Introduction
Serous papillary adenofibroma (SPAF) is a rare
benign gynecological tumor that is typically incidentally found
during the treatment of other diseases. As a variant of
adenofibroma (AF) and cystadenofibroma (CAF), it is characterized
by both fibrous and epithelial components under the microscope
(1). These characteristics also
become a barrier to imaging-based differential diagnosis (2). Surgeons and gynecologists sometimes
misdiagnose it as a serous adenoma or even an ovarian malignancy,
resulting in excessive surgery (3). Magnetic resonance imaging (MRI) is a
promising tool for differentiating SPAF from cancers (2). Certain experts have summarized that
the solid part of AF and CAF shows a distinguishably lower T2 and
diffusion-weighted imaging (DWI) signal intensity and gains a
higher apparent diffusion coefficient value than malignant
neoplasms (2,4). SPAF is mainly located in the ovary,
but it can be found in the fallopian tube and vulva as well
(5-7).
Most patients are asymptomatic unless the mass compresses the
surrounding organs (7). Due to the
rarity of SPAF, no specific guidelines are available and clinicians
usually resect unilateral or even bilateral adnexa in practice.
Early diagnosis and prevention are crucial for the treatment of
SPAF (3,7,8).
While most SPAF cases are benign, delayed diagnosis may lead to
malignant misidentification, causing unnecessary harm and fertility
impairment. The present study aimed to summarize the clinical and
pathological characteristics of 13 patients with SPAF encountered
at the Second Affiliated Hospital Zhejiang University School of
Medicine (Hangzhou, China) and provide information to contribute to
the further elucidation of SPAF. It was found that the clinical
characteristics of SPAF were atypical. Conventional imaging methods
such as ultrasound and CT scan offered limited evidence and a
varied performance. It was also found that the MRI appearance of
SPAF was uniform. In certain cases, MRI can show protrusions or
papillae on the cyst wall. Immunohistochemistry (IHC) results of
available slices showed that SPAF was hormone-sensitive with high
expression of estrogen receptor (ER), progesterone receptor (PR)
and human epidermal growth factor receptor 2 (Her-2).
Patients and methods
Data collection
The present study was a retrospective single-center
observational study. A total of 13 female patients with SPAF who
underwent surgery at the Second Affiliated Hospital of Zhejiang
University School of Medicine (Hangzhou, China) from January 2018
to July 2023 were enrolled, with ages ranging from 25 to 66 years
and a median age of 47 years. Preoperative laboratory results,
radiological and sonographic imaging and slices of the surgical
specimens were available after obtaining ethical approval.
Laboratory tests were conducted according to manufacturers'
instructions at the time of sample acquisition. Imaging features
and diagnostic reports were generated by professional doctors
according to the Ovarian-Adnexal Reporting and Data System criteria
(9,10).
The inclusion criteria for this study were as
follows: i) Pathologically diagnosed as SPAF; and ii) data from
inpatient system since 2015. The exclusion criteria for this study
were: i) Patients with other gynecological tumors or malignancies;
ii) patients without abundant data, e.g., outpatients; and iii)
patients with severe complications.
IHC staining and analysis
Following ethical approval, the tissue slices were
uniformly collected and subjected to IHC staining and analysis on
November 30th, 2023. IHC staining was performed by Zhongshan Golden
Bridge Biotechnology Co. Ltd. The following steps were all
performed at a laboratory room temperature of 26˚C. Briefly, the
acquired paraffin-embedded slices were dewaxed with xylene three
times for 10 min each and dehydrated with a graded series of
ethanols four times for 2 min each. Subsequently, the slices were
placed in a pressure cooker (103˚C) containing EDTA buffer for 2.5
min after washing five times with PBS for 2 min each. The slices
were then incubated with 3% hydrogen peroxide for 10 min and washed
five times with distilled water and PBS for 2 min each. Primary
antibodies were then incubated at 37˚C for 1 h, and secondary
antibody (cat. no. PV-8000D; no dilution; Zhongshan Goldenbridge
Biotechnology Co., Ltd.) was added after washing five times with
PBS for 2 min each. It should be noted that the secondary antibody
for Her-2 was different (cat. no. PV-8000; no dilution; Zhongshan
Goldenbridge Biotechnology Co., Ltd.). Diaminobenzidine (cat. no.
ZLI-9018; Zhongshan Goldenbridge Biotechnology Co., Ltd.) was added
and incubated for 8 min. After washing with running water,
hematoxylin and acid-fast differentiation solution (Zhongshan
Goldenbridge Biotechnology Co., Ltd.) was used for counterstaining.
The final pathology results were obtained by professional
pathologists according to the current diagnostic criteria for p53
(cat. no. ZM-0408; no dilution; Zhongshan Goldenbridge
Biotechnology Co., Ltd.), Ki-67 (cat. no. ZM-0166; no dilution;
Zhongshan Goldenbridge Biotechnology Co., Ltd.), BRAF (cat. no.
ZA-0668; no dilution; Zhongshan Goldenbridge Biotechnology Co.,
Ltd.), Wilms' tumor gene 1 (WT-1; cat. no. ZA-0559; no dilution;
Zhongshan Goldenbridge Biotechnology Co., Ltd.), ER (cat. no.
ZA-0102; no dilution; Zhongshan Goldenbridge Biotechnology Co.,
Ltd.), PR (cat. no. ZA-0255; no dilution; Zhongshan Goldenbridge
Biotechnology Co., Ltd.) and Her-2 (cat. no. ZM-0065; 1:150
dilution; Zhongshan Goldenbridge Biotechnology Co., Ltd.).
