Introduction
Comprehensive cancer genome profiling (CGP) tests
were approved by the public health insurance (PHI) system in Japan
in June 2019, to identify genotype-matched therapies (GMTs) for
patients with advanced solid tumors who fail to respond to standard
therapies or for whom there are no appropriate standard therapies
(1). Cancer genetic medicine is
becoming part of routine clinical practice for patients with
metastatic prostate cancer. The PROfound trial was conducted in
patients with metastatic castration-resistant prostate cancer
(mCRPC) who had deleterious alterations in prespecified genes
related to homologous recombination repair (2). Olaparib was listed in Japanese PHI on
December 2020 for patients with mCRPC with BRCA1 or
BRCA2 deleterious alterations, confirmed by
BRACAnalysis® or CGP tests using
FoundationOne® CDx (F1CDx) or FoundationOne®
Liquid CDx (F1LCDx) CGP. The results of the PROpel and TALAPRO-2
trials were also approved by the Pharmaceuticals and Medical
Devices Agency in Japan for patients with mCRPC with BRCA
deleterious alterations (3,4).
The advent of cancer genetic medicine is an
opportunity to recognize the importance of GMT for advanced
prostate cancer and to validate the existence of hereditary cancers
in patients with prostate cancer. When managing patients with
metastatic prostate cancer, physicians must have the ability to
interpret the results of CGP tests and identify appropriate GMTs
for those patients. In addition, the need for genetic counseling
(GC) has increasingly been recognized as the opportunity for CGP
tests has increased in daily clinical practice.
In the present study, we investigated the status and
issues regarding genetic medicine using publicly reimbursed CGP
tests in patients with metastatic prostate cancer who failed to
respond to standard therapies or for whom there were no appropriate
standard therapies. We also investigated the status of GC and
confirmatory germline testing for the secondary findings of cancer
susceptibility genes identified by tumor-only sequencing.
Materials and methods
Patients and study design
This single-institution retrospective study
evaluated the records of patients with metastatic prostate cancer
who underwent CGP tests at Kitasato University Hospital (Kanagawa,
Japan) between August 2019 and November 2024. Patients with
metastatic prostate cancer who showed resistance to docetaxel or
second-generation androgen receptor signaling inhibitors (ARSIs),
including abiraterone, apalutamide, enzalutamide, and darolutamide,
were subjected to CGP testing. Patients with androgen-indifferent
prostate cancer, such as neuroendocrine prostate cancer, also
underwent CGP testing. A total of 86 male patients were included,
with a median age of 73.5 years (range, 46-85). High-volume disease
was defined according to the CHAARTED criteria to evaluate the
metastatic status (5).
Pathological slides for patients who underwent
biopsy or radical prostatectomy at a facility other than our
institution were reviewed by our institutional pathologists.
Gleason scores were obtained from surgical specimens for patients
who underwent radical prostatectomy, or from biopsy specimens at
initial diagnosis for patients who did not undergo radical
prostatectomy. All patients had histologically confirmed prostate
cancer.
The Kitasato University Medical Ethics Organization
approved this study (B21-110). All methods were carried out in
accordance with relevant guidelines and regulations. Informed
consent was obtained in the form of an opt-out option on the
website and posters.
CGP tests and actionable genomic
alterations
F1CDx and F1LCDx were used for CGP tests in this
study. F1CDx is a tissue-based hybrid capture next-generation
sequencing (NGS) assay, and F1LCDx is a liquid biopsy-based NGS
assay. Both tests analyze 324 cancer-related genes and selected
intronic regions of 36 genes, designed to detect base
substitutions, insertions/deletions, copy number alterations, gene
rearrangements, microsatellite instability (MSI), and tumor
mutation burden (TMB). Sequencing covers the entire coding regions
of the included genes and selected intronic regions relevant for
fusion detection (1). CGP testing
was carried out using F1LCDx if the quality or quantity of an
archival formalin-fixed paraffin-embedded tissue sample was
inadequate for DNA analysis. CGP testing was only covered by
Japanese PHI once in a person's lifetime at the time of this study,
and individuals could thus not undergo both F1CDx and F1LCDx tests
under PHI.
The evidence level was categorized as A to F based
on the clinical practice guidelines for CGP tests in cancer
diagnosis and treatment (Edition 2.0) issued by the Joint Consensus
of Japanese Society of Medical Oncology, Japan Society of Clinical
Oncology, and Japanese Cancer Association. When GMT was identified
by CGP tests, actionable genomic alterations were defined as
alterations at or above evidence level D (1,6,7).
GC
If the patient wanted to know the results of the
possible germline gene alterations detected by the CGP test, the
physician in charge of genomic medicine disclosed if the patient
had secondary findings of cancer susceptibility genes identified by
tumor-only sequencing. The patients were advised to receive GC from
the physician in charge of genomic medicine. If a patient was
willing to receive GC, our hospital provided a system in which the
patient called the Genetics Division and made an appointment. After
receiving GC from clinical geneticists and certified genetic
counselors, the patient then decided if they wanted to undergo
confirmatory germline tests.
Statistical analyses
Three different grouping strategies were employed in
this study based on the analytical objectives. First, to evaluate
clinical characteristics and the utility of testing modalities,
patients were grouped according to the type of CGP test received:
F1CDx and F1LCDx. Second, for the analyses of progression-free
survival (PFS) and prostate-specific antigen (PSA) 50% decline,
patients were stratified based on the type of post-CGP treatment:
GMT/PHI group and subsequent systemic therapy (SST) group. Third,
for the overall survival (OS) analysis, patients who received SST
or transitioned to best supportive care (BSC) were categorized as
the non-GMT group, and their outcomes were compared with those of
the GMT/PHI group. SST was defined as any prostate cancer treatment
administered after CGP testing that was not genomically matched
therapy. CRPC, PSA progression, and radiographic progression were
defined according to the Prostate Cancer Working Group 3.
Radiographic progression was evaluated according to Response
Evaluation Criteria in Solid Tumors (RECIST 1.1) on computed
tomography scan or the 2 + 2 rule on bone scintigraphy (8,9).
Clinical progression was defined as cancer pain requiring
administration of opioids, the need to initiate subsequent therapy,
radiation therapy, surgical intervention for complications due to
tumor progression, or deterioration in Eastern Cooperative Oncology
Group (ECOG) performance to ≥ grade 3. A castrate testosterone
level was defined as ≤50 ng/dl.
PFS was defined as the time from initiation of GMT
or SST to first occurrence of any progression or death from any
cause, whichever occurred first. The events required to estimate
PFS were PSA progression, radiographic progression, clinical
progression, or any cause of death. Declines in serum PSA levels
≥50% were compared by χ2 test. OS was calculated from
the date of genetic testing to last follow-up. One patient was
confirmed as positive by BRACAnalysis® and started
olaparib before CGP testing, and was considered as receiving GMT in
PHI. PFS and OS were calculated from the start date of olaparib
administration and BRACAnalysis® testing, respectively,
and the best PSA response to olaparib was assessed in this
patient.
