Introduction
Gastric cancer is the fifth most common cancer
globally, with the fifth highest rates of mortality (1). Notably, most patients with gastric
cancer present with stages IB to IIIC of the disease, according to
the American Joint Committee on Cancer (AJCC). At present, the gold
standard of surgical treatment involves total gastrectomy combined
with D2 lymph node dissection, followed by gastrointestinal tract
reconstruction (2-5). The 5-year
survival rates following R0 resection vary, ranging from 89.9% for
stage IB disease to 20.2% for stage IIIC disease, as reported in
the 8th edition of the TNM classification (6). Due to the high morbidity rates
associated with gastrectomy, research has focused on improving
perioperative care and the quality of life of patients (7,8).
Moreover, despite its curative intent, total gastrectomy often
leads to persistent post-gastrectomy syndromes that can
significantly impair short and long-term quality of life (9-11). Importantly,
quality of life (HRQoL) has also been identified as an independent
prognostic factor for survival, underscoring its importance in
postoperative care (12,13).
Results of previous studies demonstrated that
minimizing surgical intervention may be associated with improved
rates of recovery (7,8,14).
The Enhanced Recovery after Surgery (ERAS) guidelines offer
globally recognized perioperative recommendations for various
surgeries. Notably, the guidance recommends against the use of
drain placement to reduce complications, such as pain, delayed
mobilization and infection (8).
Historically, drain placement remained a routine intervention
during gastrectomy (15), with
literature reporting usage rates of 57.7-62.8% (16-18). In 2014,
Mortensen et al (19)
introduced the ERAS guidelines for gastrectomy in gastric cancer,
based on two meta-analyses (including 400 cases) and two
prospective trials. Results of these studies revealed no
significant differences in hospital stay, morbidity rates,
mortality rates or complications between patients with and without
drains (20-23). However, due
to heterogeneity in surgery type and data collection, the evidence
was considered insufficient.
Although the ERAS guidelines provide clear
recommendations for the use of drains in surgeries such as
pancreaticoduodenectomies and colectomies (24,25),
their usage in gastrectomy for gastric cancer remains the topic of
debate. Japanese guidelines support the routine use of drains
following total gastrectomy, with removal by the fifth
post-operative day (26). Results
of previous studies in Japan also highlighted the prognostic value
of drain contents post-gastrectomy, reinforcing this practice
(15,27-31). By contrast,
Western guidelines, such as those from the American College of
Surgeons, advise against routine drain use, suggesting placement
only in exceptional cases (32).
This conflicting evidence may lead to uncertainty regarding drain
use in gastrectomy, resulting in limited adherence to ERAS
recommendations in Western practices (33,34).
The present prospective, non-randomized, controlled
clinical trial aimed to individualize drain use following
gastrectomy for the treatment of gastric cancer. The tailored
approach described in the present study is based on criteria
developed using previous literature, including established risk
factors for post-operative complications. Significantly, the
objective of the study was not to assess the benefit of drains
themselves, but rather to determine whether these predefined
clinical criteria can effectively guide the decision to place a
drain during open total gastrectomy. To the best of our knowledge,
the present study is the first to propose specific criteria
addressing routine prophylactic drain use in total gastrectomy,
aiming to provide a structured framework for clinical decision
making in high-volume centers.
Materials and methods
Study design
The DRains After Gastrectomy (DRAG) trial (Clinical
Trials.gov NCT 04288661) was a prospective,
non-randomized, controlled clinical trial involving patients
diagnosed with gastric or esophagogastric junctional neoplasm. All
surgeries were performed by experienced Foregut Surgeons in The
University Surgical Department at Hippocration General Hospital in
Athens, Greece, a leading referral centre for upper
gastrointestinal tract diseases. Patients underwent open total
gastrectomy with D2 lymph node dissection, followed by Roux-en-Y
gastrointestinal tract reconstruction. This was performed in
accordance with a pre-defined, ERAS-compliant perioperative
departmental protocol. The study design was conducted according to
the following sequence: i) A literature review was carried out to
identify commonly reported risk factors for major intra-abdominal
complications following total gastrectomy; ii) The most
consistently cited risk factors were selected to form the basis for
the drain placement criteria; iii) Eligible patients were screened
according to predefined preoperative and intraoperative criteria;
iv) The final decision regarding drain placement was made
intraoperatively-after completion of the resection and
reconstruction, and immediately prior to abdominal wall
closure-based on the selected criteria; v) All patients were
followed for 30 days postoperatively, in line with the
institutional monitoring protocol.
As aforementioned, the first step involved a
comprehensive review of the literature, focused on the use of
gastrectomy for the treatment of gastric cancer, and on the
identification of risk factors associated with post-operative
complications, such as esophagojejunal anastomotic leak or
bleeding. The present review included 11 retrospective studies and
two randomized controlled trials, and these highlighted numerous
risk factors associated with the aforementioned complications. Of
these, the most consistently reported factors were selected for
subsequent analyses. The following criteria were suggested for the
placement of a drain, as the presence of these risk factors
signified an increased risk of post-operative complications
(15,35-47): i) Chronic
cardiopulmonary disease; ii) chronic oral steroid use (≥5 mg/day
prednisone equivalent for >1 month); iii) intraoperative
hemodynamic instability requiring vasopressors; iv) intraoperative
blood loss exceeding 250 ml (mean amount of estimated blood loss in
all included studies), referring to intra-operative non-traumatic,
non-vascular blood loss; v) vessel injury involving the celiac axis
or the associated branches; vi) injury to adjacent structures, such
as the pancreas, spleen or duodenum; vii) tension at the
anastomosis, or the requirement for anastomosis reconstruction; and
viii) concerns regarding the integrity of the duodenal stump,
particularly due to potential staple misfiring or compromised
tissue quality.
All patients that met the eligibility criteria
underwent pre-operative evaluations, including clinical
examinations, blood tests, imaging studies and consultations with
specialist clinicians. The eligibility criteria included the
following: Histologically confirmed gastric adenocarcinoma; planned
open total gastrectomy with D2 lymphadenectomy; no evidence of
distant metastasis (M1); absence of massive ascites or severe
cachexia; no unplanned organ resections; no severe cardiovascular,
respiratory, renal, hepatic, or psychiatric conditions; not
currently enrolled in another clinical trial; and no
contraindications such as pregnancy or poor compliance with
clinical protocols. Intraoperative criteria that ruled out
eligibility included findings of unresectable or metastatic disease
and cases requiring conversion to subtotal gastrectomy.
Intra-operatively, all dissections were carried out
with an ultrasonic vibration scalpel to minimise blood loss. Saline
was used for irrigating the surgical field, and volume was
monitored and excluded from the total calculated blood loss.
Notably, the protocol followed by the University Surgical
Department at Hippocration General Hospital does not routinely
incorporate exploratory laparoscopy or peritoneal cytology. In some
cases, high procedural complexity may necessitate lymphadenectomy
beyond a standard D2 dissection (48). However, to maintain uniformity,
only patients undergoing D2 gastrectomy were included in the
present study.
Final allocation to the drain or non-drain group was
made at the end of the procedure and prior to abdominal wall
closure. The decision for drain was based on the aforementioned
criteria. The patients for whom drain placement was decided,
received a standard 10-mm (~30 Fr) silicone tube drain near the
esophagojejunal anastomosis (Shanghai International Holding Corp
GmbH). By contrast, no drain was placed in the patients who did not
meet the criteria. A CONSORT flowchart illustrating patient
enrolment, exclusion, and group allocation is provided in Fig. 1.
According to the departmental protocol, patients
were gradually mobilized directly following surgery, when feasible.
At 48 and 72 h post-surgery, an oral gastrografin fluoroscopy study
was performed for each patient, for the detection of early
anastomotic leaks. Following a routine radiological study, patients
were initiated on a liquid diet, which was subsequently advanced to
pureed food on the fourth post-operative day, and a soft diet on
the fifth post-operative day. In the drain group, drains were
monitored daily for 24-h output volume and fluid characteristics,
including amylase and bilirubin concentrations. They were removed
from patients in the drain group on the fifth post-operative day,
when drainage volume was <50 ml within the preceding 48 h. Drain
output was considered excessive if the volume exceeded 100 ml on
postoperative day five (18,26)
or if the drain fluid amylase level was greater than three times
the upper limit of normal serum amylase on or after postoperative
day three, consistent with the definition of a postoperative
pancreatic fistula (49). Patients
were discharged on the fifth post-operative day when no
complications were observed.
Drain and non-drain groups were compared within the
following cohorts: i) The overall study population; ii) patients
with complications; and iii) patients without complications.
Primary outcomes were as follows: i) Pain level
assessment during the first 5 post-operative days, using the Visual
Analog Scale (VAS). Notably, the VAS is a 10-point scale, with 0
representing no pain and 10 indicating the highest level of pain. A
score of >7 is considered high (50); ii) post-operative nausea and
vomiting (PONV) within the first 5 post-operative days; iii)
initiation of feeding, carried out between 48 and 72 h
post-surgery. According to the ERAS-compliant institutional
protocol, initiation of feeding beyond the third post-operative day
is considered delayed; iv) post-operative bowel mobilization,
primarily measured by the time to the first passage of flatus; v)
patient mobilization, following a specific post-operative
mobilization schedule for each day. Failure to meet the specific
criteria was indicative of a delay in mobilization; and vi) length
of hospital stay, referring to the time from the day of the
operation to the day of discharge.
Secondary outcomes included: i) mortality, referring
to death occurring within 30 days post-surgery; ii) readmissions
due to surgical complications, occurring within 30 days
post-surgery, and iii) reoperations due to surgical complications,
occurring within 30 days post-surgery.
The present study was conducted in accordance with
the principles outlined in the Declaration of Helsinki (51) and the Guidelines of Good Clinical
Practice (52). The final study
protocol and the informed consent form for participant inclusion
were approved by the Institutional Review Board of Hippocration
General Hospital (approval no. 1678/31-01-2020; Athens, Greece).
