Introduction
Esophageal cancer, a leading global cause of
cancer-related mortality, accounts for over 600,000 annual
diagnoses and 540,000 deaths worldwide. Significant epidemiological
disparities exist, with squamous cell carcinoma predominating in
Asian and sub-Saharan African populations, while adenocarcinomas
prevail in Western nations. Although contemporary multimodal
approaches combining surgery, chemoradiotherapy, and emerging
immunotherapies have advanced treatment paradigms, five-year
survival rates remain below 30%, primarily attributed to frequent
late-stage presentation and molecular complexity. Critical clinical
barriers persist, including the absence of reliable early detection
methods, therapeutic resistance, frequent post-treatment
recurrence, and metastatic dissemination.
Overall survival (OS) in patients with advanced or
metastatic esophageal squamous cell cancer (ESCC) is generally poor
after first-line therapy, and there is no effective treatment after
tumor progression. The median progression-free survival (PFS) in
these patients is ~7 months after first-line immunotherapy plus
chemotherapy (1), while PFS is
only 2 months after second-line immunotherapy alone (2). The management of later-line therapies
for advanced esophageal cancer requires highly individualized
approaches, tailored to the patient's performance status, tumor
burden, and history of prior treatment. In this report, the case of
a patient with advanced ESCC who exhibited a lengthy PFS after
fourth-line therapy with anlotinib combined with S-1 after failure