Statistical analysis
All data were recorded and processed with Office
2019 (Microsoft Corporation). Count data were presented as n (%).
Hypothesis testing is not applicable in this study.
Results
Clinical characteristics
The mean patient age was 43 years (range, 25-66
years). A total of four patients were menopausal. The mean body
mass index of the included patients was 22.97 kg/m2
(range, 15.79-28.19 kg/m2). According to the World
Health Organization criteria for Asian populations (11), 7 patients were overweight, while 2
were underweight. Nearly all SPAF were located in the ovary, except
for one case, where it was located in the fallopian tube (Table I).
 | Table IDetails and laboratory data of the
cases (n=13). |
Table I
Details and laboratory data of the
cases (n=13).
| Case no. | Age, years | Location | Manifestation | CA125, U/ml | E2,
pmol/l | PRL, mIU/l | Menopausal state | BMI,
kg/m2 |
|---|
| 1 | 49 | Ovary | Null | 24.7 | - | - | No | 24.22 |
| 2 | 25 | Ovary | Dysmenorrhea | 11.2 | 170.36 | 218.46 | No | 22.88 |
| 3 | 33 | Ovary | Null | 16.5 | 1,101.37 | 2,604.14 | No | 16.66 |
| 4 | 26 | Ovary | Irregular
menstruation and dysmenorrhea | 43.5 | 244.46 | 206.13 | No | 15.79 |
| 5 | 32 | Ovary | Intermenstrual
bleeding | 21.6 | 249.71 | 248.72 | No | 25.78 |
| 6 | 52 | Ovary | Null | 13.8 | 145.31 | 534.38 | No | 20.89 |
| 7 | 47 | Ovary | Null | 17 | 376.04 | - | No | 22.07 |
| 8 | 53 | Ovary | Null | 57.2 | 524.76 | 2,326.64 | Yes | 25.00 |
| 9 | 36 | Ovary | Null | 46.2 | - | 75.64 | No | 28.19 |
| 10 | 66 | Ovary | Null | 20.6 | 78.06 | - | Yes | 26.37 |
| 11 | 28 | Fallopian tube | Null | - | - | 75.43 | No | 22.67 |
| 12 | 60 | Ovary | Null | 17.7 | 138.17 | - | Yes | 23.44 |
| 13 | 52 | Ovary | Null | 818 | 55.43 | 150.25 | Yes | 24.70 |
Ovarian tumors are asymptomatic, particularly at
early stages (12,13). In the present study, 10 patients
were asymptomatic, of whom 40% were incidentally discovered during
surgeries for other gynecological diseases, such as uterine myoma,
adenomyosis and cervical cancer. Among those with symptoms, two
patients had menstrual problems and two patients had mild
dysmenorrhea.
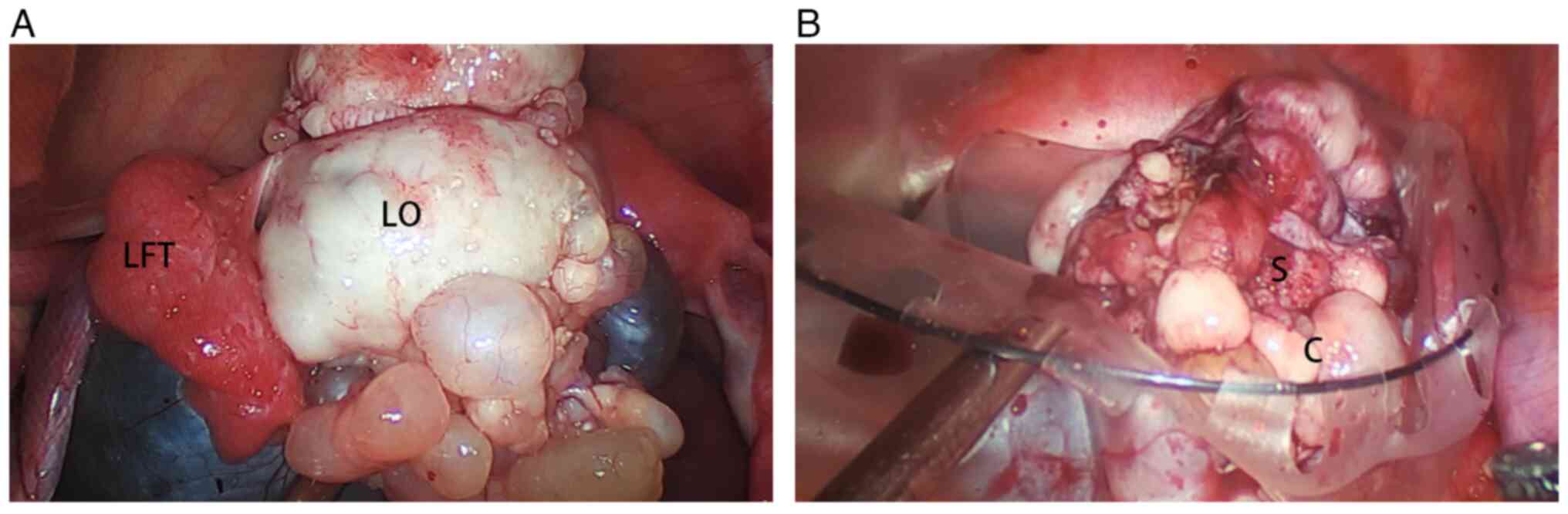
Images of a typical gross appearance were found
during surgery (Fig. 1A). Grossly,
SPAF is a mixed neoplasm comprising cysts and solid components, but
not all cases visually exhibit this characteristic. The resected
specimen can help to visualize the solid components more clearly.
