Introduction
Pancreatic ductal adenocarcinoma (PDAC) has a poor
prognosis, particularly PDAC with peritoneal metastases (1-4).
PDAC with peritoneal metastases makes it difficult to maintain
chemotherapy because of cancerous ascites, bowel obstruction,
nutritional status deterioration, and poor performance status
(5). Recently, multidisciplinary
treatments combining chemotherapy and surgery have been introduced
for PDAC with peritoneal metastases. Additionally, long-term
survival is achieved by conversion surgery (CS) after
intraperitoneal (ip) and intravenous (iv) chemotherapy (1,3,6).
Yamamoto et al (1) reported
that implementation of chemotherapy including ip chemotherapy may
improve survival in patients with PDAC with peritoneal
dissemination because of the high proportion of patients in which
conversion surgery is performed. For example, the authors reported
a conversion rate of 23% in the ip paclitaxel (ipPTX) group
compared with a conversion rate of 4% in the control group
(P=0.005). Herein, we report a case of distal pancreatectomy
following ip and iv chemotherapy for PDAC with peritoneal
metastases that achieved long-term survival after CS, indicating
the efficacy of ip and iv chemotherapy combined with CS for PDAC
with peritoneal metastases.
Case report
A 62-year-old man was referred to our hospital for
ip and iv chemotherapy after being diagnosed with unresectable
pancreatic body adenocarcinoma with peritoneal dissemination via
laparotomy at another hospital. His cancer antigen 19-9 (CA19-9)
level increased to 4,678 U/ml. The tumor mass was obscure on plain
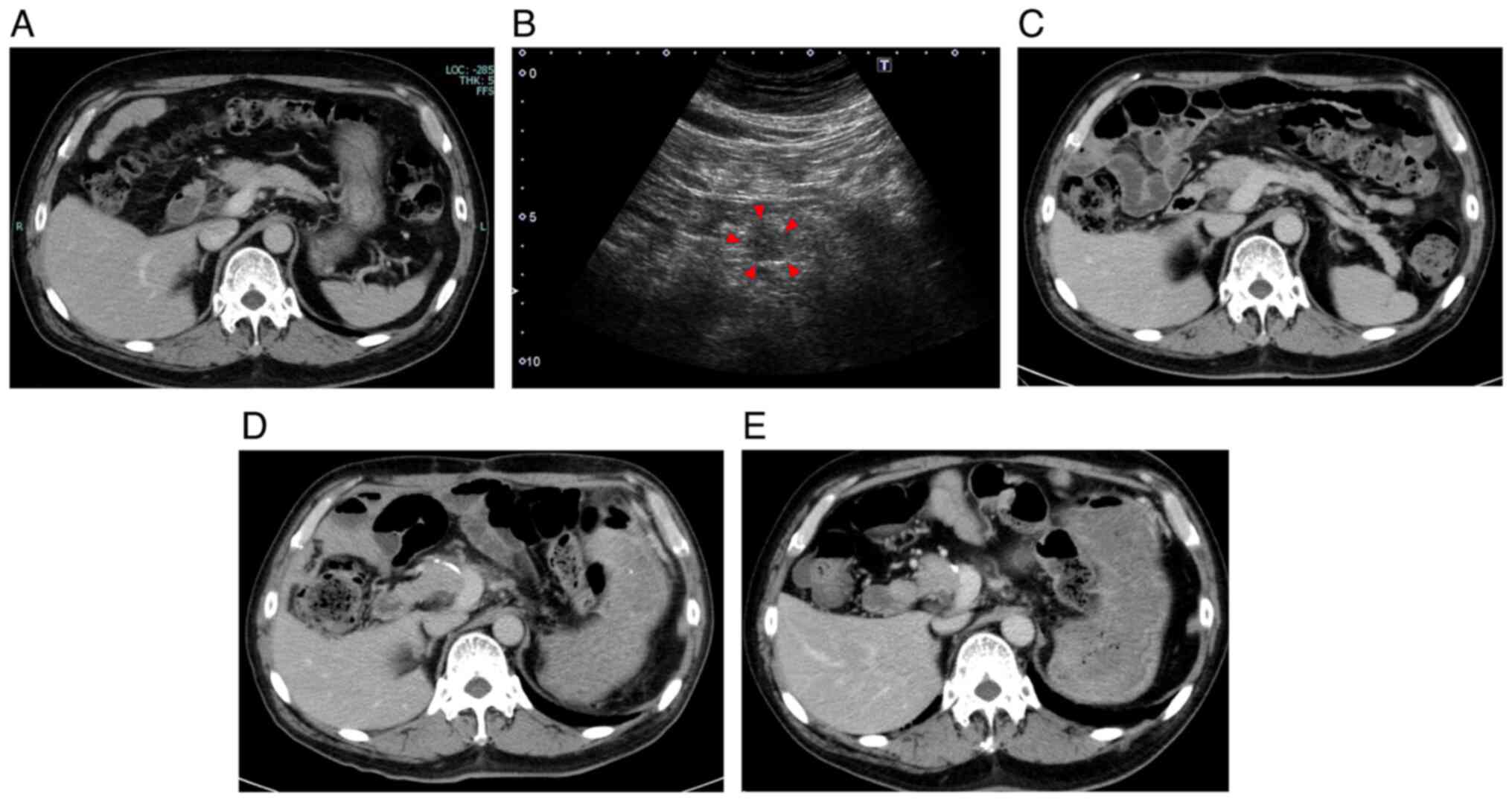
computed tomography (CT) (Fig. 1A)
because the patient could only undergo plain CT due to contrast
media allergy. The tumor could not be identified on positron
emission tomography-CT, or magnetic resonance imaging; however, a
16.3 mm hypoechoic mass was identified in the pancreatic body on
abdominal ultrasonography (Fig.