of treatment with platinum-based chemotherapy, radiotherapy and
immunotherapy, is presented.
Case presentation
A 59-year-old male was admitted to Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China) in September 2017, with dysphagia for 1
month. He had a medical history of hypertension and coronary
atherosclerotic heart disease, as well as a personal history of
alcohol consumption and smoking. The white blood cell (WBC) count
was 17.39x109/l, while a barium X-ray examination
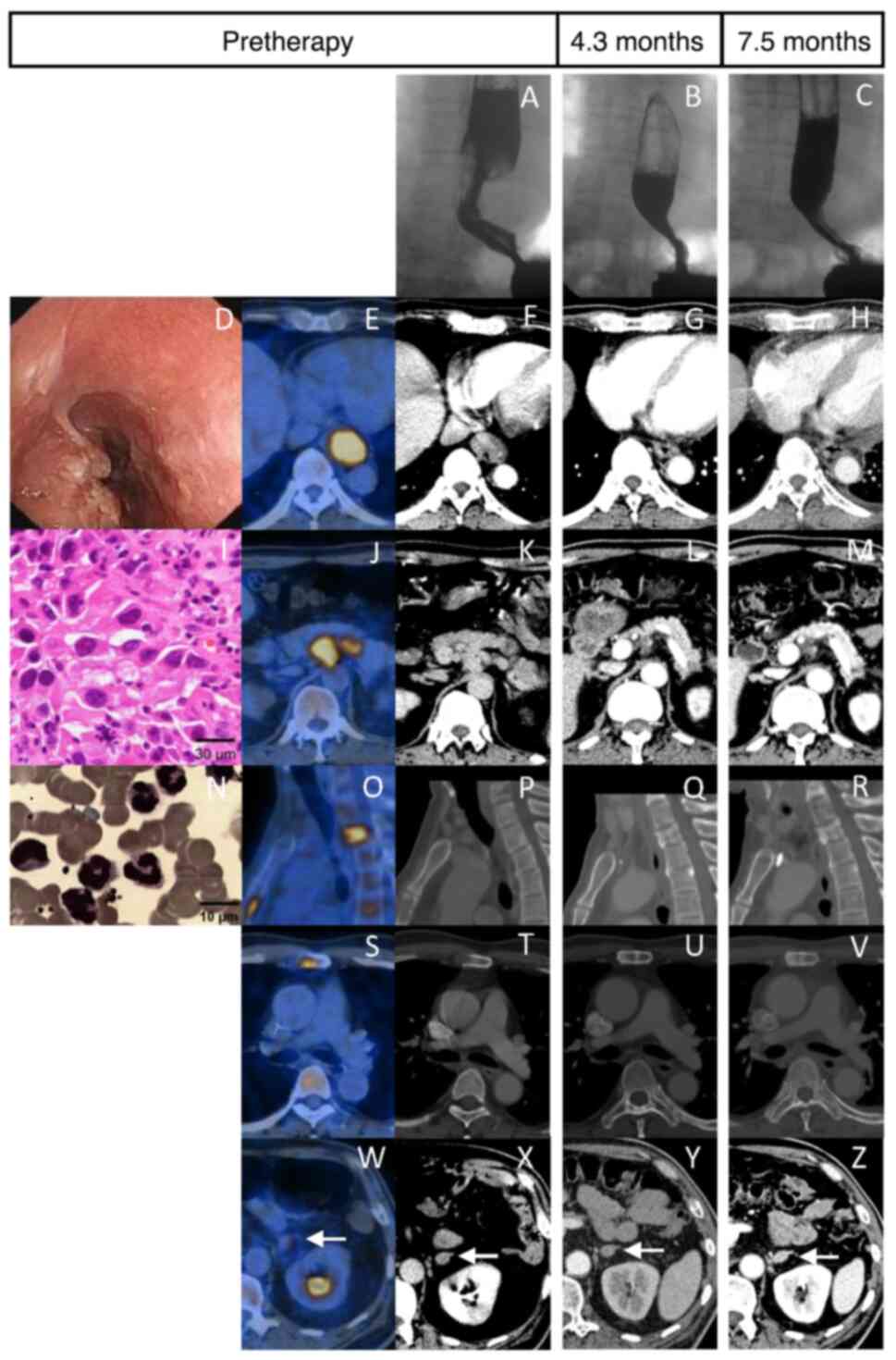
revealed an 8-cm stenosis in the lower esophagus (Fig. 1A). A gastroscopy identified an
ulcerous esophageal mass 38-43 cm from the incisors (Fig. 1D), which was biopsied and confirmed
as ESCC with poor differentiation (Fig. 1I). A PET scan showed high [18F]
fluorodeoxyglucose avidity at multiple sites [lower esophagus,
maximal standardized uptake value (SUVmax)=16.3; celiac lymph
nodes, SUVmax=8.0-11.6; second thoracic vertebra and sternum,
SUVmax=5.0-10.2; left adrenal gland, SUVmax=4.4] (Fig. 1E, J, O,
S and W). Thus, the diagnosis was ESCC with
multiple metastases (cT2N2M1 according to AJCC 7th edition).
Despite multiple intravenous antibiotics administered over a period
of >20 days to combat suspected infection, the WBC count reached
20.45x109/l. Moreover, bone marrow cytology indicated
active proliferation of nucleated cells and a high granulocyte
ratio with normal cell morphology (Fig. 1N). Due to the fact that the
positive rate and score of neutrophil alkaline phosphatase in