The demarcation between the cyst (Fig.
1B, ‘C’) and solid component (Fig.
1B, ‘S’) was undefined.
Laboratory test characteristics
Carbohydrate antigens and sex hormones are two
common laboratory tests for patients with ovarian masses detected
before surgery. In the present study, 12 patients underwent blood
tests for carbohydrate antigens (Table
I). Among these patients, 8 had elevated carbohydrate antigen
levels. The most common elevated carbohydrate antigen was CA125
(50%), followed by SCC (25%), CA199 (12.5%), CA153 (12.5%) and
CA211 (12.5%). Of note, one patient had a significantly elevated
CA125 level (818 U/ml, upper limit: 35 U/ml). We considered this as
supportive evidence that demonstrated a likelihood of misdiagnosis
as malignancy. After surgery, the CA125 level of this patient
descended to 73.3 U/ml.
The potential effect of SPAF on sex hormone levels
was then explored. A total of 10 patients had been tested for sex
hormone levels. Among the patients, 4 were menopausal or
peri-menopausal. The average estrogen (E2) level in menopausal
patients was 199.1 pmol/l (normal range: <118.2 pmol/l for
menopausal women). The other 6 patients were premenopausal with
regular menstrual periods. The highest E2 level of the
pre-menopausal patients was 1101.37 pmol/l and the average E2 level
was 381.21 pmol/l (normal range: 71.6-529.2 pmol/l in follicular
phase, 204.8-786.1 pmol/l in luteal phase). Follicle-stimulating
hormone (FSH, normal range: 2.5-10.2 IU/l in follicular phase,
3.4-33.4 IU/l in ovulation period, 1.5-9.1 IU/l in luteal phase,
23.0-116.3 IU/l in menopausal women) levels of menopausal patients
ranged from 32.06 to 45.53 IU/l, while the FSH level of
peri-menopausal patients ranged from 3.49 to 18.59 IU/l, which was
consistent with the menstrual state. The mean prolactin level (PRL;
normal range: 59.00-619.00 mIU/l for non-pregnant women,