PFS and OS were estimated by the Kaplan-Meier method
and compared using the Gehan-Breslow-Wilcoxon test. Multivariate
Cox proportional hazards regression models were performed to
evaluate the factors influencing PFS. Differences were considered
statistically significant at P<0.05. All reported P-values are
two-sided. All analyses were performed using Stata version 15
(Stata Corp., College Station, TX, USA) and GraphPad Prism version
8 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patient profiles
Table I summarizes
the clinical characteristics of the 86 patients in the present
study. To assess the patient characteristics and the impact of each
testing modality on clinical utility, the patients were divided
into two groups based on the type of CGP test: F1CDx (n=63) and
F1LCDx (n=23). The median age of the overall population was 73.5
years, with 73 years for patients who underwent F1CDx and 74 years
for those who underwent F1LCDx. The median time to CRPC for the
overall population was 16 months, with 14.1 months for F1CDx and
24.4 months for F1LCDx. The detection of deleterious BRCA
alteration for the overall population was 16.2% (14/86), with 22.2%
(14/63) for F1CDx and 0% (0/23) for F1LCDx.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | F1CDx (n=63) | F1LCDx (n=23) | Total (n=86) |
|---|
| Median age (range),
years | 73 (46-85) | 74 (57-84) | 73.5 (46-85) |
| Median PSA at
initial diagnosis (range), ng/ml | 95.5
(1.13-9,747) | 21.0
(6.86-8,537) | 55.0
(1.13-9,747) |
| Metachronous
(TxNxM0) | 21 (33.3) | 14 (60.9) | 35 (40.6) |
|
Radical
prostatectomy | 10 (15.9) | 5 (21.7) | 15 (17.4) |
|
Prostate
radiotherapy | 9 (14.3) | 8 (34.8) | 17 (19.8) |
| Synchronous
(TxNxM1) | 42 (66.7) | 9 (39.1) | 51 (59.4) |
|
High volume
(CHAARTED criteria) | 31 (49.2) | 8 (34.7) | 39 (45.3) |
| Gleason score | | | |
|
7 | 5 (7.9) | 2 (8.7) | 7 (8.1) |
|
8 | 16 (25.4) | 8 (34.8) | 24 (27.9) |
|
9 or 10 | 42 (66.7) | 12 (52.2) | 54 (62.8) |
|
Unknown | 0 (0.0) | 1 (4.3) | 1 (1.2) |
| Median time from
initiation of ADT to CGP tests (range), months | 32.8
(0.5-238.5) | 73.3
(24.7-151.4) | 40.6
(0.5-238.5) |
| Median time to CRPC
(range), months | 14.1
(1.9-98.6) | 24.4
(1.4-88.2) | 16.0
(1.4-98.6) |
| Deleterious
BRCA alteration | 14 (22.2) | 0 (0) | 14 (16.2) |
|
BRCA1 | 1 | 0 | 1 |
|
BRCA2 | 13 | 0 | 13 |
| MSI-high and
TMB-high | 3 (4.8) | 0 (0.0) | 3 (3.5) |
| BRAF
V600 | 0 (0.0) | 1 (4.3) | 1 (1.2) |
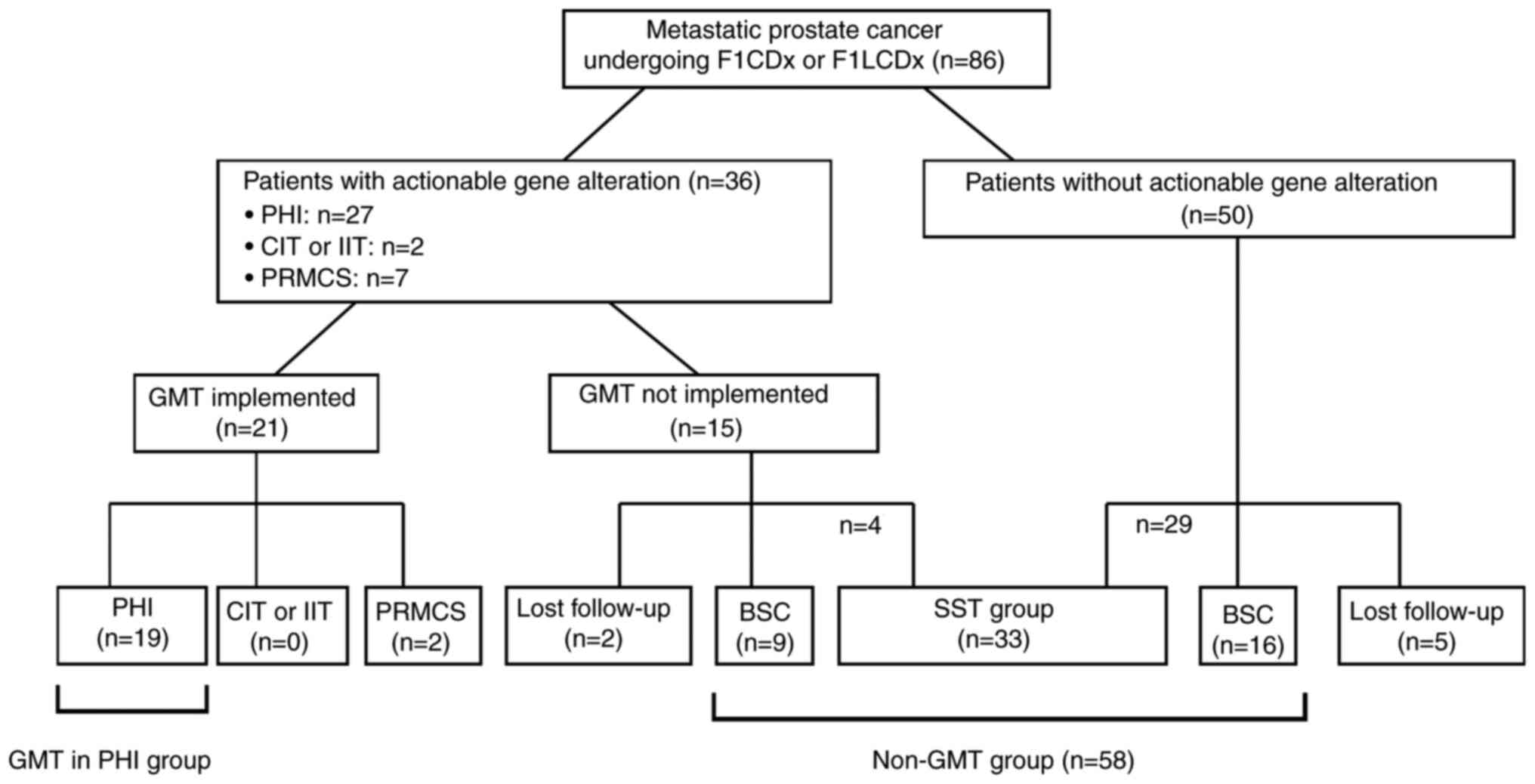
The patient profiles after CGP testing are shown in
Fig. 1. Based on expert panel
discussion, GMT was recommended in 36 patients (41.8%), including
27 in PHI, two in phase I or II clinical trials, and seven in
patient-requested medical care system phase II (PRMCS). GMT was
actually implemented in 21 patients (24.4%), including 19 (22.1%)
in PHI, none in clinical trials, and two (2.3%) in PRMCS. Of the 36
patients for whom GMT was recommended, 15 (17.4%) did not receive
the GMT due to deterioration of their general condition including
prostate cancer death (n=9), ineligibility for clinical trial
participation (n=1), the long distance to the clinical trial sites
(n=1), unwilling to undergo the GMT (n=2), transfer to other
institutions with loss to follow-up (n=2). Additionally, four
patients received SST other than the recommended GMT.
