, such as
juvenile angiofibroma, inverted papilloma, vascular tumors such as
hemangioma, and malignant lesions. This resemblance complicates
diagnosis, highlighting the need for a comprehensive evaluation for
accurate differentiation (3-5).
Studies have highlighted specific imaging features on computed
tomography (CT) and magnetic resonance imaging (MRI) scans that
help distinguish ANPs from other sinonasal tumors, including
juvenile angiofibroma, inverted papilloma and malignancies
(4-6).
Recognition of these features is crucial to avoid unnecessary,
extensive surgical interventions and to guide appropriate clinical
management.
This study aimed to describe the clinical and
radiological features of six pathologically confirmed cases of
ANP.
Patients and methods
Patient recruitment
This retrospective study was approved by the
Institutional Review Board of Chonnam National University Hwasun
Hospital (Hwasun, Korea; approval no. CNUHH-2023-127). It included
six patients diagnosed with ANP at Hwasun National University
Hospital (Hwasun, Korea) between December 2010 and May 2022. This
study included six patients with pathologically confirmed ANP.
Clinical and radiological features were reviewed and all patients
underwent endoscopic sinus surgery for tumor removal.
Imaging
CT was performed in all cases using standard
paranasal sinus protocols, while four patients also underwent
preoperative MRI. CT was conducted using a 64-slice
multidetector-row CT scanner (GE Healthcare). The scanning
parameters were as follows: Section thickness, 3 mm; beam pitch,
0.8; gantry rotation time, 0.5 sec; table speed, 30.7 2 mm per
rotation; reconstruction interval, 3 mm; tube voltage, 120 kV; and
tube current, 120-36 mA. The MRI was conducted on a 3.0T Siemens
MAGENTOM Skyra scanner (Siemens Healthineers), including
T1-weighted, T2-weighted and contrast-enhanced T1 sequences with 3
mm slice thickness. Sinus opacification was evaluated using the
Lund-Mackay scoring system (range: 0-24) (7). The Lund-Mackay scoring system, a
validated method for assessing sinus opacification, assigns a score
of 0 (no opacification), 1 (partial) or 2 (complete) to each of six
sinus regions on both sides, including the maxillary, anterior and
posterior ethmoid, frontal and sphenoid sinuses, and the
osteomeatal complex. The total score ranges from 0 to 24.
Information on the patient's symptoms, symptom duration, medical
history and imaging examinations before surgery was collected, as
well as data regarding follow-up observation and recurrence after
surgery. Clinical and imaging data were independently reviewed by
two rhinologic surgeons with 22 and 38 years' experience in
otorhinolaryngology and 1 radiologist with 22 years' experience in
head and neck radiology. Histologically, ANPs are characterized by
edematous and fibrotic stroma containing numerous dilated,
thin-walled blood vessels, often accompanied by areas of
infarction, hemorrhage, and thrombus formation (2,3). No
statistical analyses were performed due to the small sample size
and the descriptive nature of the study.
Follow-up
Postoperative follow-up was conducted one and two
weeks after the operation, followed by visits at three months, six
months and annually. Endoscopic examinations were performed at each
visit to monitor for recurrence.
Statistical analysis
Data management was performed using R version 4.2.3
(R Foundation for Statistical Computing). Values are expressed as
the mean ± standard deviation or n (%).
Results
Clinical characteristics
The clinical data of six patients were reviewed,
including sex, age, symptom duration, medical and surgical history,
lesion location and size, follow-up duration and recurrence.
Details are presented in Tables I
and II.
 | Table ISummary of the six patients with
angiomatous nasal polyps. |
Table I
Summary of the six patients with
angiomatous nasal polyps.
| Variable | Value |
|---|
| Sex
(male/female) | 3:3 |
| Age, years | 42±28.4 (12-77) |
| Laterality
(left/right) | 4:2 |
| Symptom duration,
months | 10.8±4.5 (1-24) |
| Common symptoms | |
|
Nasal
obstruction | 6(100) |
|
Rhinorrhea | 5 (83.3) |
|
Epistaxis | 3(50) |
|
Hyposmia | 2 (33.3) |
|
Cheek
swelling | 1 (16.7) |
|
Epiphora | 1 (16.7) |
| Comorbidities | |
|
Hypertension | 1 (16.7) |
|
Smoking | 2 (33.3) |
|
Prior sinus
surgery | 2 (33.3) |
|
History of
trauma | 0 (0) |
| Endoscopic sinus
surgery approach | 6(100) |
| Tumor size, cm | 3.57±0.8
(2.7-5.0) |
| Follow-up duration,
months | 63.5±17.4
(15-132) |
| Recurrence | 0 (0) |
| Origin site | |
|
Maxillary
sinus | 4 |
|
Middle
turbinate | 1 |
|
Superior
turbinate | 1 |
| Lund-Mackay
score | 11.5±2.5 (5-20) |
 | Table IIClinical characteristics of six
patients with angiomatous nasal polyps. |
Table II
Clinical characteristics of six
patients with angiomatous nasal polyps.