38.00-430.00 mIU/l for menopausal women) in the 10 patients was
604.34 mIU/l. Notably, 3 patients had elevated PRL levels. One of
the three patients underwent pituitary MRI revealing a Rathke's
cyst, with PRL concentration reaching 2,604.14 mIU/l, approximately
four times the upper reference limit.
Radiological and sonographic
characteristics
Ultrasound is the most widely used technology in
gynecology clinics. All patients completed the ultrasound test
before surgery. Only 1 patient had a negative result due to a small
fallopian tube SPAF. Among those with positive ultrasound findings,
two had developed multicystic tumors. The echoes of the cysts
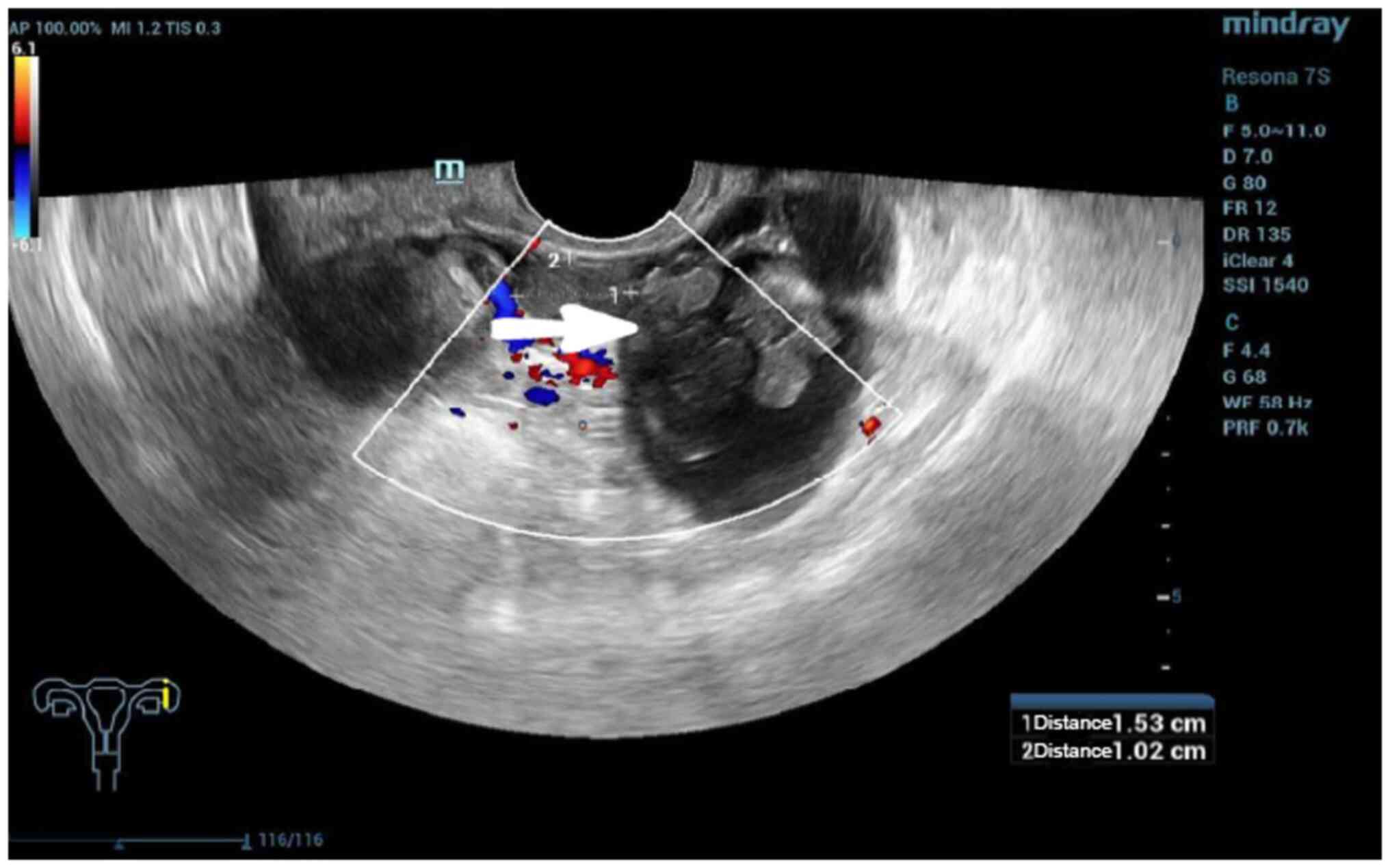
varied from spotted to high-echoes. The papillae of the SPAF were
small and difficult to detect. Only two sonographies revealed SPAF
papillae (Fig. 2). Clinically, the
appearance of SPAF is not classical. Therefore, most sonographers
(76.92%) did not draw a conclusion regarding the suspected disease,
while the other three mentioned differential diseases were
teratomas, cystadenomas and myomas (data not shown).
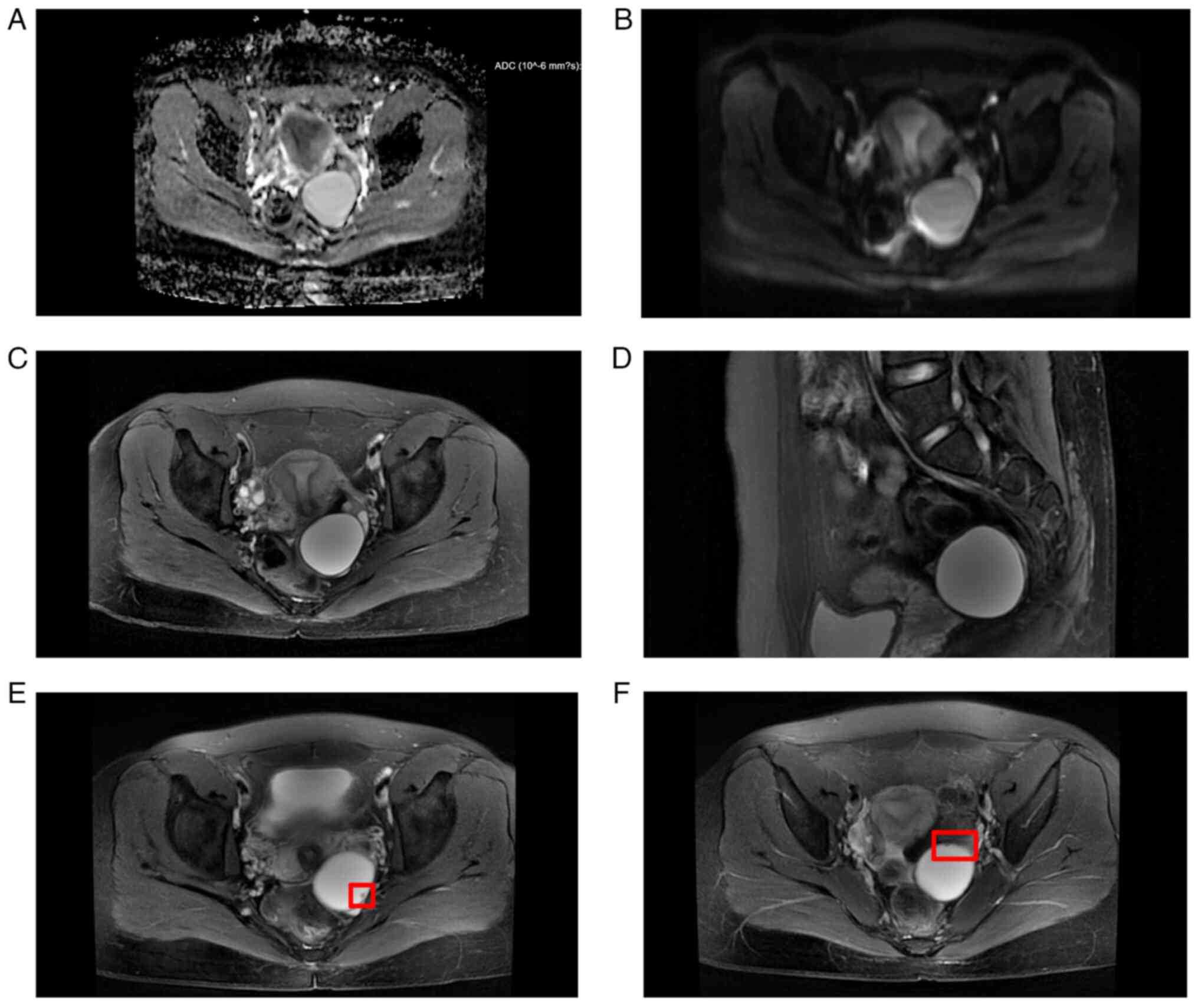
The MRI appearances were more uniform than
ultrasound (Table II). A total of
6 patients underwent MRI. Due to the small tumor volume, 1 MRI
didn't reveal any SPAF appearance and SPAF was detected during
surgery. Apart from this, 4 patients showed adnexa cyst and 1
patient displayed a pelvic mass on MRI. Low signal intensity on
T1-weighted imaging (T1WI) and high signal intensity on T2WI were
observed in these patients. All MRI results indicated restricted
diffusion in the DWI phase. In addition, one of them observed small
protrusions, which may be the papillae of the SPAF (Fig. 3). However, the radiologist
initially considered the mass to be a serous cystadenoma.
