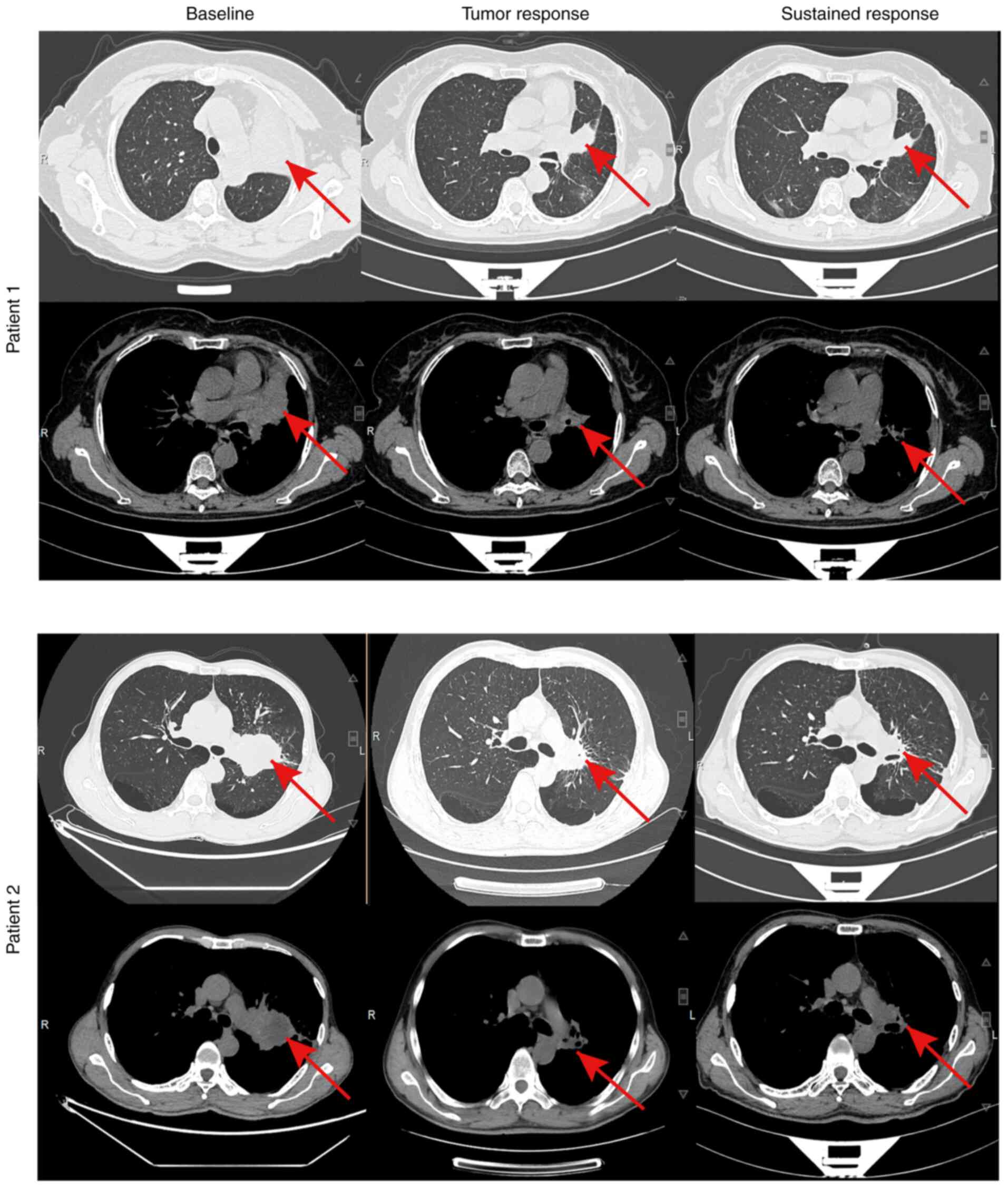
|
1
|
Beaudet M, Lacasse Y and Labbé C:
Palliative systemic therapy given near the end of life for
metastatic non-small cell lung cancer. Curr Oncol. 29:1316–1325.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ichikawa M, Muramatsu N, Matsunaga W,
Ishikawa T, Okuda T, Okamoto H and Gotoh A: Effects of inhalable
gene transfection as a novel gene therapy for non-small cell lung
cancer and malignant pleural mesothelioma. Sci Rep.