| Patient no. | Sex | Age, years | Side | Duration of symptoms,
months | Symptoms Nasal
obstruction | Epistaxis | Rhinorrhea | Hyposmia | Visual
disturbance | Cheek swelling | Epiphora | History | Trauma or
surgery | CT | MRI | Mass size, cm | FU duration,
months | Recurrence |
|---|
| 1 | F | 22 | Left | 24 | Y | Y | Y | N | N | N | N | None | None | Y | Y | 3.6 | 52 | N |
| 2 | M | 68 | Left | 1 | Y | N | N | N | N | N | N | None | Sinus surgery 30 yrs
ago | Y | Y | 2.7 | 132 | N |
| 3 | M | 17 | Left | 12 | Y | N | Y | N | N | N | N | None | Sinus surgery 1 yr
ago | Y | Y | 3.3 | 48 | N |
| 4 | M | 56 | Right | 3 | Y | N | Y | Y | N | N | N | HTN | None | Y | N | 2.7 | 96 | N |
| 5 | F | 12 | Left | 1 | Y | Y | Y | N | N | N | N | None | None | Y | N | 4.1 | 15 | N |
| 6 | F | 77 | Right | 24 | Y | Y | Y | Y | N | Y | Y | None | None | Y | Y | 5.0 | 38 | N |
The cohort consisted of three men (50%) and three
women (50%) aged 12-77 years (mean: 42±28.4 years). All patients
had unilateral lesions, with four on the left (66.7%) and two on
the right (33.3%), and no contralateral sinonasal involvement.
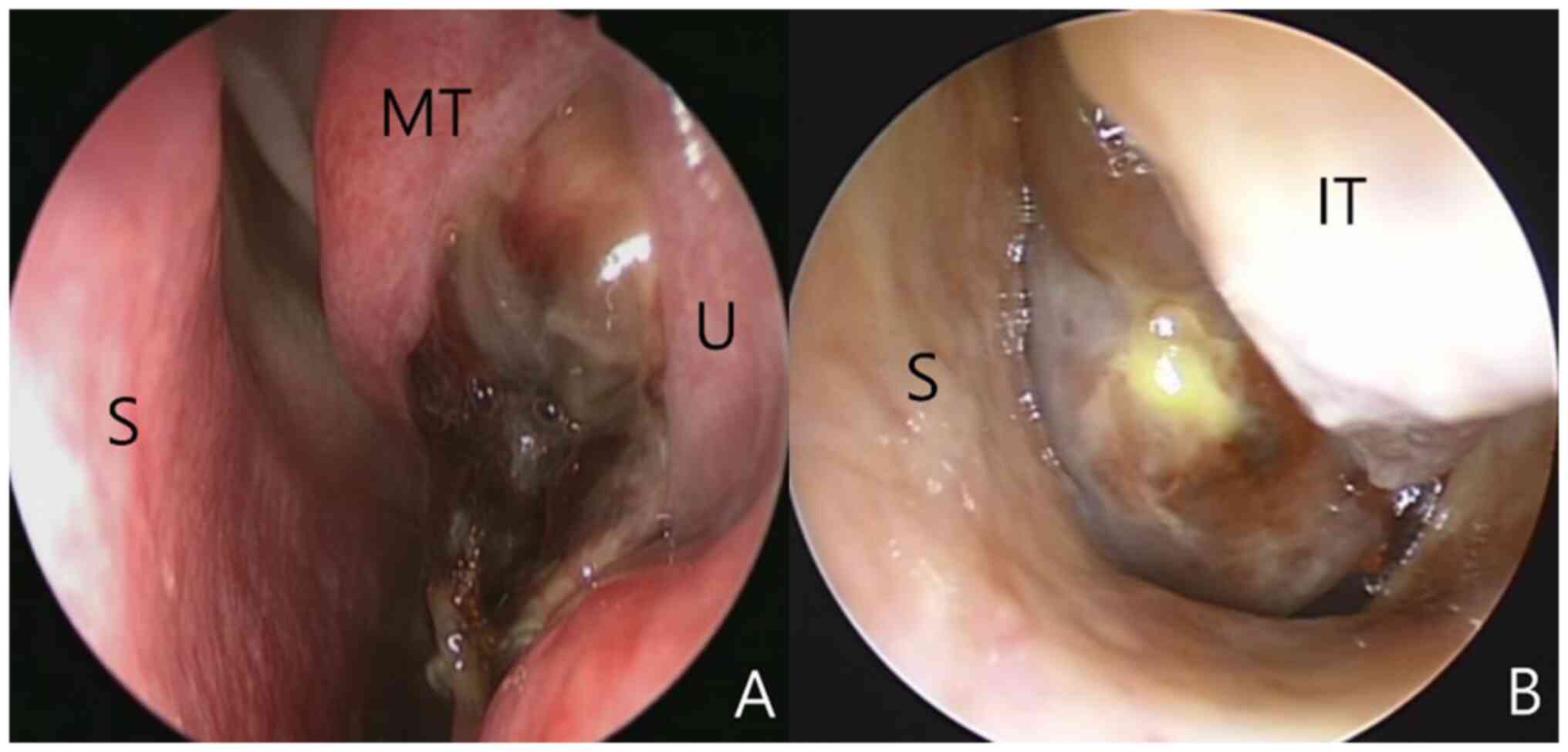
Endoscopic examination revealed polypoid masses with necrotic
changes in the nasal cavity (Fig.
1). Nasal obstruction was the most common symptom, present in
all cases. Rhinorrhea occurred in five patients (83.3%), followed
by epistaxis (n=3, 50%), hyposmia (n=2, 33.3%), and cheek swelling