 | Table IIComparison of imaging features
observed on ultrasound and MRI. |
Table II
Comparison of imaging features
observed on ultrasound and MRI.
| Imaging
feature | Ultrasound
detection (performed in 13 cases) | MRI detection
(performed in 6 cases) |
|---|
| Well-defined
borders | 9(69) | 5(83) |
| Cystic
components | 8(62) | 5(83) |
| Papillae | 2(15) | 1(17) |
| T2
hyperintensity | N/A | 5(83) |
| T1
hypointensity | N/A | 5(83) |
| DWI
hyperintensity | N/A | 5(83) |
Ultrasound and MRI are both widely used in
gynecological imaging. However, based on the retrospective analysis
of a small SPAF sample performed in the present study, it was found
that MRI is more accurate and consistent than ultrasound in
displaying lesion characteristics (Table II). Specifically, MRI more
accurately identified well-defined borders (83 vs. 69%), cystic
components (83 vs. 62%) and papillae (17 vs. 15%).
Pathological characteristics
Overall, Ki-67, ER, PR, Her-2, BRAF, p53 and WT-1
were tested to determine hormone sensitivity and their association
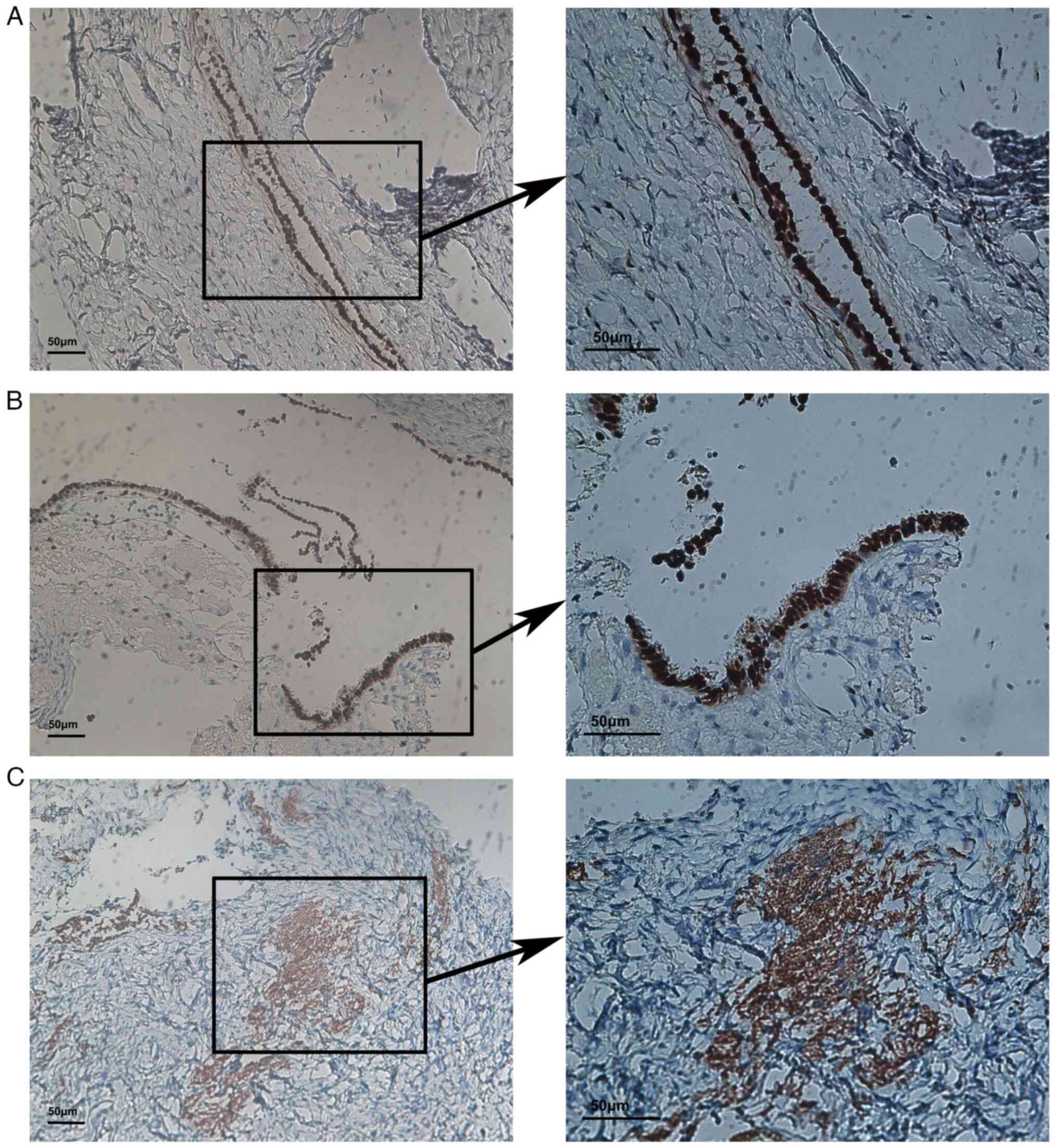
with malignancy of SPAF and prognosis of patients. The IHC images
for all the indicators can be found in Figs. 4 and S1. SPAF appeared to be sex
hormone-sensitive, as most slices were positive for ER (12/13), PR
(13/13) and Her-2 (10/13) (Fig.