12(8634)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang W, Gao Y, Li X, Zhang J, Liu T, Feng
X, Pan H, Yang X, Xie S, Feng X, et al: Postoperative survival of
EGFR-TKI-targeted therapy in non-small cell lung cancer patients
with EGFR 19 or 21 mutations: a retrospective study. World J Surg
Oncol. 15(197)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dong Y, Li Q, Miao Q and Li D: Erlotinib
as a salvage treatment after gefitinib failure for advanced
non-small-cell lung cancer patients with brain metastasis: A
successful case report and review. Medicine (Baltimore).
100(e26450)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu Y, Guo YB, Xu D, Zhang J, Liu ZG, Wu
X, Yang XY, Chang DD, Xu M, Yan J, et al: A computed tomography
(CT)-derived radiomics approach for predicting primary co-mutations
involving TP53 and epidermal growth factor receptor (EGFR) in
patients with advanced lung adenocarcinomas (LUAD). Ann Transl Med.
9(545)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu HE, Vuppalapaty M, Wilkerson C, Renier
C, Chiu M, Lemaire C, Che J, Matsumoto M, Carroll J, Crouse S, et
al: Detection of EGFR mutations in cfDNA and CTCs, and comparison
to tumor tissue in non-small-cell-lung-cancer (NSCLC) patients.
Front Oncol. 10(572895)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen H, Yang D, Wang Y, Tao H, Luo Y, Wu
A, Li S, Yang Z and Chen M: Activation of the Hedgehog pathway
mediates resistance to epidermal growth factor receptor inhibitors
in non-small cell lung cancer. J Cancer. 13:987–997.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu M, Xie Y, Ni S and Liu H: The latest
therapeutic strategies after resistance to first generation
epidermal growth factor receptor tyrosine kinase inhibitors (EGFR
TKIs) in patients with non-small cell lung cancer (NSCLC). Ann
Transl Med. 3(96)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Peng KC, Su JW, Xie Z, Wang HM, Fang MM,
Li WF, Chen YQ, Guan XH, Su J, Yan HH, et al: Clinical outcomes of
EGFR+/METamp+ vs. EGFR+/METamp-untreated patients with advanced
non-small cell lung cancer. Thorac Cancer. 13:1619–1630.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Calabrese F, Pezzuto F, Lunardi F,
Fortarezza F, Tzorakoleftheraki SE, Resi MV, Tiné M, Pasello G and
Hofman P: Morphologic-molecular transformation of oncogene addicted
non-small cell lung cancer. Int J Mol Sci. 23(4164)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qin K, Hong L, Zhang J and Le X: MET
amplification as a resistance driver to TKI therapies in lung
cancer: Clinical challenges and opportunities. Cancers (Basel).
15(612)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng W, Xie Q, Liu S, Ji Y, Li C, Wang C
and Jin L: Krüppel-like factor 4 promotes c-Met
amplification-mediated gefitinib resistance in non-small-cell lung
cancer. Cancer Sci. 109:1775–1786. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu X, Zheng X, Yang S, Wang L, Hao X, Cui
X, Ding L, Mao L, Hu P and Shi Y: First-in-human phase I study of
BPI-9016M, a dual MET/Axl inhibitor, in patients with non-small
cell lung cancer. J Hematol Oncol. 13(6)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu YL, Zhang L, Kim DW, Liu X, Lee DH,
Yang JCH, Ahn MJ, Vansteenkiste JF, Su WC, Felip E, et al: Phase
Ib/II study of capmatinib (INC280) plus gefitinib after failure of
epidermal growth factor receptor (EGFR) inhibitor therapy in
patients with EGFR-mutated, MET factor-dysregulated non-small-cell
lung cancer. J Clin Oncol. 36:3101–3109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y,
Zhao J, Kim DW, Soo RA, Kim SW, Pan H, et al: Tepotinib plus
gefitinib in patients with EGFR-mutant non-small-cell lung cancer
with MET overexpression or MET amplification and acquired
resistance to previous EGFR inhibitor (INSIGHT study): An
open-label, phase 1b/2, multicentre, randomised trial. Lancet
Respir Med. 8:1132–1143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Overbeck TR, Cron DA, Schmitz K, Rittmeyer
A, Körber W, Hugo S, Schnalke J, Lukat L, Hugo T, Hinterthaner M,
et al: Top-level MET gene copy number gain defines a subtype of
poorly differentiated pulmonary adenocarcinomas with poor
prognosis. Transl Lung Cancer Res. 9:603–616. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu YL, Guarneri V, Voon PJ, Lim BK, Yang
JJ, Wislez M, Huang C, Liam CK, Mazieres J, Tho LM, et al:
Tepotinib plus osimertinib in patients with EGFR-mutated
non-small-cell lung cancer with MET amplification following
progression on first-line osimertinib (INSIGHT 2): A multicentre,
open-label, phase 2 trial. Lancet Oncol. 25:989–1002.