and epiphora (each n=1, 16.7%). None of the patients experienced
any visual disturbances. The symptoms lasted 1 to 24 months (mean:
10.8±4.5 months). One patient had hypertension (16.7%) and two were
smokers (33.3%). Furthermore, two patients had prior endoscopic
sinus surgery (33.3%), though none had a history of trauma. All
patients underwent endoscopic sinus surgery for polyp removal.
Tumor sizes ranged from 2.7 to 5.0 cm (mean: 3.57±0.8 cm).
Postoperative follow-up included routine endoscopic examinations to
evaluate recurrence. The mean follow-up duration was 63.5±17.4
months (range: 15-132 months), with no recurrences observed.
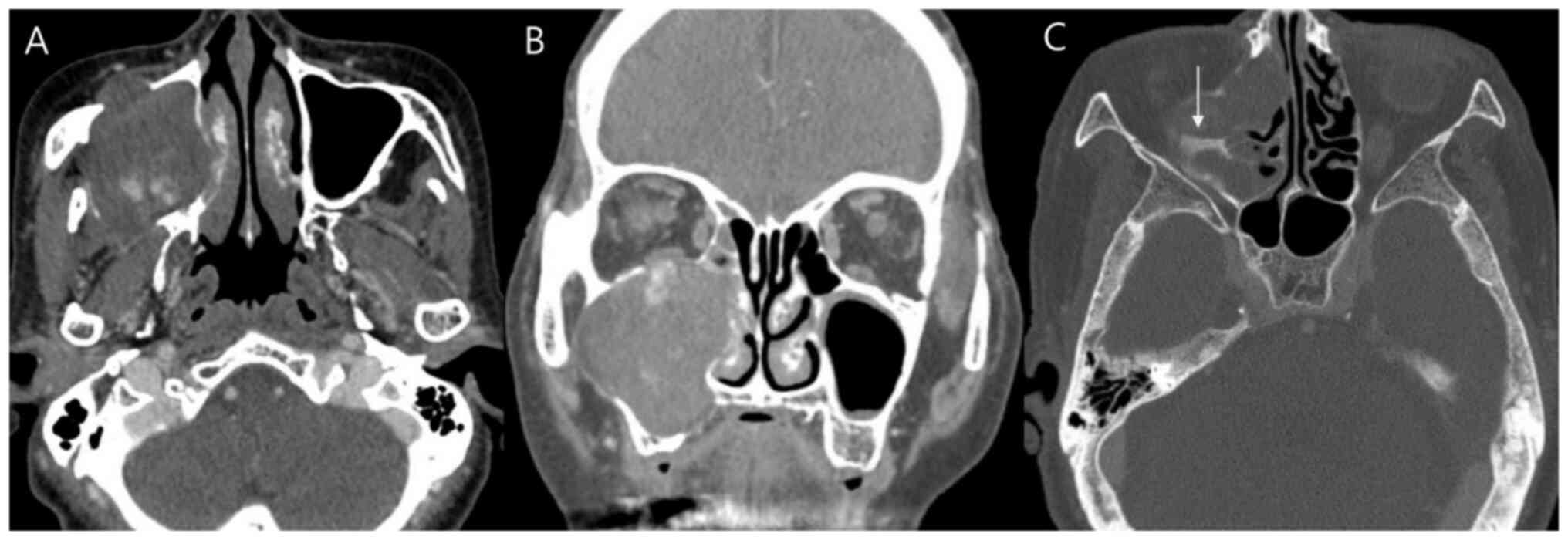
CT findings
Preoperative contrast-enhanced CT scans were
performed for all six patients. The imaging characteristics are
summarized in Tables I and
III. The maxillary sinus was the
most common site of origin (n=4, 66.7%; representative case
presented in Fig. 2), followed by
the middle turbinate and superior turbinate (each n=1, 16.7%). The
mean Lund-Mackay score was 11.5±2.5 (range: 5-20). All cases
featured involvement of the nasal cavity, osteomeatal complex,
posterior choana and ipsilateral ethmoid sinus. The maxillary sinus
was affected in four cases (66.7%), while the sphenoid sinus was
involved in one case (16.7%); no frontal sinus involvement was
observed. One lesion extended into the nasopharynx (16.7%), two
involved the orbital floor and one reached the infratemporal fossa