Among the 50 (58.2%) patients in whom no actionable
gene alteration was found, 29 received some kind of SST for
prostate cancer. Sixteen patients (18.6%) switched to BSC due to
the absence of aggressive prostate cancer treatment. Five patients
(5.8%) lost to follow-up due to transfer to other institutions.
A total of 33 patients (38.4%) received some form of
SST for prostate cancer other than GMT. Twenty-five patients
(29.1%) switched to BSC due to the absence of aggressive prostate
cancer treatment. Seven patients (8.1%) lost to follow-up.
Oncological outcomes
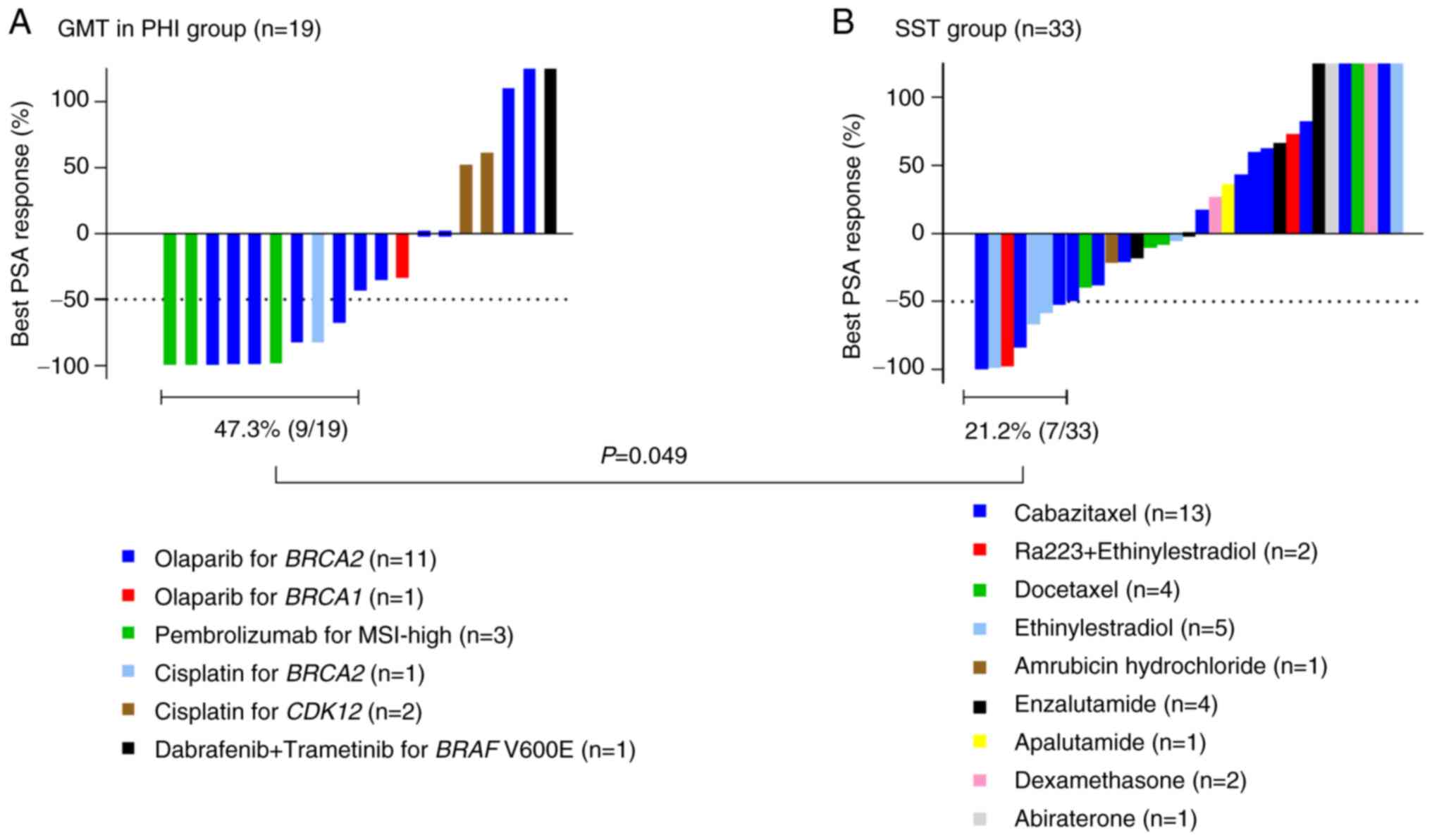
The PSA response in the GMT/PHI group and the SST
group based on the results of CGP testing is shown in Fig. 2. Because individual data for GMT in
PRMCS could not be disclosed, only data for GMT in PHI are
presented in Fig. 2A. The
treatments details for the 19 patients in the GMT/PHI group are
also shown in Fig. 2A, the
detailed oncological outcomes for the GMT/PHI group are shown in
Table SI, and details of the
treatments in the 33 patients in the SST group are shown in
Fig. 2B. Declines in serum PSA
levels ≥50% occurred more frequently in the GMT/PHI group (47.3%,
9/19) than in the SST group (21.2%, 7/33) (P=0.049). PFS in the
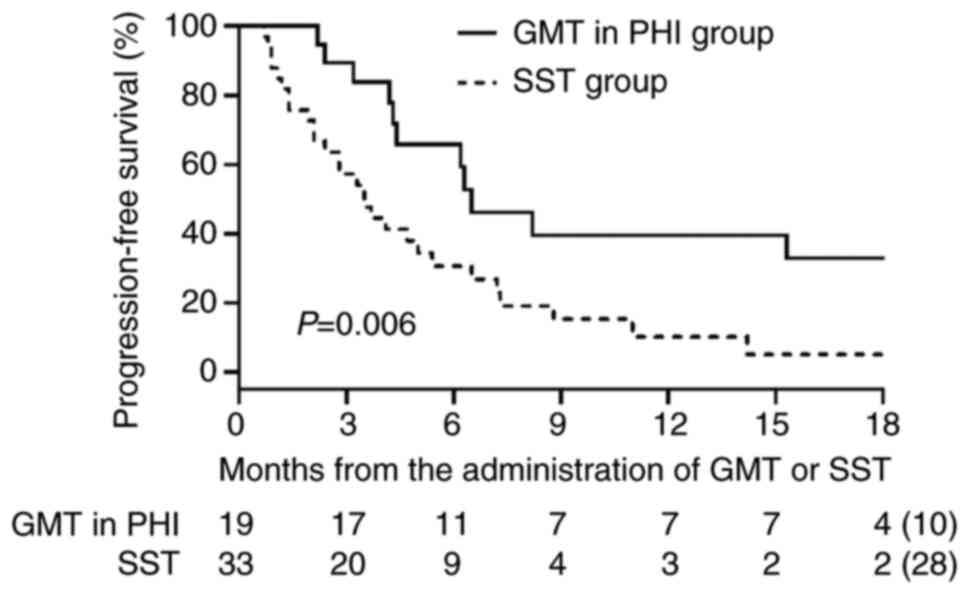
GMT/PHI and SST groups is shown in Fig. 3. PFS was significantly longer in