The IRB also conducted regular assessments as required, to ensure
ongoing compliance with lawful medical practice throughout the
trial.
Statistical analysis
Statistical analysis was performed using R software
(version, 4.3.0; R foundation for Statistical Computing).
Descriptive statistics for quantitative data included descriptive
characteristics, and these were expressed as the median and
minimum/maximum values and quartile 1 (Q1) to quartile 3 (Q3)
range, and/or for completeness reasons the mean ± standard
deviation (SD). Descriptive statistics for qualitative data were
expressed as the frequency of occurrence and the relevant
percentage. Comparisons were performed between patients with
drainage and those without drainage, and additional analyses were
performed between patients with and without complications.
Qualitative data were analysed using Chi-squared and Fisher's exact
tests when required and quantitative data were analysed using the
Mann Whitney U test, as data normality was not always confirmed
(via the Shapiro Wilk test). P<0.05 was considered to indicate a
statistically significant difference. For time to event analysis,
that is to visualize and report time to gut mobilization and days
of hospitalization, the Kaplan-Meier estimator was applied.
To address potential confounding and strengthen
causal inference between study groups, a propensity score matching
(PSM) approach was applied using R version 4.4.0(53). The binary clinical covariates were
blood loss, vessel damage, anastomosis, stump and respiratory
issues, the criterion of adjacent structures was not involved since
none of the patients had such issues in addition drainage volume
was also not possible to account as only two patients had abnormal
drainage volume. Eventually, the application of a drain was
modelled using logistic regression to estimate the propensity
scores and matching was performed using 1:1 nearest neighbour
matching without replacement through the MatchIt package (54) especially developed for the R
environment. Covariate balance was evaluated using standardized
mean differences. Moreover, to explore potential impact of possible
confounders, a Rosenbaum sensitivity analysis was conducted using
the rbounds package (55).
Finally, subgroup analysis was performed between patients with and
without a drain, separately for the patients that had complications
and those who did not experience such complications.
Results
Comparison of the overall study
population
The present study was conducted from February 2020
to March 2023. Of the 60 eligible participants, 40 were assigned to
the drain group, while 20 were included in the non-drain group, as
per the aforementioned criteria. The mean age of patients was
71±9.6 years. Pre-operative histological diagnosis primarily
identified intestinal-type gastric cancer in 46.6% of cases, with
the body of the stomach being the most common location of observed
lesions (43.3%). In addition, 25% (n=15) of patients had undergone
pre-operative chemotherapy due to nodal positivity (N≥1) at
diagnosis, or due to unresectability observed during exploratory
laparotomy (Table I). As there
were no patients on chronic steroids or patients that developed
intra-operative hemodynamic instability, these factors were not
included in the results. Results of the present study revealed no
statistically significant differences in pre-operative
characteristics between the drain and non-drain groups (Table II).
 | Table IDescriptive statistics of
perioperative characteristics. |
Table I
Descriptive statistics of
perioperative characteristics.
|
Characteristics | Value |
|---|
| Age, years | 71±9.6, median
(Q1-Q3): 73 (65-77.5), min: 48, max: 87 |
| Sex, male | 32 (53.33%) |
| Charlson score | 2.45±0.85, median
(Q1-Q3): 2 (2-2.5), min: 2, max: 5 |
| ECOG | |
|
1 | 8 (13.33%) |
|
0 | 52 (86.66%) |
| Histology | |
|
Diffuse | 22 (36.67%) |
|
Intestinal | 28 (46.67%) |
|
Other | 7 (11.67%) |
| Location | |
|
Esophago-gastric
junction | 12 (20.00%) |
|
Fundus | 5 (8.33%) |
|
Body | 26 (43.33%) |
|
Prepyloric
antrum | 16 (26.67%) |
|
Pylorus | 1 (1.67%) |
| Positive
preoperative chemotherapy | 15 (25.42%) |
| CA
positivitya | |
|
CA 19.9 | 8 (13.33%) |
|
CA 15.3 | 6 (10.00%) |
|
CA 125 | 3 (5.00%) |
|
CEA | 7 (11.60%) |
| Intraoperative
characteristics | |
|
Duration of
operation, min | 197.5±37.2, median
(Q1-Q3): 200 (180-227.5), min 120, max 180 |
|
Blood
transfusion | 8 (13.33%) |
|
FFP
transfusion | 5 (8.33%) |
|
Allergic
reaction to drugs | 0 (0.00%) |
|
Intraoperative
incidents | 7 (11.67%) |
| Criteria for drain
placement | |
|
Blood loss
of >250 ml | 20 (33.33%) |
|
Vessel
injury | 3 (5.00%) |
|
Anastomosis
concerns | 11 (18.33%) |
|
Adjacent
structures injury | 0 (0.00%) |
|
Stump
integrity concerns | 3 (5.00%) |
|
Chronic
cardiopulmonary comorbidity | 3 (5.00%) |
| Postoperative
characteristics | |
|
Swallow test
(negative) | 58 (96.67%) |
|
Visual
analogue score (high) | 34 (56.67%) |
|
Postoperative
nausea and vomiting | 29 (48.33%) |
|
Surgical
site infection | 12 (20.00%) |
|
Mobilization
delay | 22 (36.67%) |
|
Oral feeding
delay | 17 (28.33%) |
|
Gut motility
start (postoperative day) | 3.58±1.04, median
(Q1-Q3): 3 (3-4), min: 2, max 6 |
|
Intra-abdominal
complication | 9 (15.00%) |
|
Extra-abdominal
complication | 5 (8.33%) |
|
Length of
stay (post-op to exit) | 9.5±9.01, median
(Q1-Q3)6.5 (5-8), min: 5, max: 59 |
| AJCC stage | |
|
1A | 9 (15.00%) |
|
1B | 10 (16.66%) |
|
2A | 16 (26.66%) |
|
2B | 12 (20.00%) |
|
3A | 5 (8.33%) |
|
3B | 6 (10.0%) |
|
3C | 2 (3.33%) |
| Outcomes | |
|
Mortality | 3 (5.00%) |
|
Re-admission | 5 (8.33%) |
|
Re-operation | 6 (10.00%) |
 | Table IIComparison of demographics, and
preoperative and intraoperative characteristics between the two
groups. |
Table II
Comparison of demographics, and
preoperative and intraoperative characteristics between the two
groups.
| Characteristic | Without drain
(n=20) | With drain
(n=40) | P-value |
|---|
| Age, years | 73 (66.5-77.5) | 73.5 (63-78) | 0.8138 |
| Sex (male) | 9/45.00% | 23/57.50% | 0.4180 |
| Charlson score | 2 (2-2) | 2 (2-3) | 0.1567 |
| ECOG | 0 (0-0) | 0 (0-0) | 0.5943 |
| Histology | | | |
|
Diffuse | 7/35.00% | 15/37.50% | 1.0000 |
|
Intestinal | 11/55.00% | 17/42.50% | 0.4180 |
|
Mixed | 1/5.00% | 6/15.00% | 0.4065 |
| Location | | | |
|
Esophago-gastric
junction | 6/30.00% | 6/15.00% | 0.1893 |
|
Fundus | 3/15.00% | 2/5.00% | 0.3216 |
|
Body | 7/35.00% | 19/47.50% | 0.4162 |
|
Prepyloric
antrum | 4/20.00% | 12/30.00% | 0.5408 |
|
Pylorus | 1/5.00% | 0/0.00% | 0.3333 |
| Positive
preoperative chemotherapy | 5/25.00% | 10/25.64% | 1.0000 |
| CA
positivitya | | | |
|
CA 19.9 | 2/10.00% | 6/15.00% | 0.7068 |
|
CA 15.3 | 1/5.00% | 5/12.50% | 0.6532 |
|
CA 125 | 0/0.00% | 3/7.50% | 0.5441 |
|
CEA | 3/15.00% | 4/10.00% | 0.6760 |
| Intraoperative
characteristics | | | |
|
Duration of
operation, min | 195 (160-205) | 200 (180-230) | 0.2095 |
|
Packed red
blood cells transfusion | 4/20.00% | 4/10.00% | 0.4218 |
|
Fresh frozen
plasma transfusion | 2/10.00% | 3/7.50% | 1.0000 |
|
Any allergic
reaction to drugs | - | - | - |
|
Intraoperative
incidents | 2/10.00% | 5/12.50% | 1.0000 |
The mean duration of gastrectomy was 197±37 min.
Blood transfusion was required in 13.3% of patients, and 8.3%
received fresh frozen plasma (FFP) only. The most common
intra-operative indications for drain placement were significant
blood loss (33.3%; n=20) and tension at the anastomosis site
(18.3%; n=11). Further details on intra-operative characteristics
are provided in Table I. Regarding
age distribution, Table II shows
no statistically significant difference between the two groups: the
median age was 73.5 years (IQR: 63-78) in the drain group and 73
years (IQR: 66.5-77.5) in the non-drain group (P=0.8138). No other
statistically significant differences were observed when comparing
intra-operative characteristics between the two groups (Table II).
Post-operatively, ~56% (n=34) of patients reported
severe pain during the first 5 days post-surgery. Results of the
present study also revealed that high VAS scores (>7) were
significantly less frequent in the non-drain group (15%; n=3) when
compared with the drain group (77.5%; n=31; P<0.0001; Fig. 2).