1B). There was an ambiguous hypoechoic lesion on the pancreatic
body on endoscopic ultrasonography, and the biopsy of the lesion
showed features of adenocarcinoma. The pathological findings of the
peritoneal nodules sampled during laparotomy was also
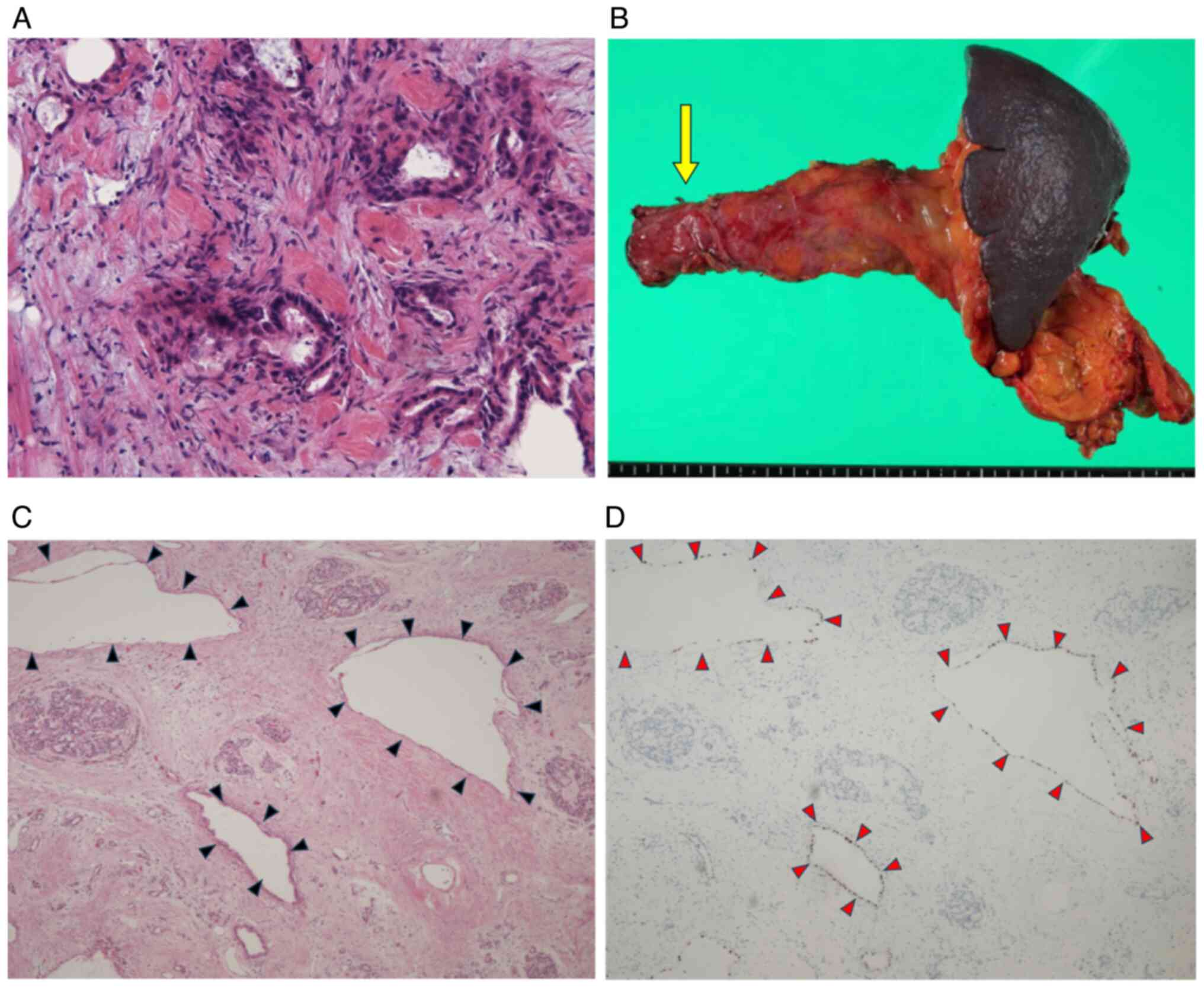
adenocarcinoma (Fig. 2A). In this
case, based on histological features and comparison with previously
confirmed pancreatic cancer, and the absence of any other findings
of cancer causing peritoneal dissemination on positron emission
tomography-CT, a diagnosis of metastatic pancreatic cancer was
established. An abdominal access device was laparoscopically
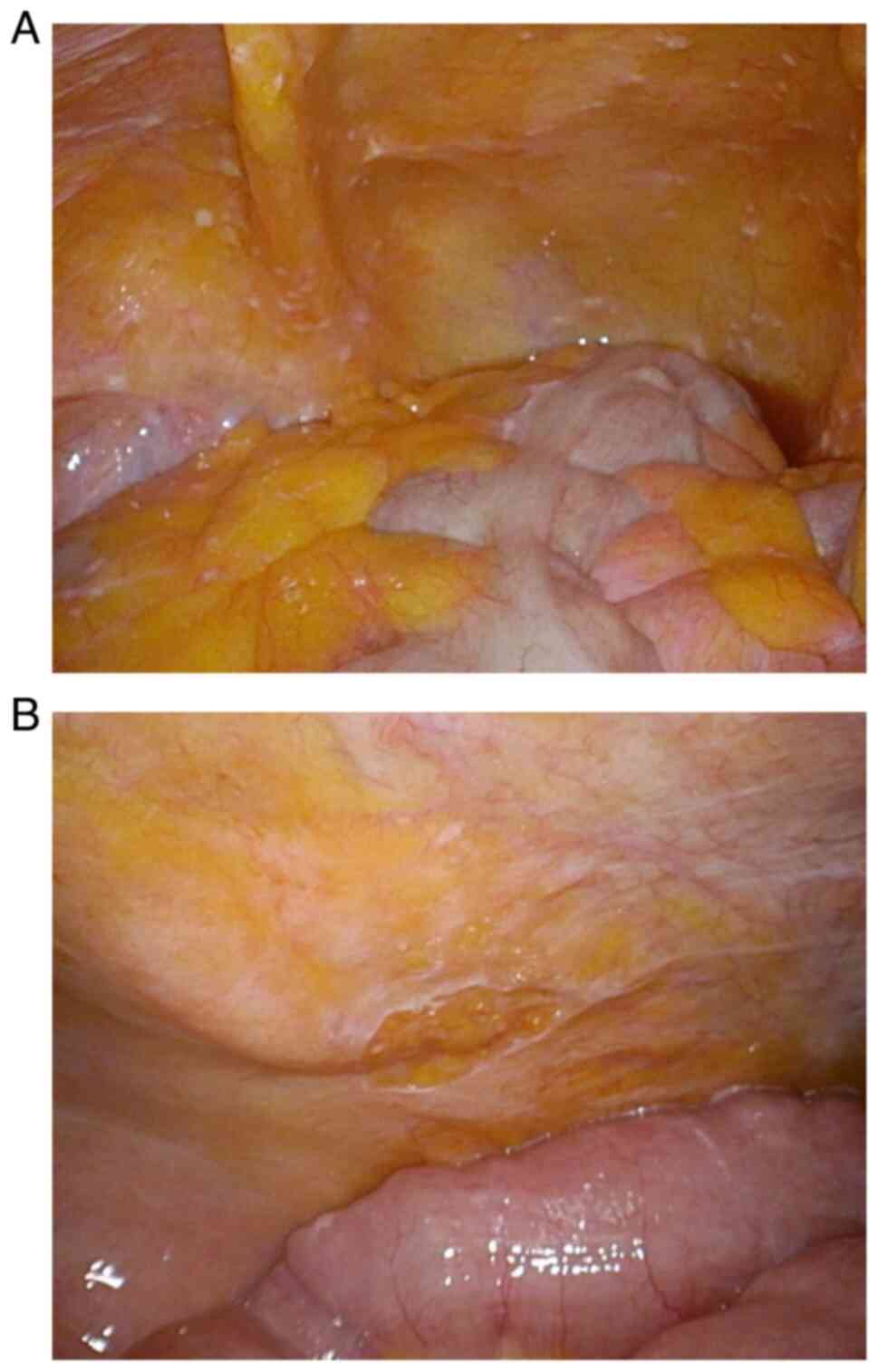
implanted, which revealed many white peritoneal nodules in the
greater omentum and lower abdomen (Fig. 3A). The peritoneal caner index (PCI)
of the patient was 16. The patient was treated with 4 cycles of
ipPTX (20 mg/m2) and S-1 (80 mg/m2/day) plus
ivPTX (50 mg/m2) for 3 months in a single-center
clinical trial. After confirming negative peritoneal washing
cytology via the abdominal access device, a histology of the second
laparoscopy-obtained sample showed peritoneal dissemination. At
that time, the PCI was as low as 1. Given the fact that residual
dissemination was still present, and the patient's general
condition was tolerable, we considered an outcome of CS to have
become clearer and decided to intensify the therapy to 4 cycles of
ipPTX (20 mg/m2) and FOLFIRINOX (5-fluorouracil, 400
mg/m2; leucovorin, 200 mg/m2; irinotecan, 180
mg/m2; and oxaliplatin, 85 mg/m2) for 5
months for greater and improved efficacy. During the ipPTX plus