the GMT/PHI group than in the SST group (median: 6.5 vs. 3.5
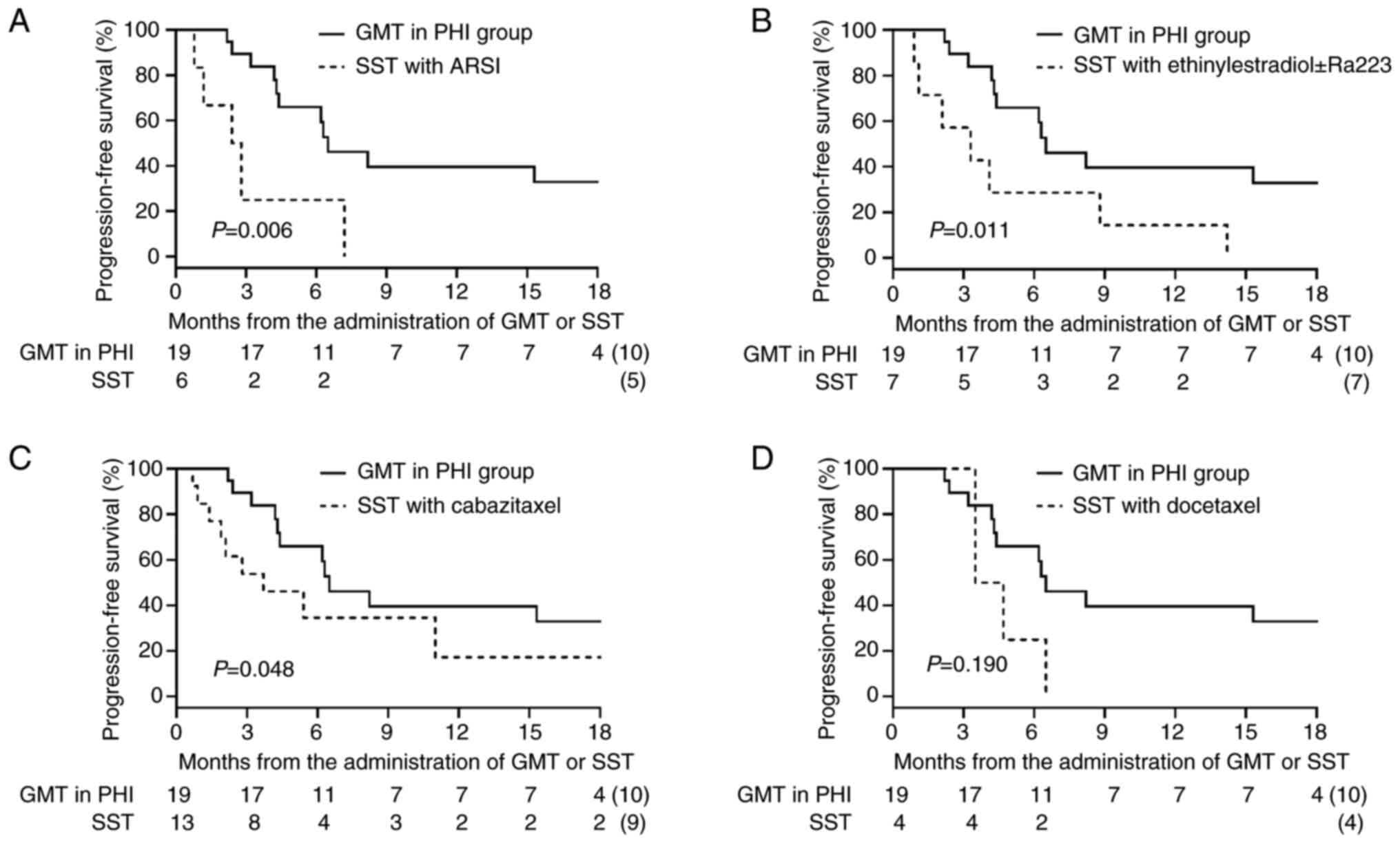
months; P=0.006). We conducted a subgroup analysis for PFS in which
each treatment regimen within the SST group was individually
compared with the GMT/PHI group (Fig.
4). The median PFS in the GMT/PHI group was significantly
longer than that in the SST subgroup treated with ARSI rechallenge
(6.5 vs. 2.6 months; P=0.006), ethinylestradiol ± Ra223 (6.5 vs.
3.3 months; P=0.011), and cabazitaxel (6.5 vs. 3.7 months;
P=0.048). In contrast, there was no statistically significant
difference in PFS between the GMT/PHI group and the SST subgroup
treated with docetaxel (6.5 vs. 4.1 months; P=0.190). After
multivariate adjustment, receiving GMT/PHI was the only factor that
showed a statistically significant association with longer PFS
(hazard ratio 0.44, 95% confidence interval 0.20-0.93) (Table II).
 | Table IIMultivariate analyses using a Cox
proportional hazards model for progression-free survival. |
Table II
Multivariate analyses using a Cox
proportional hazards model for progression-free survival.
| Variable | HR | 95% CI | P-value |
|---|
| Baseline PSA at
initiation of GMT or SST, continuous | 1.00 | 0.99-1.00 | 0.64 |
| CHAARTED criteria,
high volume vs. low volume | 0.97 | 0.50-1.89 | 0.94 |
| Time to CRPC,
continuous | 1.00 | 0.98-1.02 | 0.85 |
| GMT/PHI group vs.