peripheral blood smear were 87% and 347, respectively, leukocytosis
was considered a paraneoplastic leukemoid reaction (PLR).
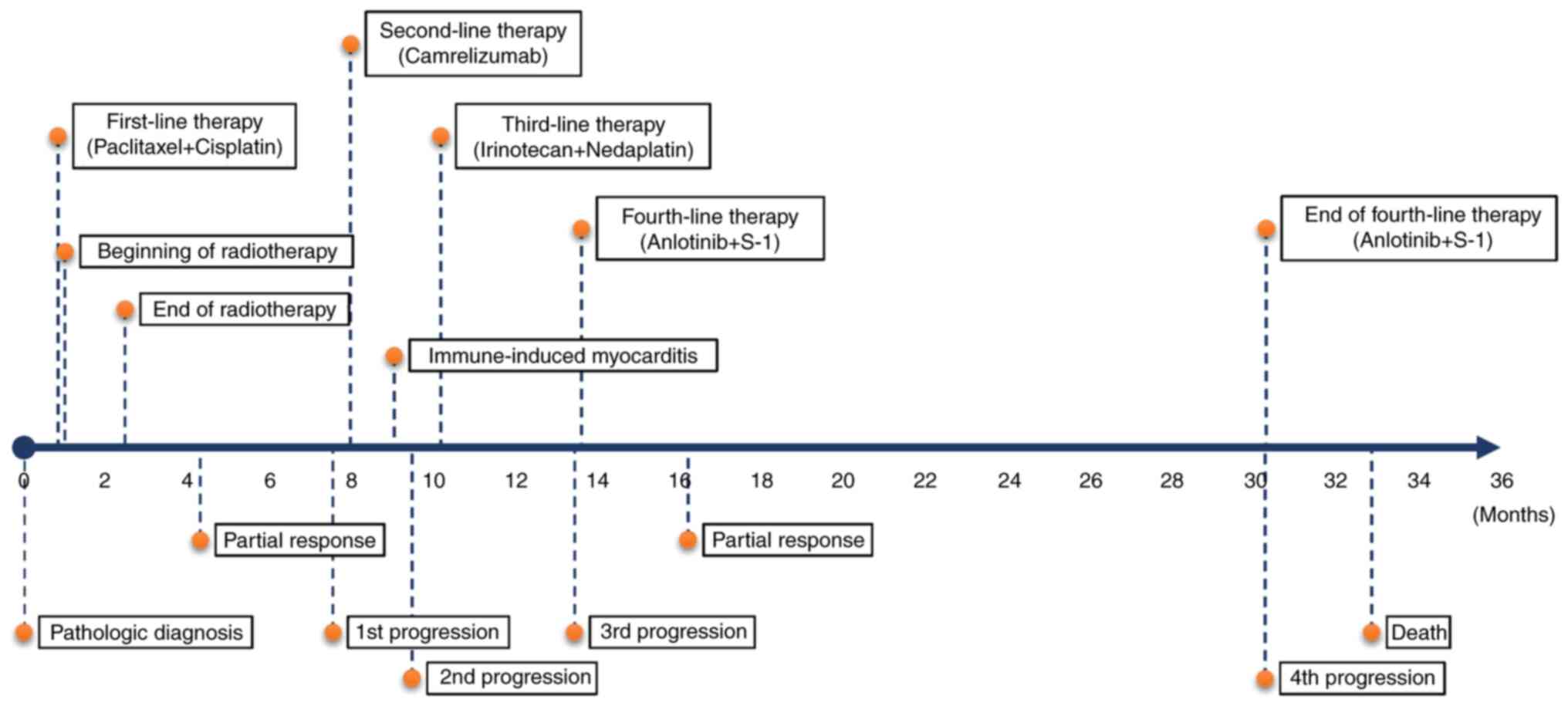
Consequently, six cycles of paclitaxel and cisplatin were
administered as first-line therapy, and leukocytosis resolved
quickly after 1 week. Meanwhile, intensity-modulated radiotherapy
was delivered to the esophageal tumor and metastatic celiac nodes
(60 Gy/30 F) and the second thoracic vertebra (40 Gy/20 F). The
patient exhibited a partial response 4.3 months after diagnosis
based on the Response Evaluation Criteria in Solid Tumors version
1.1 (Fig. 1B, G, L,
Q, U and Y).
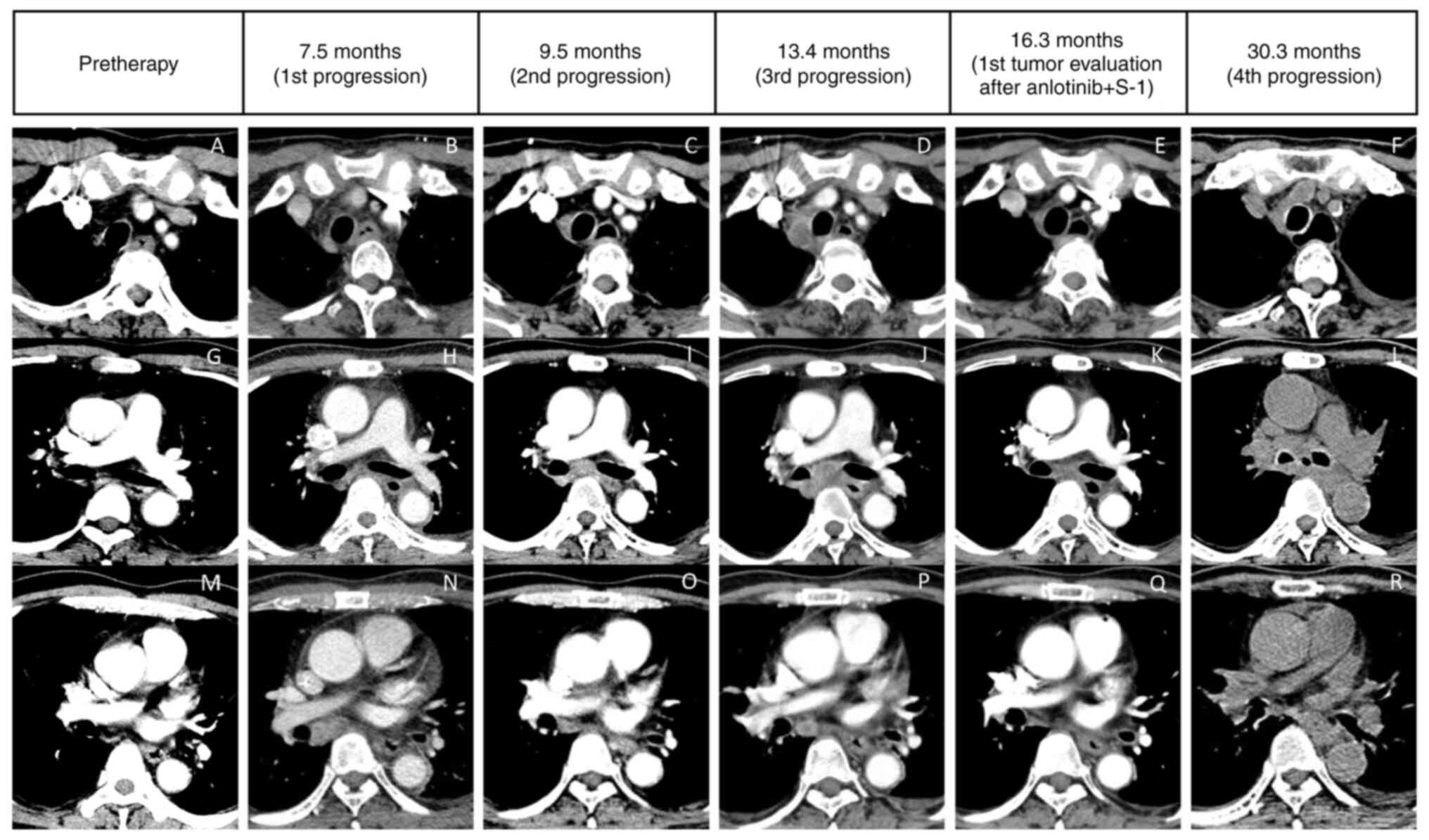
After ~7.5 months, chest computed tomography (CT)
revealed a mild compression fracture of the second thoracic
vertebra (Fig. 1R) and a marked
enlargement of the lymph node in the medial right bronchus
intermedius (Fig. 2N), indicating
progressed disease. The patient received camrelizumab as
second-line monotherapy. In the routine follow-up after two cycles,
the levels of troponin I (731.8 pg/ml), alanine aminotransferase
(110 U/l), aspartate aminotransferase (203 U/l) and creatine kinase
(>300 U/l) were found to be elevated, in combination with muscle
soreness in the lower extremities and hoarseness without chest
pain. Emergency coronary arteriography revealed no obvious
abnormality, and the patient was diagnosed with immune-induced
myocarditis, myositis and hepatitis. Although camrelizumab was