4). Low expression of Ki-67, p53 and WT-1 was also observed in
the slices, as most of the expressions of Ki-67 (9/13) and p53
(13/13) were no more than 1% or negative (Table III).
 | Table IIIImmunohistochemistry results. |
Table III
Immunohistochemistry results.
| | Ki67 (nuclear) | ER (nuclear) | PR (nuclear) | P53 (nuclear) | BRAF
(nuclear+cytoplasm) | WT-1 (nuclear) | Her-2 (membrane +
cytoplasm) |
|---|
| Patient ID | % Stained
cells | % Stained
cells | S.I. | % Stained
cells | S.I. | % Stained
cells | % Stained
cells | S.I. | S.I. | % Stained
cells | Membrane staining
integrity | S.I. |
|---|
| 1 | 1 | 90 | 2+ | 95 | 3+ | - | 90 | 1+ | + | 90 | Incomplete | 2+ |
| 2 | <1 | 60 | 2+ | 80 | 2+ | - | 10 | 1+ | + | - | - | - |
| 3 | 1 | 80 | 2+ | 95 | 3+ | 1 | - | - | + | 90 | Incomplete | 2+ |
| 4 | 2 | 90 | 2+ | 95 | 3+ | 1 | 80 | 1+ | + | 90 | Incomplete | 1+ |
| 5 | 1 | 90 | 2+ | 95 | 2+ | 1 | 80 | 1+ | + | 90 | Incomplete | 1+ |
| 6 | 2 | 80 | 2+ | 95 | 3+ | 1 | 80 | 1+ | + | 80 | Incomplete | 1+ |
| 7 | <1 | 95 | 2+ | 90 | 3+ | 1 | - | - | + | 80 | Incomplete | 2+ |
| 8 | 1 | 95 | 2+ | 90 | 3+ | 1 | 80 | 1+ | + | 90 | Incomplete | 1+ |
| 9 | <1 | 90 | 2+ | 90 | 3+ | - | 60 | 1+ | + | - | - | - |
| 10 | - | 10 | 1+ | 60 | 2+ | - | 10 | 1+ | + | - | - | - |
| 11 | 2 | 90 | 2+ | 90 | 3+ | 1 | 90 | 1+ | + | 30 | Incomplete | 1+ |
| 12 | 2 | 95 | 3+ | 30 | 2+ | 1 | 90 | 2+ | + | <10 | Complete membrane
positive | 2+ |
| 13 | <1 | 90 | 2+ | 95 | 2+ | 1 | 50 | 1+ | - | 70 | incomplete | 1+ |
Discussion
In the present study, the clinical, laboratory and
imaging characteristics of SPAF were summarized and certain
molecular markers were determined in SPAF slices. Certain
limitations in ordinary examinations were observed but hormone
sensitivity of SPAF was also found. Ultrasound is more commonly
used due to its low cost and wide availability compared to MRI.
However, numerous previous studies have validated that MRI is more
effective than ultrasound in detecting SPAF, such as in our cases
(4,14).
Benign adnexal tumors are usually asymptomatic and
most are incidentally discovered. The main aim of preoperative
examination is to exclude the possibility of malignancy. However,
>10% of patients with benign adnexal lesions still undergo
surgery (15). SPAF is a rare
benign adnexal tumor that often mimics malignancies. In the present
study, it was found that SPAF is difficult to distinguish from
other types of ovarian cysts. Therefore, it is important to
differentiate it using imaging methods. Sonography and MRI are the
most common technologies used in gynecological practice. According
to the present results, SPAF shows a cystic or cyst-solid
appearance with papillary projections on ultrasound imaging.
Generally, these appearances are considered to be signs of
malignancy. However, in those with positive ultrasound results, 8
of 11 patients showed a unilocular cyst appearance, which usually
represents benign lesions (16).
Owing to the limitations of ultrasound, MRI is another radiological
examination used for more accurate visualization. A low T2 signal
is characteristic of SPAF on MRI because of the fibrous components
in the solid part. However, SPAF showed a high T2 signal without
solid components. Typically, these signals represent simple fluid.
This may have been due to the early stage of SPAF in the patients
of the present study. By contrast, CT can visualize nearby organ
invasion more clearly. In the present study, it was found that SPAF
can appear at either a high or low intensity on CT scans, making it
difficult to diagnose. Therefore, MRI is of significant value in
preoperative diagnosis. However, radiologists and gynecologists
must also consider the cyst stage. Due to limited published
studies, it was only possible to try and predict certain MRI
features of SPAF. A serous adenofibroma is a cystic lesion with a
regular wall. SPAF should show unilocular or multilocular cysts
with solid parts and protrusions on the walls. The present results
also confirmed the presence of SPAF papillae on MRI. Further
research is needed to distinguish SPAF from other gynecological
malignancies.