SST group | 0.44 | 0.20-0.93 | 0.03 |
We defined the 25 patients who switched to BSC and
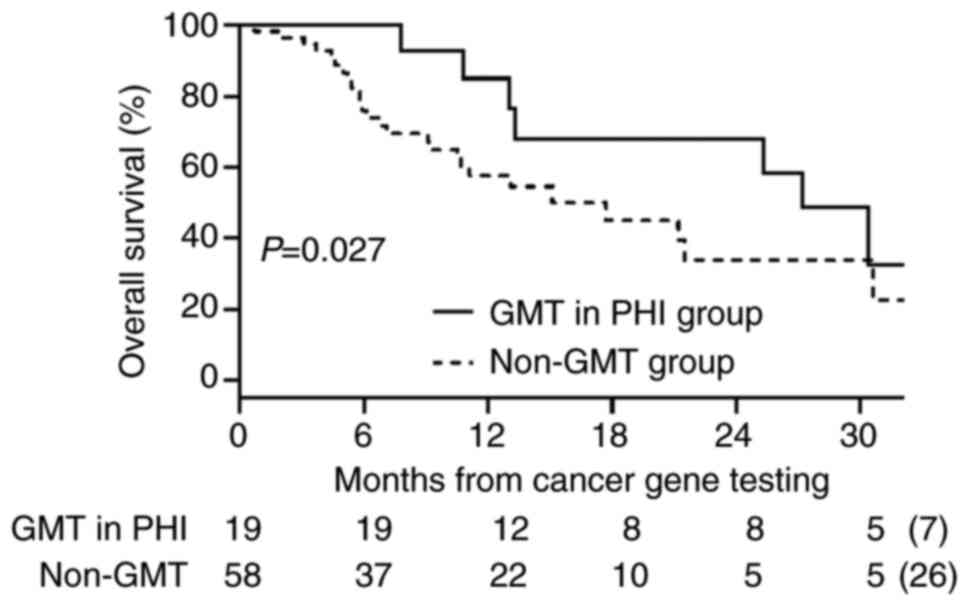
the 33 patients who received SST as the non-GMT group (n=58). OS
was significantly longer in the GMT/PHI group than in the non-GMT
group (median: 27.2 vs. 17.7 months; P=0.027) (Fig. 5).
Gene abnormalities known to be involved in cancer,
including alterations that may be targets of investigational
therapies, were summarized in Table
SII as potential actionable gene alterations identified by
F1CDx and F1LCDx (https://doi.org/10.6084/m9.figshare.29923460).
GC and confirmatory germline
testing
Table III shows
the implementation status of GC and confirmatory germline testing.
Of the 14 patients suspected of carrying deleterious germline
alterations, seven (50%) received GC, six of the seven patients
received confirmatory germline tests and two were diagnosed with
hereditary breast and ovarian cancer. Two patients underwent
confirmatory genetic testing for Lynch syndrome, but the results
were negative. The main motivation for receiving GC was the
benefits for their family, but one patient visited the genetic
division simply because his doctor told him to receive GC; however,
this patient did not undergo confirmatory germline testing. Seven
patients (50%) did not visit the genetic division because of poor
health condition (n=1), death (n=1), not feeling the need (n=1),
family opposition to GC (n=1), no relation to prostate cancer
(n=1), and unknown reasons (n=2).
 | Table IIIGenetic counseling and confirmatory
germline testing for cancer susceptibility genes. |
Table III
Genetic counseling and confirmatory
germline testing for cancer susceptibility genes.
| Alteration | Age, years | VAF, % | GC | Reasons for
receiving or not receiving GC | Germline
testing | Germline
alteration | Family history of
cancer |
|---|
| BRCA2
(A902fs*2) | Mid 40s | 64.4 | Received | Told by doctor to
receive GC | Untested | Unknown | Breast, mother;
stomach, father; uterus, grandmother |
| BRCA2
(V1447fs*1) | Early 70s | 83.6 | Received | For his family | Tested | Yes | None |
| BRCA2 (HD
exon 9-27) | Early 70s | - | Received | For his family | Tested | No | Prostate, father
and uncle; stomach, grandmother |
| BRCA2
(E2877*) | Early 50s | 76.9 | Received | For his family | Tested | Yes | Brain, aunt |
| BRCA2
M2393fs*19 | Early 80s | 36.6 | Received | For his family | Tested | No | Lung, father |
| MSI-high | Early 70s | - | Received | For his family | Tested | No | None |
| MSI-high | Mid 70s | - | Received | For his family | Tested | No | Tongue, sister |
| BRCA1 (HD,
exon 5-8) | Mid 50s | - | Not received | Poor health
condition | Untested | Unknown | None |
| BRCA2
E790fs*20 | Early 80s | 34.5 | Not received | Death | Untested | Unknown | None |
| BRCA2
(I729fs*21) | Late 70s | 25.7 | Not received | Not feeling the
need | Untested | Unknown | Uterus,
grandmother |
| BRCA2
(c.316+1G>T) | Mid 80s | 31.9 | Not received | Family
opposition | Untested | Unknown | Colon, father;
breast, aunt |
| WT1
c.1001+1G>T | Early 70s | 49.8 | Not received | No relation to
prostate cancer | Untested | Unknown | Biliary tract,
sister; stomach, sister; breast, sister; colon, cousin |
| ATM
c.5918+1G>T | Mid 70s | 71.4 | Not received | Unknown | Untested | Unknown | Pancreas, father;
stomach, uncle |
| PMS2
(E836fs*15) | Late 60s | 85.7 | Not received | Unknown | Untested | Unknown | None |
| MSH2
(Q170*) | | 66.3 | | | | | |
Discussion
The current study included patients with metastatic
prostate cancer who underwent F1CDx or F1LCDx testing after
progression to an ARSI or docetaxel, or patients for whom there was
no appropriate standard therapy due to androgen-indifferent
prostate cancer. Outcomes in terms of PFS and declines in serum PSA
levels ≥50% were significantly better in the GMT/PHI group compared
with the SST group. While approximately one in five of these
patients received GMT as a result of CGP testing, many patients who
underwent CGP tests were unable to receive GMT, highlighting the
need to address this issue in the future.
GMT was implemented in 21 patients (24.4%),
including 19 (22.1%) in PHI and two (2.3%) in PRMCS, but a total of
33 patients (38.4%) received some form of SST other than GMT,
because no actionable gene alterations were detected, or if gene
alterations were detected, the patients were unable to access GMT.
The timing of CGP testing is sometimes delayed in Japan since the
CGP testing is limited to patients with mCRPC who developed
resistance to docetaxel or ARSIs. As a result, patients experienced
disease progression at the time of disclosure of CGP results and
were no longer able to receive the recommended GMT. In addition,
accessibility to GMT through clinical trials is also one of the
issues that should be addressed. Some patients were unable to
receive treatment because of a lack of accurate information
regarding the eligibility criteria for clinical trials and the long
distance to the clinical trial sites. Sharing clinical trial
information among the hospitals and expanding clinical trial sites
across Japan are necessary (1).