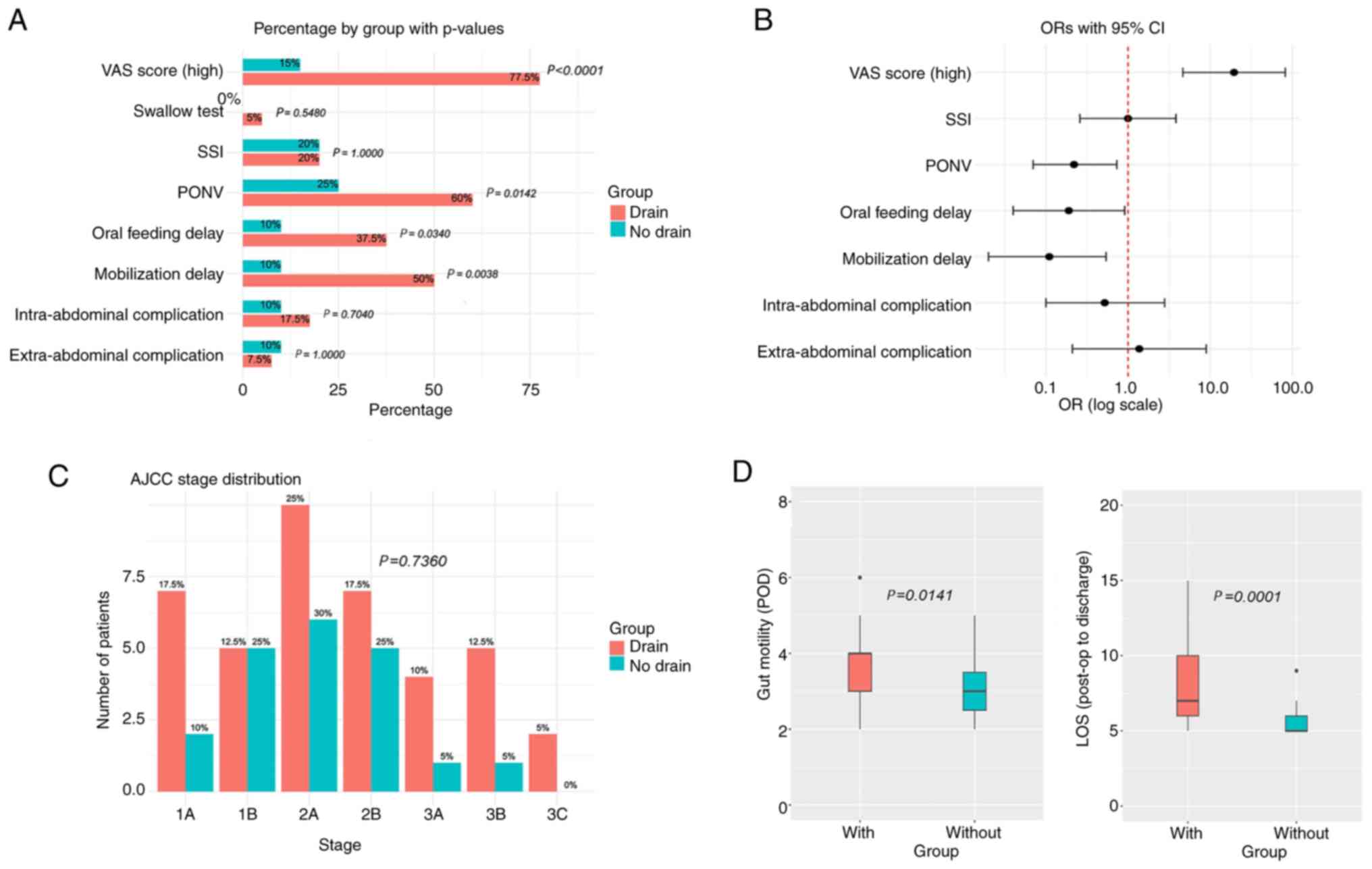 | Figure 2Comparison between postoperative
characteristics and short-term outcomes between the two groups. (A)
Proportions of several study outcomes with corresponding P-values
(χ2 test or Fisher's exact test). (B) OR and 95% CIs for
the characteristics. A valid OR could not be calculated for the
swallow test. Characteristics for which the 95% CI does not cross
the vertical red line are considered statistically significant. (C)
Bar charts showing the number and percentage of patients at each
AJCC stage for both study groups (P-value based on Fisher's exact
test). (D) Box-and-whisker plots for time to gut mobilization (in
days) and LOS. In each plot, boxes represent the interquartile
range (Q1-Q3), horizontal lines within boxes indicate the median,
whiskers show the range excluding outliers, and circles represent
outliers. P-values are derived from Kruskal-Wallis tests. AJCC,
American Joint Committee on Cancer; OR, odds ratio; CI, confidence
interval; LOS, length of hospital stay; PONV, post-operative
nausea; SSI, surgical site infections; VAS, visual analog scale;
POD, post-operative day. |
PONV was common in the overall study population,
affecting 48.3% (n=29) of patients. Significant differences were
observed between groups, with a lower incidence of PONV in the
non-drain group (25%; n=5) compared with the drain group (60%;
n=24; P=0.0142). Thus, there was a ~5X higher risk of PONV in the
drain group [odds ratio (OR), 0.22].
Surgical site infections (SSI) were observed in 12
patients (20%), with no statistically significant difference
between the drain and non-drain groups.
Timely mobilization was impeded in 37% (n=22) of
patients in the overall study population. Delays in mobilization
were notably less frequent in the non-drain group, occurring in
only 10% (n=2) of patients, compared with 50% (n=20) in the drain
group (P=0.0038). Notably, these results were statistically
significant.
In 28.3% of patients (n=17), feeding initiation was
delayed, despite the recommendation that feeding should be
initiated 48-72 h post-surgery. Notably, the delay in feeding
initiation was less common in the non-drain group, occurring in 10%
(n=2) of patients, compared with 37.5% (n=15) in the drain group
(P=0.0340). These results were statistically significant.
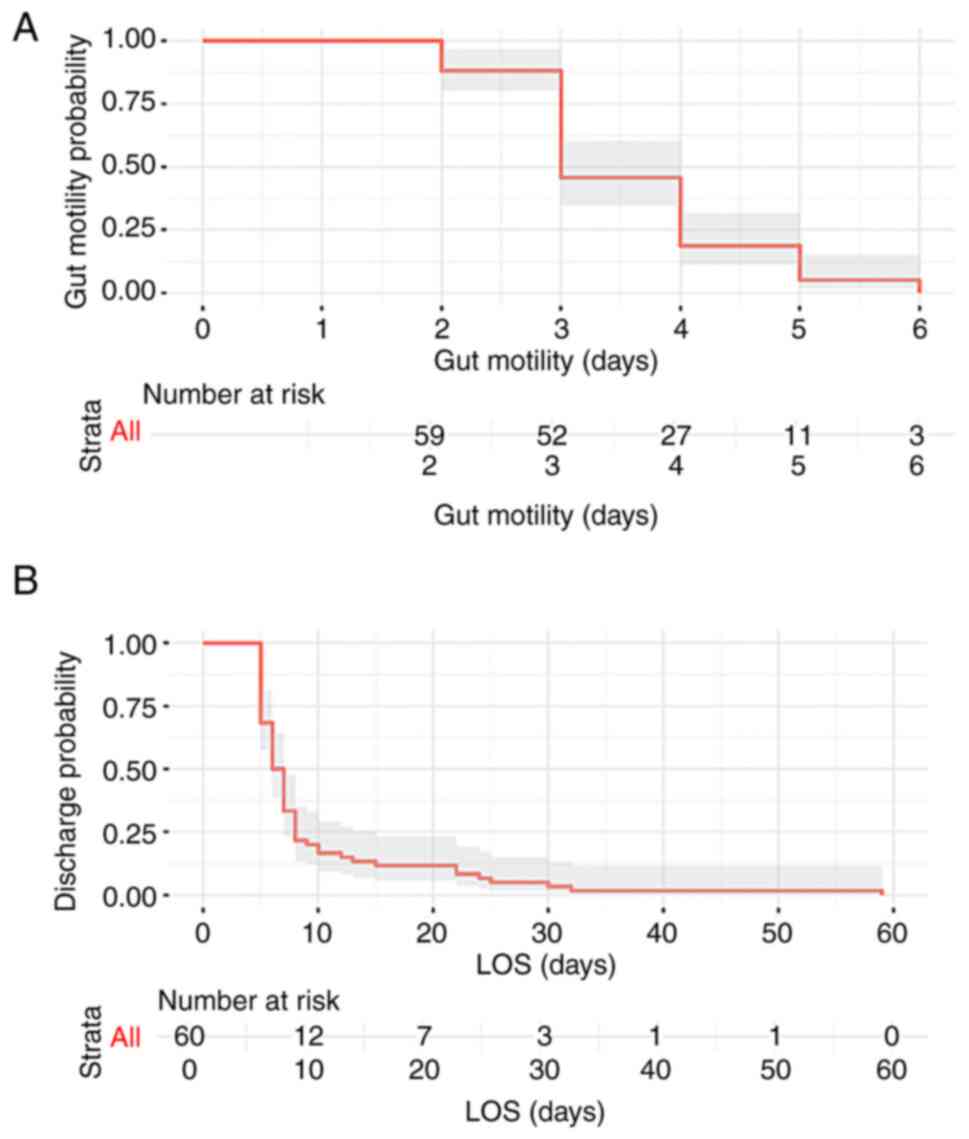
Gut motility resumed at a mean of 3.58±1 days, as
illustrated by the Kaplan-Meier plot (Fig. 3). The initiation of bowel movement
occurred significantly earlier in the non-drain group, with a
median time of 3 days, compared with 4 days in the drain group
(P=0.0142).
Intra-abdominal complications, Clavien-Dindo ≥II
(56,57) occurred in 15% (n=9) of patients,
accounting for 10% (n=2) of the non-drain group and 17.5% (n=7) of
the drain group. Notably, there was no statistically significant
difference between the two groups.