To our knowledge, no previous study has focused on
sex hormone and carbohydrate antigen testing in patients with SPAF.
Of note, in the present study, it was found that 3 of 9 patients
had elevated PRL levels. PRL is a pivotal molecule associated with
the survival and progression of gynecological cancers.
Anti-estrogen therapy is a widely applied strategy for the control
of numerous gynecological cancers, such as breast, ovarian and
endometrial cancers. However, studies have shown that PRL can
activate ER-α via p21-activated kinase 1, circumventing the effects
of anti-estrogen therapy (17). Of
note, it was found that those patients with high PRL levels had
high expression of ER and PR, two of which were also Her-2
positive. In addition, patients with normal PRL levels failed to
achieve an accurate diagnosis of SPAF, particularly in terms of
frozen-section pathology. However, patients with elevated PRL
levels can be definitively diagnosed. Therefore, PRL expression is
likely associated with the pathological appearance and hormone
sensitivity. There is evidence to demonstrate a connection between
PRL and gynecological malignancies. PRL can phosphorylate
STAT3/STAT5 and promote ovarian cancer cell migration and colony
formation, and high PRL receptor (PRLR) expression is associated
with poor prognosis of ovarian cancer (18). PRLR was also found to be
co-expressed with ER and to promote the metabolism of HeLa cells.
However, the exact effect of PRL has yet to be determined. Due to
the small sample size of the present study, it is necessary to
increase the sample size to evaluate the role of PRL in the
development of SPAF.
Ki-67 and BRAF are two common oncogenes that are
involved in cell proliferation. As a typical marker of genital
tract tumors, Ki-67 usually represents cell proliferation and is
used for risk evaluation (19).
Clinically, Ki-67 expression can help predict the prognosis of
patients with numerous cancer types (20). BRAF is an oncogene that encodes the
B-Raf protein and is expressed in several types of tumor (21). The present results showed that SPAF
tumors had low Ki-67 and BRAF expression levels. This indicates
that SPAF may not be aggressive. Mutations in KRAS, BRAF and Her-2
are thought to be markers of low-grade serous carcinoma (22). In the present study, SPAF showed
high expression of Her-2. Therefore, more attention should be paid
to diagnosis and prevention. The treatment of gynecological
malignancies is complex and expensive. Usually, doctors use
comprehensive combination therapy to control malignancy
progression. Therefore, early intervention is necessary,
particularly in those patients with Her-2 mutation. Low expression
levels of p53 and WT-1 indicate a favorable prognosis in patients.
Mutations in p53 are caused by multiple cellular stresses and are
associated with high-risk tumors (23). WT-1, at the same time, is also a
classical marker for cancers in several tissues. To identify risk
factors and prevent SPAF recurrence in clinical practice, the
present results suggest that hormone therapy may be promising.
Patients seem to have hyperestrogenaemia and hyperprolactinemia.
IHC staining also showed high expression of ER, PR and Her-2 in the
SPAF tissues. Control of sex hormone intake and anti-sex hormone
therapy may be applied to patients who were already diagnosed with
SPAF and underwent surgery.
Nevertheless, the present study has limitations,
primarily its small sample size. It is therefore recommended that
future large-scale or multi-center studies are performed. Building
on these findings, it may be worthwhile to establish a relationship
between MRI features, hormone levels (particularly PRL) and disease
progression in patients with SPAF and develop an integrated
MRI-hormone diagnostic algorithm to optimize preoperative
diagnostic accuracy. Furthermore, prospective studies may clarify
the impact of postoperative anti-sex hormone therapy on prognosis.
Finally, while IHC staining is necessary for identifying molecular
markers, its results are affected by multiple factors and are
subjective based on observers. Western blot analysis or PCR may be
required in future applications to validate results and improve
standardization if possible (24).
In conclusion, SPAF is a benign tumor that mimics
malignancy. Owing to its rarity, SPAF tends to be diagnosed
postoperatively. Radiologists may be advised to determine certain
characteristics of SPAF, particularly on MRI imaging. High sex
hormone levels may be another indication of SPAF. Because SPAF
shows low malignancy, more attention should be paid to its
prevention and early diagnosis. IHC staining results suggest that
SPAF is sensitive to sex hormones. This can be used by
gynecologists to help reduce recurrence in patients.
Supplementary Material
Immunohistochemistry images. Strong
positivity was encountered for (A) estrogen receptor, (B) human
epidermal growth factor receptor 2 and (C) progesterone receptor.
Minimally positive or negative staining was encountered for (D)
Ki-67, (E) Wilms' tumor gene 1 and (F) P53 (scale bars, 50
μm).
Acknowledgements
The authors acknowledge the clinical data support of
the Second Affiliated Hospital Zhejiang University School of
Medicine (Hangzhou, China).
Funding
Funding: This work was supported by Zhejiang Traditional Chinese
Medicine Administration (grant no. 2024038810).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All authors contributed to the study conception and
design. Material preparation, experimental result interpretation,
data collection and analysis were performed by XZ and CY. YL and XL
checked and confirmed the authenticity of the raw data. The first
draft and final edit of the manuscript were prepared by XZ and all
authors commented on previous versions of the manuscript. XW edited
all tables and figures. YL and XL searched relevant articles and
references. ZY directed the data analysis. LW revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was performed in line with the principles
of the Declaration of Helsinki. Approval was granted by the Ethics
Committee of the Second Affiliated Hospital Zhejiang University
School of Medicine (Hangzhou, China; approval no. 20231165). Based
on specific criteria, the Ethics Committee granted a formal waiver
of informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jones AMK, Yue WY, Marcus J and Heller DS:
Pitfalls of frozen section in gynecological pathology: A case of
ovarian serous surface papillary adenofibroma imitating malignancy.