2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al:
Phase III study of afatinib or cisplatin plus pemetrexed in
patients with metastatic lung adenocarcinoma with EGFR mutations. J
Clin Oncol. 41:2869–2876. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou S, Ren F and Meng X: Efficacy of
immune checkpoint inhibitor therapy in EGFR mutation-positive
patients with NSCLC and brain metastases who have failed EGFR-TKI
therapy. Front Immunol. 13(955944)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang K, Wu L, Zheng X, Xu Y, Miao Q,
Zheng X, Zhang L, Huang C and Lin G: Chemotherapy versus
personalized therapy for EGFR mutant lung adenocarcinoma resistance
to EGFR-tyrosine kinase inhibitors: A retrospective dual-center
study. BMC Pulm Med. 24(96)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gridelli C, Ciardiello F, Gallo C, Feld R,
Butts C, Gebbia V, Maione P, Morgillo F, Genestreti G, Favaretto A,
et al: First-line erlotinib followed by second-line
cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung
cancer: The TORCH randomized trial. J Clin Oncol. 30:3002–3011.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Auliac JB, Chouaid C, Greillier L, Monnet
I, Le Caer H, Falchero L, Corre R, Descourt R, Bota S, Berard H, et
al: Randomized open-label non-comparative multicenter phase II
trial of sequential erlotinib and docetaxel versus docetaxel alone
in patients with non-small-cell lung cancer after failure of
first-line chemotherapy: GFPC 10.02 study. Lung Cancer. 85:415–419.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma
Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol.
16:1512–1522. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S,
Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized,
double-blind, placebo-controlled, multicenter, phase III study of
first-line carboplatin/paclitaxel plus bevacizumab or placebo in
chinese patients with advanced or recurrent nonsquamous
non-small-cell lung cancer. J Clin Oncol. 33:2197–2204.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma L, Diao B, Huang Z, Wang B, Yu J and
Meng X: The efficacy and possible mechanisms of immune checkpoint
inhibitors in treating non-small cell lung cancer patients with
epidermal growth factor receptor mutation. Cancer Commun (Lond).
41:1314–1330. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun
Y, Ji Y, Wang Z, Shi J, Lu J, et al: AENEAS: A randomized phase III
trial of aumolertinib versus gefitinib as first-line therapy for
locally advanced or metastaticnon-small-cell lung cancer with EGFR
exon 19 deletion or L858R mutations. J Clin Oncol. 40:3162–3171.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y,
Liu C, Zhu S, Zhang X, Li Y, et al: Furmonertinib (AST2818) versus
gefitinib as first-line therapy for Chinese patients with locally
advanced or metastatic EGFR mutation-positive non-small-cell lung
cancer (FURLONG): A multicentre, double-blind, randomised phase 3
study. Lancet Respir Med. 10:1019–1028. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Asami K, Ando M, Nishimura T, Yokoi T,
Tamura A, Minato K, Mori M, Ogushi F, Yamamoto A, Yoshioka H, et
al: A randomized phase II study of docetaxel or pemetrexed with or
without the continuation of gefitinib after disease progression in
elderly patients with non-small cell lung cancer harboring EGFR
mutations (JMTO LC12-01). Thorac Cancer. 13:1827–1836.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen Y, Liu Z, An N, Zhang J, Meng W, Wang
W, Wu X, Hu X, Chen Y and Yin W: Platelet-derived mitochondria
attenuate 5-FU-induced injury to bone-associated mesenchymal stem
cells. Stem Cells Int. 2023(7482546)2023.PubMed/NCBI View Article : Google Scholar
|