Among the intra-abdominal complications observed,
the most common was esophagojejunal anastomotic leak, affecting
four patients in the drain group. In total, two patients required
reoperation with hybrid intra-operative stent suturing (58), one patient received endoscopic
stenting, and one patient succumbed during the study period. The
second most common complication was post-operative bleeding,
occurring in two patients. Notably, one patient experienced
bleeding on Day 0, and bleeding was evident in the drain. This
patient required reoperation. In addition, one patient experienced
delayed bleeding on Day 9, and this was managed non-surgically. A
duodenal stump leak was identified in one patient via computed
tomography on Day 7, and this patient was managed conservatively.
Necrotizing pancreatitis developed in one patient from the
non-drain group due to a pancreatic leak detected on Day 3, and
this led to reoperation. Notably, this patient died during the
study period. Post-operative ileus requiring nasogastric tube
placement occurred in one patient. A detailed summary of the
aforementioned findings is provided in Table I.
Extra-abdominal complications occurred in 8.3% (n=5)
of patients, comprising 5% (n=2) of the non-drain group and 10%
(n=3) of the drain group. All extra-abdominal complications
involved the respiratory system, including three cases of
atelectasis, one pleural effusion and one case of respiratory
failure in a patient who also experienced the pancreatic leak.
However, there was no statistically significant difference between
the drain and non-drain groups.
The mean length of hospital stay was 9.5±9 days
(Fig. 3). Patients in the
non-drain group experienced a significantly shorter length of stay
with a median of 5 days, compared with 7 days in the drain group
(P=0.0001).
The majority of cases were classified as stages 1B
(16.7%), 2A (26.7%) and 2B (20.0%) according to the AJCC.
Comparisons between the drain and non-drain groups revealed no
statistically significant differences in the distribution of AJCC
stages.
In the overall study population, the 30-day
post-operative outcomes included a 5% mortality rate, an 8.33%
readmission rate and a 10% reoperation rate we reviewed the ages of
the patients who died compared with those who survived. The mean
age of deceased patients was 69.7 years (76, 66 and 67 years, one
female and two males), while the mean age of survivors was 71.1
years. This indicates that there was no statistically significant
association (P>0.05) between age and mortality within our
cohort. No other statistically significant differences were
observed between the drain and non-drain groups.
Of the 40 patients who received a drain, 4 (10%)
exhibited excessive drain output by postoperative day five, and 5
(12.5%) met the criteria for postoperative pancreatic fistula.
Among those with excessive output, 2 patients-who also had a
pancreatic fistula-developed intra-abdominal complications. All
comparisons made between the two groups, including demographic,
histological and intra-operative characteristics are summarized in
Fig. 2.
Propensity score matching was feasible for 20 cases
from each group, the covariate balance as measured via standardized
mean differences had values as follows: blood loss: 1.4, vessel
damage: 0.38, anastomosis: 0.12, stump: 0.57, respiratory issues:
0.57 indicative for not perfect balance between the two categories,
however we applied additional sensitivity analysis.
The Rosenbaum sensitivity test evaluated how
sensitive the outcomes of the matched data were to a potential
presence of unmeasured confounding. Under the assumption of no
hidden bias, Gamma=1 the test yielded a P-value of 0.0899
(marginally non-significant) suggesting that, in the absence of
unmeasured confounding, there is weak evidence that the treatment
effect differs from zero. At increased Gamma=2, the bounds of the
P-value were in the range 0.0134 to 0.2635, meaning that if there
is an unmeasured factor that doubles the odds of being assigned to
non-drain group, the true P-value could vary from being
statistically significant (0.0134) to clearly non-significant
(0.2635). In summary, the sensitivity analysis indicated that there
results moderately sensitive to hidden bias.
Comparison of patients with
intra-abdominal complications
Of the 60 eligible patients, nine (15%) experienced
intra-abdominal complications, classified as Clavien-Dindo ≥II. In
the drain group, complications included one grade IIIa, three grade
IIIb, two grade II and one grade IV. In the non-drain group,
complications included one grade V and one grade II.
Patients with complications were compared with those
without complications to identify perioperative factors that may
influence or be influenced by complications. Fisher's exact test
was used for the analysis of categorical data. Results of the
present study revealed no significant differences in pre-operative
characteristics between the two groups (Table III and Fig. 4).
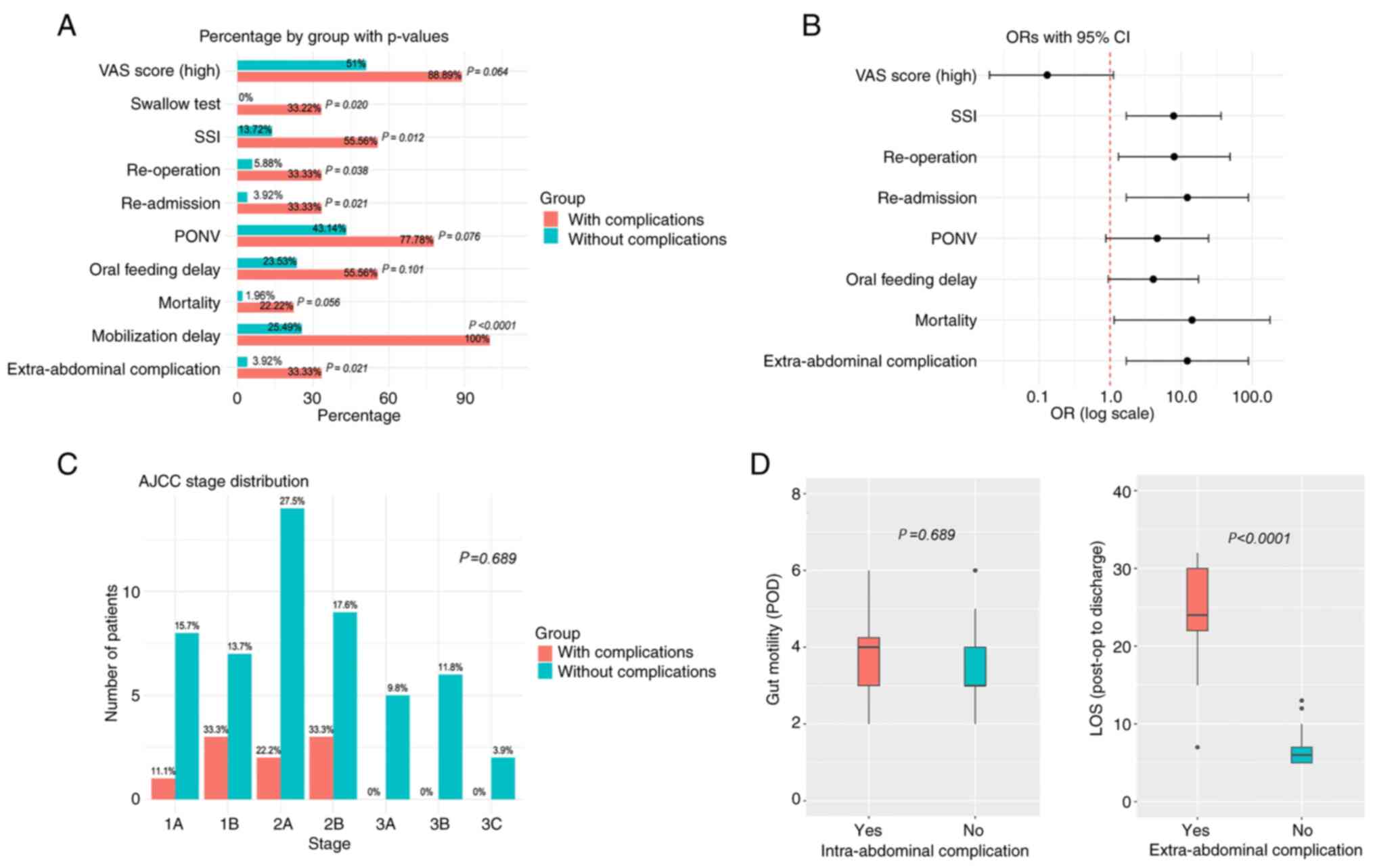 | Figure 4Comparison of postoperative
characteristics and short-term outcomes between patients with and
without intra-abdominal complications. (A) Proportions of various
study outcomes with corresponding P-values (Fisher's exact test).
(B) ORs and 95% CIs for the characteristics shown in panel A. Valid
ORs could not be calculated for the swallow test and mobilization
delay. Characteristics for which the 95% CI does not cross the
vertical red line were considered statistically significant. (C)
Bar charts displaying the number and percentage of patients at each
AJCC stage in both groups (P-value based on Fisher's exact test).
(D) Box-and-whisker plots for time to gut mobilization (in days)
and LOS. In each plot, boxes represent the interquartile range
(Q1-Q3), horizontal lines within boxes indicate the median,
whiskers denote the range excluding outliers, and circles represent
outliers. P-values are based on Kruskal-Wallis tests. AJCC,
American Joint Committee on Cancer; OR, odds ratio; CI, confidence
interval; LOS, length of hospital stay; PONV, post-operative
nausea; SSI, surgical site infections; VAS, visual analog scale;
POD, post-operative day. |
 | Table IIIPre- and intra-operative
characteristics among patients with and without intra-operative
complications. |
Table III
Pre- and intra-operative
characteristics among patients with and without intra-operative
complications.