FOLFIRINOX treatment, the patient experienced grade 3
device-related infection, grade 3 catheter-related infection, but
no hematologic toxicities or abdominal infections were observed. A
third laparoscopy and histological examination showed no peritoneal
dissemination, and peritoneal lavage cytology was negative
(Fig. 3B). The PCI values were as
low as 0. Preoperative CT revealed no new lesions (Fig. 1C), and the CA19-9 level decreased
to 63 U/ml. Distal pancreatectomy revealed no liver metastases or
peritoneal dissemination (Fig.
2B). The range of resection was determined by preoperative
imaging diagnosis on CT scan, indicating no obvious vascular
invasions, infiltrations or distant metastasis, and intraoperative
negative lavage cytology and no signs of vascular invasions or
metastasis including peritoneal metastasis. The tumor mass detected
on preoperative abdominal ultrasonography was located at the left
side of the portal vein of the pancreatic body. Given the above
findings, a standard distal pancreatectomy was performed. Operation
time was 316 min, and blood loss was 325 ml. The patient was
uneventfully discharged on postoperative day 13, and postoperative
CT showed no abnormalities (Fig.
1D). Histological diagnosis was ypT2, ypN0, ypM0, R0, and yp
Stage IB (according to the eighth edition of the Union for
International Cancer Control TNM classification). The effect of
preoperative chemotherapy was grade IIb according to the Evan's
classification (7) (Fig. 2C and D). IpPTX and S-1 were administered for 6
months as postoperative adjuvant chemotherapy for six months,
followed by ipPTX alone for six months. During postoperative