(16.7%).
 | Table IIICT findings in six cases of
angiomatous nasal polyp. |
Table III
CT findings in six cases of
angiomatous nasal polyp.
| Patient no. | Origin | LM score | Maxillary sinus | Ethmoid sinus | Sphenoid sinus | Frontal sinus | OMC | Nasal cavity | Posterior choana | Nph | Orbit floor | Infratemporal
fossa | Bone change | Homogeneity | Contrast
enhancement |
|---|
| 1 | Maxillary
sinus | 18 | Y | Y | N | N | Y | Y | Y | Y | N | N | Expansile
remodeling | Heterogeneous | Y |
| 2 | Middle
turbinate | 5 | N | Y | N | N | Y | Y | Y | N | N | N | None | Heterogeneous | Y |
| 3 | Maxillary
sinus | 5 | Y | Y | N | N | Y | Y | Y | N | N | N | Expansile
remodeling, Hyperostosis | Heterogeneous | Y |
| 4 | Superior
turbinate | 20 | N | Y | N | N | Y | Y | Y | N | N | N | Expansile
remodeling | Heterogeneous | N |
| 5 | Maxillary
sinus | 10 | Y | Y | Y | N | Y | Y | Y | N | Y | N | Expansile
remodeling, Bony destruction | Heterogeneous | Y |
| 6 | Maxillary
sinus | 11 | Y | Y | N | N | Y | Y | Y | N | Y | Y | Expansile
remodeling, Bony destruction, Hyperostosis | Heterogeneous | Y |
All lesions exhibited heterogeneous density on CT.
Contrast enhancement was seen in five cases (83.3%), while one
lesion showed no enhancement (16.7%). Bony changes were observed in
five patients (83.3%), including expansile remodeling (n=5, 83.3%),
hyperostosis (n=2, 33.3%) and bony destruction (n=2, 33.3%). Only
one patient showed no bony alteration.