Int J Surg Pathol. 27:268–270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Taylor EC, Irshaid L and Mathur M:
Multimodality Imaging Approach to Ovarian Neoplasms with Pathologic
Correlation. Radiographics. 41:289–315. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cho DH: Serous cystadenofibroma
misdiagnosed as an ovarian malignancy. BMJ Case Rep 28:.
11(e228223)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takeuchi M, Matsuzaki K and Harada M:
Ovarian adenofibromas and cystadenofibromas: Magnetic resonance
imaging findings including diffusion-weighted imaging. Acta Radiol.
54:231–236. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hsu I, Lee LH, Hsu L, Chen SU and Hsu CC:
Disordered hypothalamus-pituitary-ovary axis in heterotopic
extraovarian sex cord-stromal proliferation: A case report of
fallopian tube serous adenofibroma. BMC Womens Health.
23(243)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roshini AP, Pandarinath A and Corriea M:
Serous papillary cystadenofibroma of vulva: A histopathological
surprise. Int J Surg Case Rep. 83(105776)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tavares MA, Silva RC, Lourenço M and
Ambrósio A: Giant serous adenofibroma of the fallopian tube. BMJ
Case Rep. 13(e234267)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ulker V, Tunca AF, Akbayir O, Numanoglu C,
Yesil S and Bakir B: Serous surface papillary adenofibroma of the
ovary: Impersonator of ovarian malignancy. J Obstet Gynaecol.
34:365–366. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Andreotti RF, Timmerman D, Strachowski LM,
Froyman W, Benacerraf BR, Bennett GL, Bourne T, Brown DL, Coleman
BG, Frates MC, et al: O-RADS US risk stratification and management
system: A consensus guideline from the ACR Ovarian-adnexal
reporting and data system committee. Radiology. 294:168–185.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sadowski EA, Thomassin-Naggara I, Rockall
A, Maturen KE, Forstner R, Jha P, Nougaret S, Siegelman ES and
Reinhold C: O-RADS MRI risk stratification system: Guide for
assessing adnexal lesions from the ACR O-RADS Committee. Radiology.
303:35–47. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Global BMIMC, Di Angelantonio E,
Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de
Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, et al: Body-mass
index and all-cause mortality: Individual-participant-data
meta-analysis of 239 prospective studies in four continents.
Lancet. 388:776–786. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thull T and Kempton D: Ovarian cancer: A
review for primary care providers. JAAPA. 37:32–36. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sasamoto N and Elias KM: Early detection
of ovarian cancer. Cold Spring Harb Perspect Med.
13(a041337)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Virgilio BA, De Blasis I, Sladkevicius P,
Moro F, Zannoni GF, Arciuolo D, Mascilini F, Ciccarone F, Timmerman
D, Kaijser J, et al: Imaging in gynecological disease (16):
Clinical and ultrasound characteristics of serous cystadenofibromas
in adnexa. Ultrasound Obstet Gynecol. 54:823–830. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rocha RM and Barcelos IDES: Practical
recommendations for the management of benign adnexal masses. Rev
Bras Ginecol Obstet. 42:569–576. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Timmerman D, Ameye L, Fischerova D,
Epstein E, Melis GB, Guerriero S, Van Holsbeke C, Savelli L,
Fruscio R, Lissoni AA, et al: Simple ultrasound rules to
distinguish between benign and malignant adnexal masses before
surgery: Prospective validation by IOTA group. BMJ.
341(c6839)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Oladimeji P, Skerl R, Rusch C and
Diakonova M: Synergistic activation of ERα by estrogen and
prolactin in breast cancer cells requires tyrosyl phosphorylation
of PAK1. Cancer Res. 76:2600–2611. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alkharusi A, AlMuslahi A, AlBalushi N,
AlAjmi R, AlRawahi S, AlFarqani A, Norstedt G and Zadjali F:
Connections between prolactin and ovarian cancer. PLoS One.
16(e0255701)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jung D, Almstedt K, Battista MJ, Seeger A,
Jäkel J, Brenner W and Hasenburg A: Immunohistochemical markers of
prognosis in adult granulosa cell tumors of the ovary-a review. J
Ovarian Res. 16(50)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Davey MG, Hynes SO, Kerin MJ, Miller N and
Lowery AJ: Ki-67 as a prognostic biomarker in invasive breast
cancer. Cancers (Basel). 13(4455)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Zheng L, Yang Q and Sun J: The
evolution of BRAF activation in Non-Small-Cell lung cancer. Front
Oncol. 12(882940)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vang R, Shih IM and Kurman RJ: Ovarian
Low-grade and High-grade serous carcinoma: Pathogenesis,
clinicopathologic and molecular biologic features, and diagnostic
problems. Adv Anat Pathol. 16(267)2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu S, Liu T, Jiang J, Guo H and Yang R:
p53 mutation and deletion contribute to tumor immune evasion. Front
Genet. 14(1088455)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mebratie DY and Dagnaw GG: Review of
immunohistochemistry techniques: Applications, current status, and
future perspectives. Semin Diagn Pathol. 41:154–160.
2021.PubMed/NCBI View Article : Google Scholar
|