| Characteristic | With
intra-abdominal complications (n=9) | Without
intra-abdominal complications (n=51) | P-value | OR and 95% CI |
|---|
| Age, years | 73 (67-76) | 73 (64-78) | 0.9257 | N/A |
| Sex (male) | 6/66.67% | 26/50.98% | 0.4820 | 1.92
(0.43-8.54) |
| Charlson score | 2 (2-2) | 2 (2-3) | 0.3258 | N/A |
|
ECOG | 0 (0-0) | 0 (0-0) | 0.8329 | N/A |
|
Histology | | | | |
|
Diffuse | 3/33.33% | 19/37.26% | 1.0000 | 0.84
(0.19-3.77) |
|
Intestinal | 3/33.33% | 25/49.02% | 0.4820 | 0.52
(0.12-2.31) |
|
Other | 1/11.11% | 6/11.76% | 1.0000 | 0.94
(0.1-8.86) |
| Location | | | | |
|
Esophago-gastric
junction | 1/11.11% | 11/21.57% | 0.6713 | 0.45
(0.05-4.03) |
|
Fundus | 0/0.00% | 5/9.80% | 1.0000 | N/A |
|
Body | 6/66.67% | 20/39.22% | 0.1574 | 3.1
(0.69-13.83) |
|
Antrum | 2/22.22% | 14/27.45% | 1.0000 | 0.76
(0.14-4.08) |
|
Pylorus | 1/11.11% | 0/0.00% | 0.1500 | N/A |
| Positive
preoperative chemotherapy | 2/22.22% | 13/26.00% | 1.0000 | 0.81
(0.15-4.42) |
| CA
positivitya | | | | |
|
CA 19.9 | 0/0.00% | 8/15.69% | 0.3394 | N/A |
|
CA 15.3 | 0/0.00% | 6/11.76% | 0.5777 | N/A |
|
CA 125 | 0/0.00% | 3/5.88% | 1.0000 | N/A |
|
CEA | 1/11.11% | 6/12.00% | 1.0000 | 0.94
(0.10-8.87) |
| Intraoperative
characteristics | | | | |
|
Duration of
operation, min | 200 (190-225) | 200 (170-230) | 0.4164 | N/A |
|
Blood
transfusion | 1/11.11% | 7/13.72% | 1.0000 | 0.79
(0.08-7.28) |
|
Fresh frozen
plasma transfusion | 3/33.33% | 2/3.92% | 0.0208 | 12.25
(1.69-88.71) |
|
Allergic
reaction to drugs | - | - | N/A | N/A |
|
Intraoperative
incidents | 2/22.22% | 5/9.8% | 0.2805 | 2.63
(0.42-16.26) |
| Criteria for drain
placement | | | | |
|
Blood loss
>250 ml | 4/44.44% | 16/31.37% | 0.4644 | 1.75
(0.41-7.4) |
|
Vessel
injury | 2/22.22% | 1/1.96% | 0.0561 | 14.29
(1.14-178.87) |
|
Anastomosis
concerns | 2/22.22% | 9/17.65% | 0.6644 | 1.33
(0.24-7.51) |
|
Adjacent
structures injury | - | - | N/A | 0 (0-0) |
|
Stump
integrity concerns | 1/11.11% | 2/3.92% | 0.3914 | 3.06
(0.25-37.84) |
|
Chronic
cardiopulmonary comorbidity | 0/0% | 3/5.88% | 1.0000 | N/A |
Intra-operatively, although FFP transfusion was not
initially considered a potential risk factor for developing
intra-abdominal complications, we later observed that patients who
developed such complications had received FFP transfusion more
frequently than those without complications (P=0.0208; OR, 12.3).
In addition, vessel injury was the most common indication for drain
placement (P=0.05; OR, 14.3; Table
III).
The analysis of post-operative characteristics
revealed differences between patients with and without
intra-abdominal complications. Notably, differences were observed
in oral contrast, SSIs, patient mobilization delays,
extra-abdominal complications, length of hospital stay (LOS),
readmissions and reoperations (Fig.
4).
As aforementioned, nine patients experienced
intra-abdominal complications, with seven patients included in the
drain group and two patients included in the non-drain group. The
most common complications included anastomotic leak and bleeding,
and these significantly affected the LOS and rates of reoperation.
The median day of complication detection was 5.8 (range, 1-11;
Table SI). Although the number of
cases in this subgroup is relatively small, the statistical
analysis was performed to ensure completeness and the outcomes
revealed no statistically significant differences in the detection
of anastomotic leaks, post-operative day of complication detection,
diagnosis based on drain content, hospitalization duration,
reoperation rates or mortality between the two groups.
Drains were closely monitored for signs suggestive
of intra-abdominal complications. Notably, four patients with
complications were identified through drain content analysis, while
three were detected based on clinical presentation and blood tests.
A comparison between patients diagnosed via drain analysis and
those diagnosed through other methods revealed no significant
differences in terms of detection method, post-operative day of
diagnosis, LOS or rates of mortality (Table SII).
Comparison of patients without
intra-abdominal complications
A total of 51 patients did not exhibit any
intra-abdominal complications. A comparative analysis was performed
within this subgroup to determine whether non-abdominal adverse
effects were more prevalent in the drain or non-drain group. When
comparing the pre- and intra-operative characteristics of each
group, no statistical significance was observed (Table SIII). Results of the present study
revealed that a blood loss of >250 ml was the most common
indication for drain placement in this group (P<0.001).
Moreover, statistically significant differences in
pain score, delay of patient mobilization, oral feeding delay,
initiation of gut motility and LOS were observed between groups
post-surgery (Table IV).
 | Table IVPostoperative characteristics among
patients without intra-operative complications. |
Table IV
Postoperative characteristics among
patients without intra-operative complications.
|
Characteristics | Without drain
(n=19) | With drain
(n=32) | P-value | OR and 95% CI |
|---|
| Swallow test
(positive) | - | - | N/A | N/A |
| Visual analog scale
score (high) | 3 (15.79%) | 23 (71.88%) | <0.0001 | 0.07
(0.02-0.31) |
| Post-operative
nausea and vomiting | 5 (26.30%) | 17 (53.13%) | 0.1150 | 0.32
(0.09-1.08) |
| Surgical site
infections | 4 (21.05%) | 3 (9.38%) | 0.4021a | 2.58
(0.51-13.05) |
| Mobilization
delay | 1 (5.26%) | 12 (37.50%) | 0.0178a | 0.09
(0.01-0.78) |
| Oral feeding
delay | 1 (5.26%) | 11 (34.38%) | 0.0201a | 0.11
(0.01-0.90) |
| Gut motility
start | 3 (2, 5) | 4 (2, 6) | 0.0179 | N/A |
| Intra-abdominal
complication | - | - | N/A | N/A |
| Extra-abdominal
complication | 1 (5.26%) | 1 (3.13%) | 1.000a | 1.72
(0.10-29.24) |
| AJCC stage | | | | |
|
1A | 2 (10.53%) | 6 (18.75%) | 0.3710a | N/A |
|
1B | 5 (26.31%) | 2 (6.25%) | | |
|
2A | 6 (31.57%) | 8 (25.00%) | | |
|
2B | 4 (21.05%) | 5 (15.63%) | | |
|
3A | 1 (5.26%) | 4 (12.50%) | | |
|
3B | 1 (5.26%) | 5 (15.63%) | | |
|
3C | 0 (0.00%) | 2 (6.25%) | | |
| Length of hospital
stay (post-op to exit) | 5 (5, 9) | 7 (5, 13) | <0.0001 | N/A |
| Short-term
outcomes | | | | |
|
Mortality | 1 (5.26%) | 0 (0.00%) | 0.3732a | 5.27
(0.20-136.1) |
|
Re-admission | 0 (0.00%) | 2 (6.25%) | 0.5234a | 0.31
(0.01-6.87) |
|
Re-operation | 1 (5.26%) | 2 (6.25%) | 1.0000a | 0.83
(0.07-9.86) |
Discussion
The use of drains following elective total D2
gastrectomy remains a topic of debate in surgical practice. The
ERAS guidelines recommend against the use of drains, with reduced
recovery periods and hospital stays, and an earlier return to daily
activities when their use is avoided (7,8,19,24,25,59).
Yet, compliance with the aforementioned guidelines varies globally,
with the use of smaller drainage tubes being supported in some
practices. However, reductions in drain size exerted minimal impact
on psychological outcomes or the rates of SSIs (16,60,61).
Notably, the present study aimed to provide specific criteria to
support clinical decision making on drain placement following total
gastrectomy for the treatment of gastric cancer.
Expertise is a critical factor in the present study,
as it influences the quality of care provided. In this context, an
experienced surgeon in a high-volume centre plays a key role in
assessing post-operative risk and tailoring drain placement
(60,62).
Results of the present study revealed no significant
pre-operative or demographic differences between patients with and
without drains across all three groups. The absence of
statistically significant differences between the drain and
non-drain groups indicated that the cohorts were comparable,
enhancing the reliability and robustness of the present statistical
analyses. While the results of the present study may further the
current understanding of the use of drains, larger, multi-centre
studies with more diverse populations are required, to provide more
definitive conclusions and validate the applicability of these
criteria in different clinical settings.
Results of the present study revealed that patients
who required FFP transfusion during surgery exhibited a higher
incidence of complications, including anastomotic leaks. As it has
been previously reported, packed red blood cell transfusions may
adversely affect post-operative outcomes (63-65). Previous
studies suggest that FFP transfusion may exert immunosuppressive
effects through several mechanisms. These include downregulation of
proinflammatory cytokines such as TNF-α and upregulation of
anti-inflammatory mediators such as IL-10, resulting in attenuated
systemic inflammatory responses-reflected by lower C-reactive
protein levels and altered leukocyte counts. Additionally, soluble
HLA class I molecules and fibroblast-derived ligands present in
stored plasma can impair natural killer cell cytotoxicity and
suppress T-cell proliferation, further contributing to immune
modulation (63,66). Thus, FFP transfusion should be
considered as an intra-operative risk factor. Future studies could
include measurement of systemic inflammatory markers, such as IL-6
and CRP, to improve elucidation of the relationship between drain
use and immunomodulatory effects.
In the present study, vessel injury was identified
as a significant risk factor for post-operative complications.
Thus, vessel injury should be considered when deciding on drain
placement during gastrectomy.
By contrast, blood loss exceeding 250 ml was the
primary factor for selecting drain placement in patients who
exhibited no intra-abdominal complications. Notably, 250 ml was
selected as the minimum mean value based on the results of previous
studies (15,42-46,67-71). Results of
previous studies demonstrated an association between post-operative
intra-abdominal complications and non-vascular blood loss that does
not require transfusion. Fibrinolytic and inflammatory pathway
activation may play a role in non-vascular blood loss (67-71). However,
there were no detailed methods for estimating blood loss in each
study, which may have led to discrepancies between the reported and
actual values associated with intra-abdominal complications.