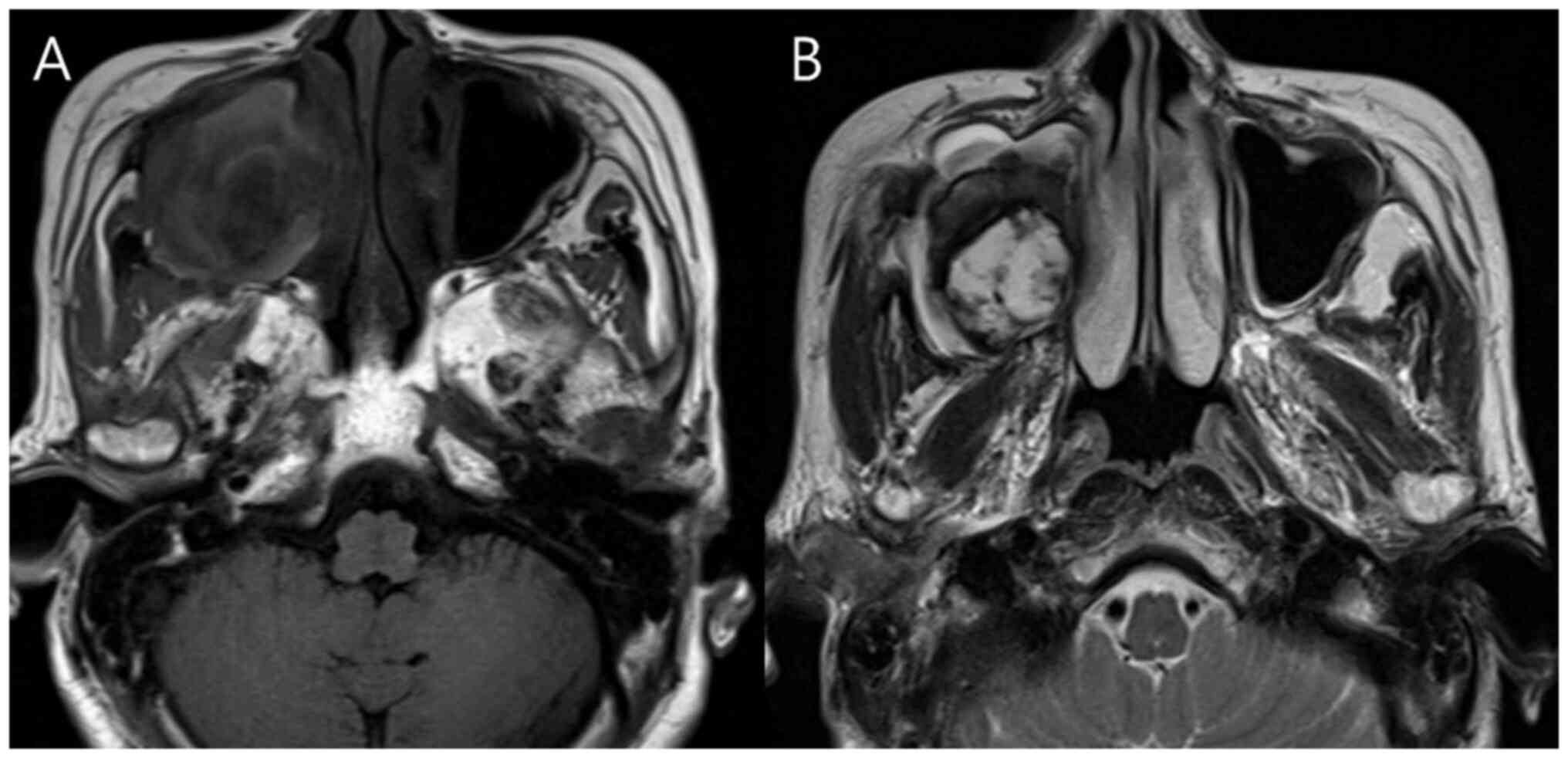
MRI findings
A total of four of the six patients (66.6%)
underwent additional MRI to evaluate suspected sinonasal malignancy
(representative case presented in Fig.
3). All four cases exhibited mild hyperintense signals on
T1-weighted images and heterogeneous hyperintense signals on
T2-weighted images. A peripheral hypointense rim on T2-weighted
images was noted in three patients (75%). Detailed MRI imaging
findings are described in Tables I
and IV.
 | Table IVMagnetic resonance imaging findings
in four cases of angiomatous nasal polyps. |
Table IV
Magnetic resonance imaging findings
in four cases of angiomatous nasal polyps.