stopped and the blood indices returned to normal after
corticosteroid therapy, the tumor continued to progress (new
subcarinal node metastasis) (Fig.
2I).
Third-line therapy included two cycles of irinotecan
and nedaplatin; however, the patient was unable to tolerate it due
to thrombocytopenia (grade 3), leukopenia (grade 2), anemia (grade
2) and weakness (grade 1), and the tumor progressed after 4 months
(upper paratracheal and subcarinal node metastases) (Fig. 2D and J).
Anlotinib (12 mg, once daily for 14 days, followed
by 7 days off) and S-1 (60 mg, twice daily for 14 days, followed by
7 days off) were administered as fourth-line therapy, and this
treatment reduced the upper paratracheal and subcarinal node
metastases after 2.9 months (Fig.
2E and K), whereas the other
lesions remained stable. Afterward, the patient underwent tumor
response assessment every two cycles via contrast-enhanced CT and
esophageal radiography. Anorexia (grade 2), skin pigmentation
(grade 2), hand-foot syndrome (grade 2), hypertension (grade 1) and
weakness (grade 1) were the main toxicities. The patient received
combination treatment with anlotinib and S-1 until dry cough and
chest distress were aggravated after 16.7 months. Chest CT revealed
the progression of subcarinal node metastases, which had invaded
the left main bronchus (Fig. 2L).
Eventually, the patient succumbed to dyspnea and pulmonary
infection. The PFS after administration of anlotinib and S-1 was
16.7 months, while the OS was 32.9 months from the pathologic
diagnosis of ESCC (Fig. 3).