Moreover, results of previous studies highlighted
multiple risk factors associated with post-operative complications,
such as advanced age (>65 years), male sex, diffuse tumor
histology, extensive lymphadenectomy, extended surgery duration and
impaired blood supply (35-40). However, the
literature is often based on retrospective studies with variation
in the type of gastric surgery, indication and patient cohort. In
the present study, the aforementioned factors were not
statistically significant; thus, further investigations are
required.
In the present study, the gastrografin swallow test
was performed 48-72 h post-surgery, and the results demonstrated a
sensitivity of 50%. This oral contrast study is routinely performed
on or after the fifth following total gastrectomy. However, results
of previous studies revealed that 46.6-63.6% (72-75) of
esophagojejunal anastomotic leaks occur prior to the scheduled
contrast study. Thus, the oral contrast study was conducted 48-72 h
following surgery in the present study, to determine the potential
for improved sensitivity in anastomotic leak detection. However,
the results of the present study were comparable with those
previously obtained, highlighting the low levels of sensitivity
(20-60%) of this technique in confirming esophagojejunal
anastomotic integrity (72-75).
In the present study, pain was determined using VAS
score. The results revealed marginal levels of statistical
significance between the drain and non-drain groups when
complications were present. However, a significant difference was
observed in patients without complications, suggesting that drain
placement may be avoidable in these cases. Results of the present
study were comparable with those previously obtained, further
highlighting that drains may negatively impact the levels of
post-operative pain (21,22,59).
Moreover, results of the present study revealed a
significant difference in PONV between the drain and non-drain
groups in the overall study population. These findings supported
the rationale for the selective use of drains, limiting them to
high-risk patients, as per the suggested criteria. Previous studies
focused on drain-related PONV in bariatric surgery (76,77);
however, studies focused on the use of drains in gastrectomy for
the treatment of gastric cancer are limited. The use of drains
should be carefully considered, as PONV may contribute to prolonged
hospital stays.
Results of the present study also highlighted that
there was no statistically significant difference in SSIs between
the drain and non-drain groups. However, in patients with
post-operative complications, the drain group exhibited a
significantly higher rate of SSI, which is comparable with the
results obtained in previous studies. No statistically significant
differences were observed between drain and non-drain groups in
patients without complications. Notably, the potential association
between drains and SSIs remains to be fully elucidated, with
several studies reporting no association (18,22,23,78),
and others suggesting that retro-grade bacterial contamination or
leakage of protein-rich ascitic fluid may play a role in SSI
development (79). Thus, the use
of drains in patients with complications should be carefully
considered to minimize the risk of SSIs.
Results of the present study revealed that
extra-abdominal complications, limited to respiratory tract
infections in the present study, were more prevalent in patients
with drains when intra-abdominal complications occurred. However,
no statistically significant difference was observed when comparing
patients without complications. Results of a previous study
demonstrated that drain-induced pain may be associated with
micro-atelectases and subsequent respiratory tract infections
(60), and a further study
revealed that drain placement may facilitate the dissemination of
pathogens (16,79). By contrast, numerous retrospective
studies focused on total and subtotal gastrectomy revealed no
statistically significant differences in the incidence of
pulmonary-related complications between groups that received
post-operative drains and those that did not (18,22).
Conflicting results highlight the requirement for further
investigations that focus on the role of post-operative drains in
pulmonary events.
Results of the present study also revealed that
drain placement in patients with no intra-abdominal complications
was associated with delays in mobilization, oral feeding and
gastrointestinal tract motility. However, statistical significance
was marginal when compared with patients with intra-abdominal
complications. Thus, delays in mobilization, oral feeding and
gastrointestinal tract motility may be associated with the specific
complication, rather than with the presence of a drain. By
contrast, the drain was considered the primary contributing factor
in the absence of complications. Previous studies focused on the
impact of drains on mobilization and gastrointestinal function
demonstrate conflicting results, with some studies reporting no
significant differences between drain and non-drain groups, and
further studies demonstrating that drains may hinder recovery
(15,16,18,20,59).
In the present study, the use of drains
significantly impacted the LOS. As expected, patients who
experienced delays in mobilization, dietary resumption and
restoration of gastrointestinal motility were less likely to be
discharged in a timely manner. These results are comparable with
those obtained previously (20-23,59,80).
Drains may delay mobilization due to a fear of dislodgement or
pain, leading to prolonged bowel motility recovery and feeding
initiation. Thus, individualized drain use is suggested.
Results of the present study also indicated that the
number of readmissions and reoperations was highest in patients in
the drain group who developed intraabdominal complications.
Notably, results of a previous study also revealed that the number
of reoperations may be associated with complications (15); however, evidence for the potential
association between readmissions and complications is conflicting
(80,81). The results of two meta-analyses
demonstrated higher readmission rates in non-drain groups,
primarily due to undetected complications during the initial
hospital stay and a poor ERAS compliance. Thus, low rates of
readmission may be attributed to the implementation of the selected
criteria, which were systematically applied to identify patients in
whom omitting the perianastomotic drain was considered both
appropriate and safe.
In the present study, the rates of mortality
differed between patients with and without intra-abdominal
complications; however, the levels of statistical significance were
marginal. Moreover, no statistically significant difference in
mortality rates was observed between the drain and non-drain
groups. Studies focused on the rates of mortality in these groups
are limited, with two previous studies reporting that there were no
statistically significant differences in mortality between the
drain and non-drain groups (22,23).
In summary, the present study demonstrated that a
criteria-based approach may aid in standardizing drain placement in
gastrectomy. The use of pre-defined criteria ensures a more
consistent decision-making process; thus, reducing variability in
surgical practice. While the study was not designed to evaluate the
benefit of drain use itself, the presence of statistically
significant differences between certain subgroups supports the
clinical relevance of a selective approach. These criteria could be
considered for application in routine clinical settings.
The present study exhibits numerous limitations. For
example, the sample size included in the present study was small,
and this was largely due to constraints imposed by the
coronavirus-19 pandemic when conducted. The small sample size may
have introduced selection bias, reduced statistical power and
limiting the generalizability of the findings, which could have
affected the observed statistical significance levels. Moreover,
the present study was not designed to assess the clinical benefit
of drain placement itself, but rather to evaluate the feasibility
and relevance of predefined criteria guiding their use in open
total gastrectomy. The small sample size further limits any
conclusions regarding efficacy of the drains and reinforces the
need for cautious interpretation, as for example the role of the
drain in the intra-abdominal complication. Thus, further
investigations with increased sample sizes are required.
Additionally, the present study focused on patients undergoing open
D2 gastrectomy, which may have led to selection bias. Notably, a
focus on these patients may have led to the exclusion of more
complex cases requiring extended lymphadenectomy, subtotal
gastrectomy, or laparoscopic approach restricting the applicability
of the results to broader populations. In addition, an absence of
randomization and blinding in the present study introduces possible
selection bias, especially in subjective outcomes (for example,
pain, PONV). However, due to the specific design and objectives of
the study, randomization was not feasible. As previously outlined,
the literature presents conflicting evidence on the use of
prophylactic drains, with opposing perspectives supported by
multiple randomized trials and studies (15-23). However,
none of the trials incorporated predefined criteria for drain
placement. Future studies that will include criteria on drain
insertion should adopt RCTs with strict baseline matching. Finally,
our study analysed some patients' parameters collectively,
potentially obscuring differences among subgroups. Future larger
studies could consider patients' tumor stage, surgical complexity,
and preoperative chemotherapy status.
In conclusion, debate is ongoing regarding the use
of drains in total gastrectomy. While national guidelines provide
recommendations for their use in clinical practice, decision-making
is the responsibility of the clinician. The present study proposed
evidence-based criteria that may lead to standardization of drain
usage, providing a practical decision-making tool that may be
applied in cases with and without complications.
To the best of our knowledge, the present study is
the first prospective study to implement literature-based criteria
for drain use in gastrectomy for the treatment of gastric cancer.
Results of the present study provide a novel theoretical basis for
drain use, and further investigations should focus on refining and
expanding the outlined criteria.
Supplementary Material
Statistical analysis outcomes for the
subgroup of patients with intra-abdominal complications.
Statistical analysis of
intra-abdominal complications: drain content as a differentiation
factor.
Pre- and intra-operative
characteristics among patients without intra-abdominal
complications.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be found
in the Clinical Trial.gov under accession number
NCT04288661 or at the following URL: https://clinicaltrials.gov/study/NCT04288661?term=drag&rank=1.
Authors' contributions
ME served as the principal investigator of the
study, contributing to its conception and design, data acquisition,
interpretation, and manuscript drafting. MD provided data
interpretation and analysis, as well as critical revisions to the
manuscript draft. AP and DK conducted data statistical analysis and
had substantial contribution to the design of the manuscript. DA
contributed to data interpretation and critical manuscript
revisions. DT was the study sponsor and surgical lead for all
procedures; alongside ME, he contributed to the study's conception
and design. TT was responsible for data acquisition and analysis.
GZ and KT contributed to data analysis and interpretation along
with critical revisions, ensuring the intellectual rigor of the
manuscript. All authors read and approved the final version of the
manuscript. Additionally, ME, DT and TT have agreed to be
accountable for all aspects of the work, so that any questions
relating to research integrity or scientific accuracy in any part
of the study are appropriately investigated and resolved. ME, DT
and TT confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the principles outlined in the Helsinki Declaration of Human Rights
and with the Guidelines of Good Clinical Practice. The final study
protocol received approval from the Institutional Review Board
(IRB) of Hippocration General Hospital (approval no.