| Patient no. | T1-weighted
image | T2-weighted
image | Peripheral
hypointense rim in T2-weighted image | Enhancement |
|---|
| 1 | Mild
hyperintense | Heterogeneous
hyperintense | Yes | Heterogeneous |
| 2 | Mild
hyperintense | Heterogeneous
hyperintense | No | Heterogeneous |
| 3 | Mild
hyperintense | Heterogeneous
hyperintense | Yes | Heterogeneous |
| 6 | Mild
hyperintense | Heterogeneous
hyperintense | Yes | Heterogeneous |
Discussion
In the present study, only six patients with ANP
were encountered during the enrolment period. ANPs occurred equally
in men and women, with a mean patient age of 42±28.4 years. The
most common symptoms were nasal obstruction (n=6), rhinorrhea
(n=5), epistaxis (n=3), hyposmia (n=2), cheek swelling (n=1) and
epiphora (n=1). All patients reported symptomatic relief
postoperatively. However, improvement was evaluated clinically
during follow-up without using a standardized symptom-scoring tool,
which represents a limitation of this study. Polyp sizes ranged
from 2.7 to 5.0 cm, with a mean diameter of 3.57±0.8 cm. The
maxillary sinus was the most frequent site of origin, accounting
for 66.7% of cases, consistent with previous reports (3,4).
The precise pathogenesis of ANPs remains elusive,
but two prevailing theories have been proposed. One hypothesis
suggests that pedicle compression by adjacent nasal structures,
particularly when the polyp arises from the maxillary sinus or
nasal cavity, leads to stasis, ischemia and necrosis. Batsakis and
Sneige (2) proposed that sinonasal
polyps are susceptible to vascular compromise at key anatomical
sites, such as the polyp pedicle, sinus ostium, posterior end of
the inferior turbinate, posterior choana and nasopharynx. An
alternative theory attributes ANP formation to hematoma
development, which may result from operation, trauma, bleeding
disorders or other causes of hemorrhage. In this scenario, sinus
hypoventilation promotes blood pooling and stagnation, eventually
leading to organized hematoma and reactive tissue changes (2,5-9).
In all six cases in the present cohort, the polyps
were located in anatomically vulnerable regions. Four originated
near the maxillary sinus ostium, while the remaining two arose from
the superior and middle turbinates, where adjacent structures could
compress the pedicle. Furthermore, all polyps extended into the
posterior choana, contributing to the likelihood of vascular
compromise. This is the first reported case series of ANPs from our
institution, and two cases demonstrated atypical imaging features,
including the absence of a peripheral hypointense rim and subtle
bone remodeling. These findings support the first hypothesis,
suggesting the development of an inflammatory polyp as the initial
event, followed by compression-induced ischemia and necrosis.
Hypertension (HTN), diabetes mellitus (DM) and
aspirin use may contribute to the pathophysiology of ANPs (10-12).
Elevated blood glucose and blood pressure can compromise blood
vessel integrity, increasing the risk of thrombosis and infarction.
While aspirin and other anticoagulants inhibit prostaglandin
synthesis, dilate blood vessels and prevent clot formation, they
may predispose to hemorrhage. In this study, only one patient had
HTN and none had DM or were on aspirin therapy. Therefore, no
significant association was observed between ANPs and HTN, DM or
aspirin use.
ANPs typically appear on CT as sinus-expanding
masses with heterogeneous density. Chronic inflammatory obstruction
may lead to bony changes such as destruction or hyperostosis
(5,6). However, these features are
non-specific to ANPs and can be seen in malignant and benign
sinonasal tumors, such as inverted papillomas, hemangiomas and
juvenile angiofibromas (13). In
the present study, CT revealed bony alterations in 83.3% of cases:
Expansile remodeling in five patients (83.3%), bony destruction in
two (33.3%) and hyperostosis in two (33.3%).
MRI has proven useful in distinguishing ANPs from
other lesions. Typically, ANPs show mild hyperintensity on
T1-weighted images and heterogeneous hyperintensity with a
peripheral hypointense rim on T2-weighted sequences. This rim is
often attributed to hemosiderin deposition from prior hemorrhage,
supporting the diagnostic value of MRI (5,13,14).
In the present study, all four cases with MRI data exhibited mild
hypointensity on T1-weighted images and marked heterogeneous
hyperintensity on T2-weighted images. A peripheral hypointense rim
was observed in three patients (75%), consistent with the typical
MRI findings for ANPs. The current findings are consistent with
previous reports (4,5), which described heterogeneous T2
signal intensity and peripheral hypointense rims as typical
findings. Unlike common nasal polyps, which are often bilateral and
exhibit minimal vascularity, ANPs tend to be unilateral, larger in
size and display prominent vascular features on imaging.
Furthermore, while inverted papillomas exhibit a cerebriform
pattern on MRI and sinonasal malignancies show aggressive bone
destruction, ANPs characteristically display benign remodeling with
the noted T2 hypointense rim (13). This study is limited by its small
sample size, retrospective design, incomplete MRI data for all
patients and absence of a control group, which may restrict broader
applicability.