Discussion
A PLR is a hematological paraneoplastic syndrome
induced by multiple solid tumors that are characterized by
leukocytosis >20,000-50,000/µl when infection, hematopoietic
growth factors, corticosteroids and leukemia are excluded. In most
patients, PLRs are generally associated with a large tumor burden
and a poor survival time of #x003C;1 year (3). The patient in the present case report
exhibited an OS of 32.9 months, indicating that effective therapy
could improve survival for patients with PLR.
Anlotinib is an oral tyrosine kinase inhibitor that
targets vascular endothelial growth factor receptors 1-3,
fibroblast growth factor receptors 1-4, platelet-derived growth
factor receptors α and β, Ret and c-Kit (4). The effectiveness of anlotinib has
been confirmed in multiple solid tumors (5). In previously treated advanced or
metastatic ESCC, anlotinib improved median PFS (3.02 vs. 1.41
months) with tolerable adverse events (incidence of grades 3-4: 37%
vs. 11%) compared with placebo (6). In patients with advanced ESCC, the
combination of anlotinib with nedaplatin and raltitrexed as
fourth-line therapy resulted in a PFS of 9 months (7). In a phase II trial involving patients
with stage IV non-small cell lung cancer, the combination of
anlotinib with S-1 in the third- or later-line treatment exhibited
promising antitumor outcome and manageable toxicity (8). Patients with ESCC in whom first-line
therapy had failed generally showed a poor performance score, poor
nutrition status and poor tolerance to subsequent therapy. As a
result, high efficacy and mild toxicity are important aspects when
selecting a drug in this setting. Both anlotinib and S-1 meet this
criterion with different toxicity profile. The patient in the
present case report had a satisfactory PFS of 16.7 months and mild
toxicities after fourth-line therapy, indicating that anlotinib
plus S-1 is a rational combination for previously treated advanced
ESCC, especially in patients intolerant of immunotherapy.
In conclusion, the combination of anlotinib with S-1
may be a competitive choice for treating patients with advanced
ESCC who are resistant to platinum-based chemotherapy, radiotherapy
and immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JY conceptualized the study, performed data curation
and visualization, conducted formal analysis and investigation,
wrote the original draft, and wrote, reviewed and edited the
manuscript. CL performed data curation and visualization and
conducted investigation. LL supervised the study, provided
resources, and wrote, reviewed and edited the manuscript. KY
conceptualized and supervised the study, provided resources, and
wrote, reviewed and edited the manuscript. JY and CL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved [approval no.
2017(82)] by the Ethics Committee of Union Hospital, Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China). Written informed consent to participate was obtained from
the patient.
Patient consent for publication
Due to the patient's death before the completion of
the present study, written informed consent for publication was
obtained from his family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang J, Xu J, Chen Y, Zhuang W, Zhang Y,
Chen Z, Chen J, Zhang H, Niu Z, Fan Q, et al: Camrelizumab versus
investigator's choice of chemotherapy as second-line therapy for
advanced or metastatic oesophageal squamous cell carcinoma
(ESCORT): A multicentre, randomised, open-label, phase 3 study.
Lancet Oncol. 21:832–842. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Granger JM and Kontoyiannis DP: Etiology
and outcome of extreme leukocytosis in 758 nonhematologic cancer
patients: A retrospective, single-institution study. Cancer.
115:3919–3923. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Syed YY: Anlotinib: First global approval.
Drugs. 78:1057–1062. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang
Z, Zhao F, Ahmad R and Zhao J: Anlotinib: A novel multi-targeting
tyrosine kinase inhibitor in clinical development. J Hematol Oncol.
11(120)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang J, Xiao J, Fang W, Lu P, Fan Q, Shu
Y, Feng J, Zhang S, Ba Y, Zhao Y, et al: Anlotinib for previously
treated advanced or metastatic esophageal squamous cell carcinoma:
A double-blind randomized phase 2 trial. Cancer Med. 10:1681–1689.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang D, Xu F, Lai X, Li Y, Gao H, Xu Y,
Chen R and Ma D: Combined treatment with anlotinib and chemotherapy
for advanced esophageal squamous cell carcinoma improved patient
survival: A case report. Am J Transl Res. 12:6578–6583.
2020.PubMed/NCBI
|
|
8
|
Xiang M, Yang X, Ren S, Du H, Geng L, Yuan
L, Wen Y, Lin B, Li J, Zhang Y, et al: Anlotinib combined with S-1
in third- or later-line stage IV non-small cell lung cancer
treatment: A phase II clinical trial. Oncologist. 26:e2130–e2135.
2021.PubMed/NCBI View Article : Google Scholar
|