CGP testing can detect gene alterations and genomic
signatures, including MSI, TMB, genome-wide loss of heterozygosity,
and homologous recombination deficiency. Although several
prospective randomized phase II trials have investigated the
efficacy of CGP testing for solid tumors, the clinical benefit of
GMT using CGP testing remains debatable (10-12).
The SHIVA trial was conducted for patients with any kind of
metastatic solid tumor refractory to standard of care. The use of
GMT outside their indications does not improve PFS compared with
treatment at physician's choice (10). The CUPISCO trial compared the
efficacy of GMT vs. standard platinum-based chemotherapy in
patients with an unfavorable cancer subset of unknown primary.
Subgroup analysis showed that patients with an actionable molecular
profile treated with GMT had longer PFS than those with an
actionable molecular profile treated with standard platinum-based
chemotherapy (hazard ratio 0.76, 95% confidence interval
0.42-0.99), with the greatest differences in patients treated with
atezolizumab for TMB-high or MSI-high, vemurafenib plus cobimetinib
for BRAF V600 or K601E alterations, or pemigatinib for
FGFR1, FGFR2, or FGFR3 alterations (12). These results support the
effectiveness of CGP testing for patients for whom there is no
appropriate standard treatment.
A previous study reported that BRCA
deleterious alterations were detected by tumor genetic testing in
approximately 10% of patients with mCRPC (2-4,13),
which was lower than the 16.2% in the current study. Mateo et
al (14) reported that
BRCA2 deleterious alterations were significantly more common
in patients who developed mCRPC in a short period, and patients
with BRCA2 deleterious alterations had the lower time to
androgen-deprivation therapy (ADT) progression compared to those
without BRCA2 deleterious alterations. Thus, this difference
may be due to the relatively short time to CRPC observed in our
patients undergoing CGP tests. In the present study, olaparib was
administered to patients with mCRPC who were resistant to at least
one kind of ARSI, with a similar treatment population to the
PROfound study (2). Three of 12
patients receiving olaparib responded without progression for
>24 months, confirming the existence of deep and durable
responders among patients with BRCA deleterious alterations,
after resistance to ARSI. Several potential biological mechanisms
have been reported to underly long-term responses to poly ADP
ribose polymerase (PARP) inhibitors. BRCA structural
variants, such as rearrangements and homozygous deletions, are
inherently resistant to reversion mutations and thus contribute to
durable responses to PARP inhibitors (15). Buonaiuto et al (16) reported that the efficacy of PARP
inhibitor is independent by BRCA domain defects or mutation
types. These findings are partly consistent with our results.
However, they are not sufficient to fully explain the mechanisms
underlying long-term responses to PARP inhibitors. The underlying
biological mechanisms need to be further explored.
The detection rate of MSI-high in prostate cancer is
approximately 3%, and a PSA response ≥50% with immune checkpoint
inhibitor (ICI) therapy has been reported to be 44-65% (17-19).
Three patients with both MSI-high and TMB-high genomic signatures
received pembrolizumab, all of whom showed a PSA response ≥50%,
while two achieved undetectable PSA levels in the present study.
Notably, in the two patients who achieved undetectable PSA levels,
the dominant lesions were confined to the lymph nodes and the
primary tumor. In contrast, one patient who died of prostate cancer
had extensive bone metastases. The efficacy of ICIs may differ
depending on the site of metastasis. In non-small cell lung cancer,
lymph node and primary lung lesions tend to show higher response
rates and better disease control with ICIs, whereas bone metastases
are consistently associated with poor responses and worse survival
outcomes (20,21). This likely reflects differences in
the tumor microenvironment (22).
In line with these findings, the differential response patterns
observed in our study may reflect the underlying immune contexture
of metastatic sites.
No patients in our study were identified as TMB-high
and microsatellite stable (MSS), in contrast to a previous study
that reported TMB-high with MSS in 0.7% of patients with prostate
cancer. Although the scientific rationale for ICIs is similar
between MSI-high and TMB-high with MSS, patients with prostate
cancer with TMB-high plus MSS did not demonstrate the same
durability to ICI treatment compared with MSI-high patients
(17).
BRAF gain-of-function alteration, RET
fusion, and NTRK fusion are rare alterations detected by CGP
testing in patients with prostate cancer. The use of molecular
targeted therapies, such as tumor-agnostic treatments, is approved
for these genetic alterations under PHI in Japan (23-26).
In the present study, we administered combination therapy with
dabrafenib and trametinib in a patient with a BRAF V600E
alteration. In this case, chest-to-pelvis computed tomography scans
revealed no primary tumors other than prostate cancer, while an
additional colonoscopy examination found no colorectal cancer and a
dermatological evaluation found no evidence of malignant melanoma.
In addition, F1LCDx identified the AR T878A alteration as a
covariate, with a variant allele frequency of 3.5%. We observed a
partial response in liver metastases on radiographic imaging. To
the best of our knowledge, this is the first reported case of the
efficacy of this combination therapy in a patient with BRAF
Class I alterations, which occur at the V600 codon and exhibit
extremely strong kinase activity by stimulating monomeric
activation of BRAF in prostate cancer (27). There have been no case reports of
successful treatment of prostate cancer with RET fusion and
NTRK fusion to date.
CGP tests increase the opportunities for GC by
identifying a proportion of patients with potential hereditary
cancers caused by cancer susceptibility genes (28). The introduction of the CGP test for
cancer treatment has changed physicians' awareness of hereditary
cancers; CGP tests can not only find eligible patients for GMT, but
can also reveal a predisposition to hereditary cancers. GC and
confirmatory germline testing play a crucial role in connecting the
early detection and early treatment of hereditary cancers in
relatives (29-32).
For metastatic prostate cancer, 11.8% of cases are reported to be
hereditary cancers associated with single DNA-repair gene mutations
(33); however, the current study
only identified two cases of hereditary cancers through GC. The
implementation status of GC after tumor-only sequencing at our
institution was insufficient to identify germline carriers of
cancer susceptibility genes (1,34).
When patients struggle with cancer treatment, it is sometimes
difficult for them to address secondary findings related to
hereditary cancers, because the GC is not related to their cancer
treatment. It may be difficult for patients to receive GC if they
need to return to the hospital to receive it, given that most
patients undergoing CGP tests are already spending a lot of time on
their cancer treatment. Considering the backgrounds of these
patients, our system in which patients were required to make their
own appointments for GC may have resulted in them missing
opportunities to receive GC. To improve the implementation of GC,
Kikuchi et al (1) reported
that a system in which GC was provided on the same day as the
disclosure of CGP results led to an increase in the number of
patients receiving GC. At our institution, there is a shortage of
certified genetic counselors and clinical geneticists who can
address the secondary findings generated by CGP testing. Increasing
the availability of these professionals would help ensure that GC
can be provided on the same day as the disclosure of CGP results.