1678/31-01-2020; Athens Greece). Informed consent was obtained from
all patients involved in the study, both for the study
participation and the results' publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC: ESMO Guidelines
Committee. Electronic address: clinicalguidelines@esmo.org. Gastric
cancer: ESMO Clinical Practice Guideline for diagnosis, treatment
and follow-up. Ann Oncol. 33:1005–1020. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang N, He HW, He YY, Gu W, Xu MJ and Liu
L: Xiaotan Sanjie recipe, a compound Chinese herbal medicine,
inhibits gastric cancer metastasis by regulating GnT-V-mediated
E-cadherin glycosylation. J Integr Med. 21:561–574. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yan L, Li W, Chen F, Wang J, Chen J, Chen
Y and Ye W: Inflammation as a mediator of microbiome
Dysbiosis-associated DNA methylation changes in gastric
premalignant lesions. Phenomics. 3:496–501. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim SG, Seo HS, Lee HH, Song KY and Park
CH: Comparison of the Differences in Survival Rates between the 7th
and 8th Editions of the AJCC TNM staging system for gastric
adenocarcinoma: A Single-institution study of 5,507 patients in
Korea. J Gastric Cancer. 17:212–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ljungqvist O, Young-Fadok T and Demartines
N: The history of enhanced recovery after surgery and the ERAS
society. J Laparoendosc Adv Surg Tech A. 27:860–862.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tanious MK, Ljungqvist O and Urman RD:
Enhanced recovery after surgery: History, evolution, guidelines,
and future directions. Int Anesthesiol Clin. 55:1–11.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park KB, Park JY, Lee SS, Chung HY and
Kwon OK: Chronological changes in quality of life and body
composition after gastrectomy for locally advanced gastric cancer.
Ann Surg Treat Res. 98:262–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ikeda M, Yoshida M, Mitsumori N, Etoh T,
Shibata C, Terashima M, Fujita J, Tanabe K, Takiguchi N, Oshio A
and Nakada K: Assessing optimal Roux-en-Y reconstruction technique
after total gastrectomy using the Postgastrectomy Syndrome
Assessment Scale-45. World J Clin Oncol. 13:376–387.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pinheiro RN, Mucci S, Zanatto RM, Picanço
Junior OM, Bottino AAG, Fontoura RP and Lopes Filho GJ: Quality of
life as a fundamental outcome after curative intent gastrectomy for
adenocarcinoma: Lessons learned from patients. J Gastrointest
Oncol. 10:989–998. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Husson O, de Rooij BH, Kieffer J,
Oerlemans S, Mols F, Aaronson NK, van der Graaf WTA and van de
Poll-Franse LV: The EORTC QLQ-C30 summary score as prognostic
factor for survival of patients with cancer in the ‘Real-World’:
Results from the Population-Based PROFILES registry. Oncologist.
25:e722–e732. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu Y and Zaydfudim VM: Quality of life
after curative resection for gastric cancer: Survey metrics and
implications of surgical technique. J Surg Re. 251:168–179.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kehlet H and Mogensen T: Hospital stay of
2 days after open sigmoidectomy with a multimodal rehabilitation
programme. Br J Surg. 86:227–230. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shimoike N, Akagawa S, Yagi D, Sakaguchi
M, Tokoro Y, Nakao E, Tamura T, Fujii Y, Mochida Y, Umemoto Y, et
al: Laparoscopic gastrectomy with and without prophylactic drains
in gastric cancer: A propensity score-matched analysis. World J
Surg Oncol. 17(144)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hirahara N, Matsubara T, Hayashi H, Takai
K, Fujii Y and Tajima Y: Significance of prophylactic
intra-abdominal drain placement after laparoscopic distal
gastrectomy for gastric cancer. World J Surg Oncol.
13(181)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee J, Choi YY, An JY, Seo SH, Kim DW, Seo
YB, Nakagawa M, Li S, Cheong JH, Hyung WJ, et al: Do all patients
require prophylactic drainage after gastrectomy for gastric cancer?
The Experience of a High-Volume Center. Ann Surg Oncol.
22:3929–3937. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kumar M, Yang SB, Jaiswal VK, Shah JN,
Shreshtha M and Gongal R: Is prophylactic placement of drains
necessary after subtotal gastrectomy? World J Gastroenterol.
13:3738–3741. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mortensen K, Nilsson M, Slim K, Schafer M,
Mariette C, Braga M, Carli F, Demartines N, Griffin SM and Lassen
K: Enhanced Recovery After Surgery (ERAS®) Group. Consensus
guidelines for enhanced recovery after gastrectomy: Enhanced
Recovery After Surgery (ERAS®) Society recommendations. Br J Surg.
101:1209–1229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alvarez Uslar R, Molina H, Torres O and
Cancino A: Total gastrectomy with or without abdominal drains. A
prospective randomized trial. Rev Esp Enferm Dig. 97:562–569.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim J, Lee J, Hyung WJ, Cheong JH, Chen J,
Choi SH and Noh SH: Gastric cancer surgery without drains: A
prospective randomized trial. J Gastrointest Surg. 8:727–732.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu HP, Zhang YC, Zhang YL, Yin LN and
Wang J: Drain versus no-drain after gastrectomy for patients with
advanced gastric cancer: Systematic review and meta-analysis. Dig
Surg. 28:178–189. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Z, Chen J, Su K and Dong Z: Abdominal
drainage versus no drainage post gastrectomy for gastric cancer.
Cochrane Database Syst Rev: CD008788, 2011.
|
|
24
|
Mahendran R, Tewari M, Dixit VK and Shukla
HS: Enhanced recovery after surgery protocol enhances early
postoperative recovery after pancreaticoduodenectomy. Hepatobiliary
Pancreat Dis Int. 18:188–193. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gustafsson UO, Scott MJ, Hubner M, Nygren
J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG,
Soop M, et al: Guidelines for perioperative care in elective
colorectal surgery: Enhanced recovery after surgery (ERAS®) society
recommendations: 2018. World J Surg. 43:659–695. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lim SY, Kang JH, Jung MR, Ryu SY and Jeong
O: Abdominal drainage in the prevention and management of major
Intra-abdominal complications after total gastrectomy for gastric
carcinoma. J Gastric Cancer. 20:376–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kanda M, Fujiwara M, Tanaka C, Kobayashi
D, Iwata N, Mizuno A, Yamada S, Fujii T, Nakayama G, Sugimoto H, et
al: Predictive value of drain amylase content for peripancreatic
inflammatory fluid collections after laparoscopic (assisted) distal
gastrectomy. Surg Endosc. 30:4353–4362. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Taniguchi Y, Kurokawa Y, Mikami J, Tanaka
K, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, Nakajima K,
Takiguchi S, et al: Amylase concentration in drainage fluid as a
predictive factor for severe postoperative pancreatic fistula in
patients with gastric cancer. Surg Today. 47:1378–1383.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kamiya S, Hiki N, Kumagai K, Honda M,
Nunobe S, Ohashi M, Sano T and Yamaguchi T: Two-point measurement
of amylase in drainage fluid predicts severe postoperative
pancreatic fistula after gastric cancer surgery. Gastric Cancer.
21:871–878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kobayashi D, Iwata N, Tanaka C, Kanda M,
Yamada S, Nakayama G, Fujii T, Koike M, Fujiwara M and Kodera Y:
Factors related to occurrence and aggravation of pancreatic fistula
after radical gastrectomy for gastric cancer. J Surg Oncol.
112:381–386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yamagata Y, Yoshikawa T, Yura M, Otsuki S,
Morita S, Katai H and Nishida T: Current status of the ‘enhanced
recovery after surgery’ program in gastric cancer surgery. Ann
Gastroenterol Surg. 3:231–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Surgeons ACo: American College of
Surgeons' Standards for Gastric Cancer Surgery 2021 [15/10/2024].
Available from: https://decisionpoint.medscape.com/oncology/viewarticle/946018.
|
|
33
|
Lombardi PM, Mazzola M, Giani A, Baleri S,
Maspero M, De Martini P, Gualtierotti M and Ferrari G: ERAS pathway
for gastric cancer surgery: Adherence, outcomes and prognostic
factors for compliance in a Western centre. Updates Surg.
73:1857–1865. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Salvans S, Grande L, Dal Cero M and Pera
M: State of the art of enhanced recovery after surgery (ERAS)
protocols in esophagogastric cancer surgery: The Western
experience. Updates Surg. 75:373–382. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gong W and Li J: Combat with
esophagojejunal anastomotic leakage after total gastrectomy for
gastric cancer: A critical review of the literature. Int J Surg.
47:18–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aurello P, Magistri P, D'Angelo F,
Valabrega S, Sirimarco D, Tierno SM, Nava AK and Ramacciato G:
Treatment of esophagojejunal anastomosis leakage: A systematic
review from the last two decades. Am Surg. 81:450–403.
2015.PubMed/NCBI
|
|
37
|
Trapani R, Rausei S, Reddavid R and
Degiuli M: ITALIAN RESEARCH GROUP FOR GASTRIC CANCER (GIRCG)
Clinical Investigators. Risk factors for esophago-jejunal
anastomosis leakage after total gastrectomy for cancer. A
multicenter retrospective study of the Italian research group for
gastric cancer. Eur J Surg Oncol. 46:2243–2247. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xing J, Liu M, Qi X, Yu J, Fan Y, Xu K,
Gao P, Tan F, Yao Z, Zhang N, et al: Risk factors for
esophagojejunal anastomotic leakage after curative total
gastrectomy combined with D2 lymph node dissection for gastric
cancer. J Int Med Res. 49(3000605211000883)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Aryaie AH, Singer JL, Fayezizadeh M, Lash
J and Marks JM: Efficacy of endoscopic management of leak after
foregut surgery with endoscopic covered self-expanding metal stents
(SEMS). Surg Endosc. 31:612–617. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Choi CW, Kang DH, Kim HW, Park SB, Kim SJ,
Hwang SH and Lee SH: Full covered self-expandable metal stents for
the treatment of anastomotic leak using a silk thread. Medicine
(Baltimore). 96(e7439)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Dallal RM, Bailey L and Nahmias N: Back to
basics-clinical diagnosis in bariatric surgery. Routine drains and
upper GI series are unnecessary. Surg Endosc. 21:2268–2271.