Complete surgical excision remains the treatment of
choice for ANPs. All six patients underwent endoscopic sinus
surgery, with a mean follow-up of 63.5±17.4 months (range: 15-132
months). No recurrences were observed.
Recognizing the imaging and clinical features of
ANPs can help avoid misdiagnosis with more aggressive sinonasal
tumors, reduce unnecessary extensive surgical procedures and allow
for more targeted diagnostic and therapeutic strategies.
In conclusion, preoperative diagnosis of ANPs
remains challenging due to their clinical and radiological
resemblance of other sinonasal tumors. The CT characteristics, such
as expansile remodeling, bony destruction and hyperostosis, may
support the diagnosis but are not specific to ANPs. Conversely, MRI
offers more distinctive signal patterns, providing greater
diagnostic specificity. Incorporating MRI into the diagnostic
workflow may enhance the accuracy of differentiating ANPs from
other sinonasal lesions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DHL and SY conducted and designed the research, and
checked and confirmed the authenticity of the raw data. DHL, SY and
SCL performed the experiments, analyzed the data and drafted the
manuscript. All authors contributed to data interpretation, table
design and manuscript revision. All authors read and approved the
final version.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board (IRB) of Chonnam National University Hwasun Hospital (Hwasun,
Korea; approval no. CNUHH-2023-127). Patient consent for
publication was waived by the IRB due to the retrospective nature
of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yfantis HG, Drachenberg CB, Gray W and
Papadimitriou JC: Angiectatic nasal polyps that clinically simulate
a malignant process: Report of 2 cases and review of the
literature. Arch Pathol Lab Med. 124:406–410. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Batsakis JG and Sneige N: Choanal and
angiomatous polyps of the sinonasal tract. Ann Otol Rhinol
Laryngol. 101:623–625. 1992.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sheahan P, Crotty PL, Hamilton S, Colreavy
M and McShane D: Infarcted angiomatous nasal polyps. Eur Arch
Otorhinolaryngol. 262:225–230. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang YZ, Yang BT, Wang ZC, Song L and Xian
JF: MR evaluation of sinonasal angiomatous polyp. AJNR Am J
Neuroradiol. 33:767–772. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zou J, Man F, Deng K, Zheng Y, Hao D and
Xu W: CT and MR imaging findings of sinonasal angiomatous polyps.
Eur J Radiol. 83:545–551. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Unlu HH, Mutlu C, Ayhan S and Tarhan S:
Organized hematoma of the maxillary sinus mimicking tumor. Auris
Nasus Larynx. 28:253–255. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hopkins C, Browne JP, Slack R, Lund VJ,
Topham J, Reeves BC, Copley LP, Brown P and van der Meulen JH:
Complications of surgery for nasal polyposis and chronic
rhinosinusitis: The results of a national audit in England and
Wales. Laryngoscope. 116:1494–1499. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee HK, Smoker WR, Lee BJ, Kim SJ and Cho
KJ: Organized hematoma of the maxillary sinus: CT findings. AJR Am
J Roentgenol. 188:W370–W373. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoon TM, Kim JH and Cho YB: Three cases of
organized hematoma of the maxillary sinus. Eur Arch
Otorhinolaryngol. 263:823–826. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tam YY, Wu CC, Lee TJ, Lin YY, Chen TD and
Huang CC: The clinicopathological features of sinonasal angiomatous
polyps. Int J Gen Med. 9:207–212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suryawanshi M, Saindani S, Bhatta S,
Suryawanshi R, Sawarkar S and Bhola G: Angiomatous nasal polyp: The
great emulator-a case report. Indian J Otolaryngol Head Neck Surg.
74 (Suppl 3):S4730–S4733. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dai LB, Zhou SH, Ruan LX and Zheng ZJ:
Correlation of computed tomography with pathological features in
angiomatous nasal polyps. PLoS One. 7(e53306)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim EY, Kim HJ, Chung SK, Dhong HJ, Kim
HY, Yim YJ, Kim ST, Jeon P and Ko YH: Sinonasal organized hematoma:
CT and MR imaging findings. AJNR Am J Neuroradiol. 29:1204–1208.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
De Vuysere S, Hermans R and Marchal G:
Sinochoanal polyp and its variant, the angiomatous polyp: MRI
findings. Eur Radiol. 11:55–58. 2001.PubMed/NCBI View Article : Google Scholar
|