In addition, there remains a lack of awareness among patients and
their families regarding hereditary cancers. This highlights the
need for broader educational efforts to improve understanding of
genetic risk.
Our study had several potential limitations,
including its retrospective nature. First, this was a
single-institution study, and the results thus lack adequate power
to make generalizations. Treatment strategies vary among
physicians, and these differences may have influenced our results.
Second, olaparib was not used in combination with an ARSI. The
results of the PROpel and TALAPRO-2 trials were recently approved
by the Pharmaceuticals and Medical Devices Agency in Japan
(3,4). Combined therapy with a PARP inhibitor
and an ARSI showed clinically meaningful improvement as a
first-line therapy in patients with mCRPC with BRCA
deleterious alterations who showed resistance to ADT alone or ADT
plus traditional hormonal therapy. The present study did not
reflect these new prospects for PARP inhibitors for mCRPC. Third, a
multivariate Cox proportional hazards analysis was performed to
adjust for baseline characteristics in the analysis for PFS.
However, we could not fully eliminate all potential confounders
such as age, ECOG performance status, treatment lines, and tumor
burden. These confounders are likely to influence the
interpretation of our results. Future studies with better control
of these variables are warranted to validate our findings. Fourth,
the small number of patients and short follow-up period may have
been insufficient to address the efficacy of genetic medicine and
further studies with longer follow-up and data from multiple
institutions involving more patients are needed. Although this
study included a small number of cases and the experience was
limited, GMT based on the results of CGP tests has had an impact on
our daily clinical practice.
In conclusion, CGP testing offers new hope for
patients with metastatic prostate cancer for whom there is
currently no appropriate subsequent therapy. However, most patients
do not receive GMT in our daily practice. The implementation of GC
for cancer susceptibility genes is thus insufficient and requires
improvement. Further developments in CGP testing are needed to
improve the clinical outcomes, including the development of novel
GMTs and the optimization of timings for CGP testing.
Supplementary Material
Oncological outcomes for GMT in PHI
group (n=19).
Summary of potential actionable gene
alterations.
Acknowledgements
We thank Dr Susan Furness from Edanz (https://jp.edanz.com/ac) for editing a draft of this
manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HT and DK designed the methodology. HT, AW, TY, and
JS analyzed the data. KIT, SS, SH, TS, KT, NA, RK and KM collected
the data. DK and HT wrote the original draft. FT and KM interpreted
the data and supervised. HT and DK confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The Kitasato University Medical Ethics Organization
approved this study (approval no. B21-110). The present study was
in line with the Declaration of Helsinki (1995; as revised in
Edinburgh in 2000).
Patients consent for publication
Informed consent was obtained in the form of an
opt-out option.
Competing interests
Dr Tsumura received personal fees from AstraZeneca
during the study period and personal fees from Bayer, Janssen,
Astellas, and Nippon Shinyaku outside the submitted work. Dr Satoh
received personal fees from AstraZeneca during the study period and
personal fees from Janssen, Bayer, Astellas, and Nippon Shinyaku
outside the submitted work.
References
|
1
|
Kikuchi J, Ohhara Y, Takada K, Tanabe H,
Hatanaka K, Amano T, Hatanaka KC, Hatanaka Y, Mitamura T, Kato M,
et al: Clinical significance of comprehensive genomic profiling
tests covered by public insurance in patients with advanced solid
cancers in Hokkaido, Japan. Jpn J Clin Oncol. 51:753–761.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Bono J, Mateo J, Fizazi K, Saad F,
Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al:
Olaparib for metastatic castration-resistant prostate cancer. N
Engl J Med. 382:2091–2102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saad F, Clarke NW, Oya M, Shore N,
Procopio G, Guedes JD, Arslan C, Mehra N, Parnis F, Brown E, et al:
Olaparib plus abiraterone versus placebo plus abiraterone in
metastatic castration-resistant prostate cancer (PROpel): Final
prespecified overall survival results of a randomised,
double-blind, phase 3 trial. Lancet Oncol. 24:1094–1108.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Agarwal N, Azad AA, Carles J, Fay AP,
Matsubara N, Heinrich D, Szczylik C, Giorgi UD, Joung JY, Fong PCC,
et al: Talazoparib plus enzalutamide in men with first-line
metastatic castration-resistant prostate cancer (TALAPRO-2): A
randomised, placebo-controlled, phase 3 trial. Lancet. 402:291–303.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic Hormone-Sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Clinical practice guidance for
next-generation sequencing in cancer diagnosis and treatment
(edition 2.1). Available from: http://www.jca.gr.jp/researcher/topics/2020/files/20200518.pdf
Accessed June 27, 2023.
|
|
7
|
Koguchi D, Tsumura H, Tabata K, Shimura S,
Satoh T, Ikeda M, Watanabe A, Yoshida T, Sasaki J, Matsumoto K and
Iwamura M: Real-world data on the comprehensive genetic profiling
test for Japanese patients with metastatic Castration-resistant
prostate cancer. Jpn J Clin Oncol. 53:569–576. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Clinical Practice Guidance for Prostate
Cancer. Available from: https://jua.members-web.com/topics1/uploads/attach/topic.
Accessed December 1, 2023.
|
|
9
|
Scher HI, Morris MJ, Stadler WM, Higano C,
Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, et
al: Trial design and objectives for Castration-resistant prostate
cancer: Updated recommendations from the prostate cancer clinical
trials working group 3. J Clin Oncol. 34:1402–1418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Le Tourneau C, Delord JP, Goncalves A,
Gavoille C, Dubot C, Isambert N, Campone M, Trédan O, Massiani MA,
Mauborgne C, et al: Molecularly targeted therapy based on tumour
molecular profiling versus conventional therapy for advanced cancer
(SHIVA): A multicentre, open-label, proof-of-concept, randomised,
controlled phase 2 trial. Lancet Oncol. 16:1324–1334.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Belin L, Kamal M, Mauborgne C, Plancher C,
Mulot F, Delord JP, Gonçalves A, Gavoille C, Dubot C, Isambert N,
et al: Randomized phase II trial comparing molecularly targeted
therapy based on tumor molecular profiling versus conventional
therapy in patients with refractory cancer: Cross-over analysis
from the SHIVA trial. Ann Oncol. 28:590–596. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Krämer A, Bochtler T, Pauli C, Shiu KK,
Cook N, Janoski de Menezes J, Pazo-Cid RA, Losa F, Robbrecht DGJ,
Tomášek J, et al: Molecularly guided therapy versus chemotherapy
after disease control in unfavourable cancer of unknown primary
(CUPISCO): An open-label, randomised, phase 2 study. Lancet.