2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Haverkamp L, Weijs TJ, van der Sluis PC,
van der Tweel I, Ruurda JP and van Hillegersberg R: Laparoscopic
total gastrectomy versus open total gastrectomy for cancer: A
systematic review and meta-analysis. Surg Endosc. 27:1509–1520.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J,
Du X, Huang H, Hu J, Li G, et al: Morbidity and mortality of
laparoscopic vs open total gastrectomy for clinical stage I gastric
cancer: The CLASS02 multicenter randomized clinical trial. JAMA
Oncol. 6:1590–1597. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao
Y, Chen J and Yu P: Short-term surgical outcomes of a randomized
controlled trial comparing laparoscopic versus open gastrectomy
with D2 lymph node dissection for advanced gastric cancer. Surg
Endosc. 32:2427–2433. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao B, Huang X, Lu H, Zhang J, Luo R, Xu
H and Huang B: Intraoperative blood loss does not independently
affect the survival outcome of gastric cancer patients who
underwent curative resection. Clin Transl Oncol. 21:1197–1206.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Misawa K, Kurokawa Y, Mizusawa J,
Takiguchi S, Doki Y, Makino S, Choda Y, Takeno A, Tokunaga M, Sano
T, et al: Negative impact of intraoperative blood loss on long-term
outcome after curative gastrectomy for advanced gastric cancer:
Exploratory analysis of the JCOG1001 phase III trial. Gastric
Cancer. 25:459–467. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen Cardenas SM, Santhanam P,
Morris-Wiseman L, Salvatori R and Hamrahian AH: Perioperative
evaluation and management of patients on glucocorticoids. J Endocr
Soc. 7(bvac185)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Marrelli D, Piccioni SA, Carbone L,
Petrioli R, Costantini M, Malagnino V, Bagnacci G, Rizzoli G,
Calomino N, Piagnerelli R, et al: Posterior and Para-Aortic
(D2plus) Lymphadenectomy after Neoadjuvant/conversion therapy for
locally Advanced/oligometastatic gastric cancer. Cancers.
16(1376)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bassi C, Marchegiani G, Dervenis C, Sarr
M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink
MG, et al: The 2016 update of the international study group (ISGPS)
definition and grading of postoperative pancreatic fistula: 11
Years After. Surgery. 161:584–591. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jensen MP, Chen C and Brugger AM:
Interpretation of visual analog scale ratings and change scores: A
reanalysis of two clinical trials of postoperative pain. J Pain.
4:407–414. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
World Medical A. World medical association
declaration of helsinki: Ethical principles for medical research
involving human subjects. JAMA. 310:2191–2194. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
(ICH) ICfH: Guideline for good clinical
practice E6(R2), ICH, 2016. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf.
|
|
53
|
Team RDC: R: A language and environment
for statistical computing. Foundation for Statistical Computing,
2010.
|
|
54
|
Ho D, Imai K, King G and Stuart EA:
MatchIt: Nonparametric preprocessing for parametric causal
inference. J Statistical Software. 42:1–28. 2011.
|
|
55
|
Keele L: An overview of rbounds: An R
package for Rosenbaum bounds sensitivity analysis with matched
data. White Paper Columbus, OH. 1(15)2010.
|
|
56
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J,
Slankamenac K, Bassi C, et al: The Clavien-dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Eleftheriou MM, Triantafyllou Τ, Bananis
K, Chrysikos D and Theodorou D: Hybrid intraoperative sutured
stenting for anastomotic leak after total gastrectomy. J Clin Med
Img Case Rep. 3(1502)2023.
|
|
59
|
Desiderio J, Trastulli S, D'Andrea V and
Parisi A: Enhanced recovery after surgery for gastric cancer
(ERAS-GC): Optimizing patient outcome. Transl Gastroenterol
Hepatol. 5(11)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Rekavari SG and Mahakalkar C: Prophylactic
Intra-abdominal drains in major elective surgeries: A comprehensive
review. Cureus. 16(e54056)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pang HY, Chen LH, Chen XF, Yan MH, Chen ZX
and Sun H: Prophylactic drainage versus non-drainage following
gastric cancer surgery: A meta-analysis of randomized controlled
trials and observational studies. World J Surg Oncol.
21(166)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Marano L, Verre L, Carbone L, Poto GE,
Fusario D, Venezia DF, Calomino N, Kaźmierczak-Siedlecka K, Polom
K, Marrelli D, et al: Current trends in volume and surgical
outcomes in gastric cancer. J Clin Med. 12(2708)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Nakaseko Y, Haruki K, Shiba H, Horiuchi T,
Saito N, Sakamoto T, Gocho T and Yanaga K: Impact of fresh frozen
plasma transfusion on postoperative inflammation and prognosis of
colorectal liver metastases. J Surg Res. 226:157–165.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ecker BL, Simmons KD, Zaheer S, Poe SL,
Bartlett EK, Drebin JA, Fraker DL, Kelz RR, Roses RE and Karakousis
GC: Blood transfusion in major abdominal surgery for malignant
tumors: A trend analysis using the national surgical quality
improvement program. JAMA Surg. 151:518–525. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ferraris VA, Davenport DL, Saha SP, Austin
PC and Zwischenberger JB: Surgical outcomes and transfusion of
minimal amounts of blood in the operating room. Arch Surg.
147:49–55. 2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bednarsch J, Czigany Z, Lurje I, Trautwein
C, Ludde T, Strnad P, Gaisa NT, Barabasch A, Bruners P, Ulmer T, et
al: Intraoperative transfusion of fresh frozen plasma predicts
morbidity following partial liver resection for hepatocellular
carcinoma. J Gastrointest Surg. 25:1212–1223. 2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li ZW, Shu XP, Wen ZL, Liu F, Liu XR, Lv
Q, Liu XY, Zhang W and Peng D: Effect of intraoperative blood loss
on postoperative complications and prognosis of patients with
colorectal cancer: A meta-analysis. Biomed Rep.
20(22)2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Shah A, Palmer AJR and Klein AA:
Strategies to minimize intraoperative blood loss during major
surgery. Br J Surg. 107:e26–e38. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Simões CM, Carmona MJC, Hajjar LA, Vincent
JL, Landoni G, Belletti A, Vieira JE, de Almeida JP, de Almeida EP,
Ribeiro U Jr, et al: Predictors of major complications after
elective abdominal surgery in cancer patients. BMC Anesthesiol.
18(49)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sugisawa N, Tokunaga M, Tanizawa Y, Bando
E, Kawamura T and Terashima M: Intra-abdominal infectious
complications following gastrectomy in patients with excessive
visceral fat. Gastric Cancer. 15:206–212. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Takebayashi K, Murata S, Kaida S,
Yamaguchi T, Otake R, Miyake T, Ueki T, Kojima M, Iida H, Maehira
H, et al: Adverse impact of postoperative intra-abdominal
infectious complications on cancer Recurrence-related survival
after curative gastric cancer surgery. Am J Surg. 224:949–954.
2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lamb PJ, Griffin SM, Chandrashekar MV,
Richardson DL, Karat D and Hayes N: Prospective study of routine
contrast radiology after total gastrectomy. Br J Surg.
91:1015–1019. 2004.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Aday U, Gündeş E, Çiyiltepe H, Çetin DA,
Gülmez S, Senger AS, Değer KC and Duman M: Examination of
anastomotic leak with aqueous contrast swallow after total
gastrectomy: Should it be carried out routinely? Contemp Oncol
(Pozn). 21:224–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li SS, Costantino CL and Mullen JT:
Morbidity and mortality of total gastrectomy: A comprehensive
analysis of 90-Day outcomes. J Gastrointest Surg. 23:1340–1348.
2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
El-Sourani N, Bruns H, Troja A, Raab HR
and Antolovic D: Routine use of contrast swallow after total
gastrectomy and esophagectomy: Is it justified? Pol J Radiol.
82:170–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Parisi A, Desiderio J, Cirocchi R and
Trastulli S: Enhanced recovery after surgery (ERAS): A systematic
review of randomised controlled trials (RCTs) in bariatric surgery.
Obes Surg. 30:5071–5085. 2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Davey MG, Donlon NE, Fearon NM, Heneghan
HM and Conneely JB: Evaluating the impact of enhanced recovery
after surgery protocols on surgical outcomes following bariatric
Surgery-A systematic review and Meta-analysis of randomised
clinical trials. Obes Surg. 34:778–789. 2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Feng F, Ji G, Li JP, Li XH, Shi H, Zhao
ZW, Wu GS, Liu XN and Zhao QC: Fast-track surgery could improve
postoperative recovery in radical total gastrectomy patients. World
J Gastroenterol. 19:3642–3648. 2013.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Albanopoulos K, Alevizos L, Linardoutsos
D, Menenakos E, Stamou K, Vlachos K, Zografos G and Leandros E:
Routine abdominal drains after laparoscopic sleeve gastrectomy: A
retrospective review of 353 patients. Obes Surg. 21:687–691.
2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Ding J, Sun B, Song P, Liu S, Chen H, Feng
M and Guan W: The application of enhanced recovery after surgery
(ERAS)/fast-track surgery in gastrectomy for gastric cancer: A
systematic review and Meta-analysis. Oncotarget. 8:75699–75711.
2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lee Y, Yu J, Doumouras AG, Li J and Hong
D: Enhanced recovery after surgery (ERAS) versus standard recovery
for elective gastric cancer surgery: A meta-analysis of randomized
controlled trials. Surg Oncol. 32:75–87. 2020.PubMed/NCBI View Article : Google Scholar
|