404:527–539. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sandhu S, Moore CM, Chiong E, Beltran H,
Bristow RG and Williams SG: Prostate cancer. Lancet. 398:1075–1090.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mateo J, Seed G, Bertan C, Rescigno P,
Dolling D, Figueiredo I, Miranda S, Nava Rodrigues D, Gurel B,
Clarke M, et al: Genomics of lethal prostate cancer at diagnosis
and castration resistance. J Clin Invest. 30:1743–1751.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Swisher EM, Kristeleit RS, Oza AM, Tinker
AV, Ray-Coquard I, Oaknin A, Coleman RL, Burris HA, Aghajanian C,
O'Malley DM, et al: Characterization of patients with long-term
responses to rucaparib treatment in recurrent ovarian cancer.
Gynecol Oncol. 163:490–497. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Buonaiuto R, Neola G, Caltavituro A,
Longobardi A, Mangiacotti FP, Cefaliello A, Lamia MR, Pepe F,
Ventriglia J, Malapelle U, et al: Efficacy of PARP inhibitors in
advanced high-grade serous ovarian cancer according to BRCA domain
mutations and mutation type. Front Oncol.
14(1412807)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lenis AT, Ravichandran V, Brown S, Alam
SM, Katims A, Truong H, Reisz PA, Vasselman S, Nweji B, Autio KA,
et al: Microsatellite instability, tumor mutational burden, and
response to immune checkpoint blockade in patients with prostate
cancer. Clin Cancer Res. 30:3894–3903. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abida W, Armenia J, Gopalan A, Brennan R,
Walsh M, Barron D, Danila D, Rathkopf D, Morris M, Slovin S, et al:
Prospective genomic profiling of prostate cancer across disease
states reveals germline and somatic alterations that may affect
clinical decision making. JCO Precis Oncol.
2017(PO.17.00029)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Barata P, Agarwal N, Nussenzveig R,
Gerendash B, Jaeger E, Hatton W, Ledet E, Lewis B, Layton J,
Babiker H, et al: Clinical activity of pembrolizumab in metastatic
prostate cancer with microsatellite instability high (MSI-H)
detected by circulating tumor DNA. J Immunother Cancer.
8(e001065)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Landi L, D'Inca F, Gelibter A, Chiari R,
Grossi F, Delmonte A, Passaro A, Signorelli D, Gelsomino F, Galetta
D, et al: Bone metastases and immunotherapy in patients with
advanced non-small-cell lung cancer. J Immunother Cancer.
7(316)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang K, Li J, Bai C, Sun Z and Zhao L:
Efficacy of immune checkpoint inhibitors in Non-small-cell lung
cancer patients with different metastatic sites: A systematic
review and meta-analysis. Front Oncol. 10(1098)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu YJ, Chang XS, Zhou R, Chen YD, Ma HC,
Xiao ZZ, Qu X, Liu YH, Liu LR, Li Y, et al: Bone metastasis
attenuates efficacy of immune checkpoint inhibitors and displays
‘cold’ immune characteristics in Non-small cell lung cancer. Lung
Cancer. 166:189–196. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hong DS, DuBois SG, Kummar S, Farago AF,
Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin
JD, Federman N, et al: Larotrectinib in patients with TRK
fusion-positive solid tumours: A pooled analysis of three phase 1/2
clinical trials. Lancet Oncol. 21:531–540. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Doebele RC, Drilon A, Paz-Ares L, Siena S,
Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, et al:
Entrectinib in patients with advanced or metastatic NTRK
fusion-positive solid tumours: Integrated analysis of three phase
1-2 trials. Lancet Oncol. 21:271–282. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shimoi T, Sunami K, Tahara M, Nishiwaki S,
Tanaka S, Baba E, Kanai M, Kinoshita I, Shirota H, Hayashi H, et
al: Dabrafenib and trametinib administration in patients with BRAF
V600E/R or non-V600 BRAF mutated advanced solid tumours (BELIEVE,
NCCH1901): A multicentre, open-label, and single-arm phase II
trial. EClinicalMedicine. 69(102447)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Subbiah V, Wolf J, Konda B, Kang H, Spira
A, Weiss J, Takeda M, Ohe Y, Khan S, Ohashi K, et al:
Tumour-agnostic efficacy and safety of selpercatinib in patients
with RET fusion-positive solid tumours other than lung or thyroid
tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial.
Lancet Oncol. 23:1261–1273. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Owsley J, Stein MK, Porter J, In GK, Salem
M, O'Day S, Elliott A, Poorman K, Gibney G and VanderWalde A:
Prevalence of class I-III BRAF mutations among 114,662 cancer
patients in a large genomic database. Exp Biol Med (Maywood).
246:31–39. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ueki A, Yoshida R, Kosaka T and
Matsubayashi H: Clinical risk management of breast, ovarian,
pancreatic, and prostatic cancers for BRCA1/2 variant carriers in
Japan. J Hum Genet. 68:517–526. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Page EC, Bancroft EK, Brook MN, Assel M,
Al Battat MH, Thomas S, Taylor N, Chamberlain A, Pope J, Ni
Raghallaigh H, et al: Interim results from the IMPACT study:
Evidence for prostate-specific antigen screening in BRCA2 mutation
carriers. Eur Urol. 76:831–842. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Marchetti C, De Felice F, Palaia I,
Perniola G, Musella A, Musio D, Muzii L, Tombolini V and Panici PB:
Risk-reducing salpingo-oophorectomy: A meta-analysis on impact on
ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2
mutation carriers. BMC Womens Health. 14(150)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Usui Y, Taniyama Y, Endo M, Koyanagi YN,
Kasugai Y, Oze I, Ito H, Imoto I, Tanaka T, Tajika M, et al:
Helicobacter pylori, Homologous-recombination genes, and gastric
cancer. N Engl J Med. 388:1181–1190. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lubinski J, Kotsopoulos J, Moller P, Pal
T, Eisen A, Peck L, Karlan BY, Aeilts A, Eng C, Bordeleau L, et al:
MRI surveillance and breast cancer mortality in women with BRCA1
and BRCA2 sequence variations. JAMA Oncol. 10:493–499.
2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pritchard CC, Mateo J, Walsh MF, De Sarkar
N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R,
et al: Inherited DNA-repair gene mutations in men with metastatic
prostate cancer. N Engl J Med. 375:443–453. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kawamura M, Shirota H, Niihori T, Komine
K, Takahashi M, Takahashi S, Miyauchi E, Niizuma H, Kikuchi A, Tada
H, et al: Management of patients with presumed germline pathogenic
variant from tumor-only genomic sequencing: A retrospective
analysis at a single facility. J Hum Genet. 68:399–408.
2023.PubMed/NCBI View Article : Google Scholar
|