chemotherapy, he experienced grade 2 enterocolitis, and grade 2
laryngitis, but no other specific adverse events. For the specific
follow-up measures after the surgery, we performed physical exams,
contrast-enhanced CT, and monitored CA19-9 using blood tests.
Initially, the patient underwent non-contrast CT due to an allergy
to contrast media, but following premedication with 300 mg
hydrocortisone, contrast-enhanced CT could be safely performed. The
last follow-up at 84 months after CS confirmed no evidence of
recurrence, including CT showing no new lesions (Fig. 1E) and no increase in CA19-9 levels.
The patient survived without recurrence for 7 years after CS.
Discussion
The prognosis of patients with PDAC with peritoneal
metastases is extremely poor. A population-based analysis conducted
between 2005 and 2015 reported that peritoneal metastases was
observed in 7.7% of patients with PDAC, and median survival time
(MST) of these cases was 3.4 months for the pancreatic head, 2.3
months for the body, and 2.2 months for the tail (8). Peritoneal metastases are associated
with the development of intestinal obstruction, massive ascites,
and malnutrition, leading to poor performance status, which may in
turn partially or completely prevent the patient from undergoing
chemotherapy (3,8,9).
Patients with PDAC with peritoneal metastases are
generally treated with the same systemic chemotherapeutic regimens
as those with other distant metastases (5). The standard regimens for unresectable
PDAC are fluorouracil plus leucovorin, irinotecan, and oxaliplatin
(FOLFIRINOX) (10) or gemcitabine
plus nab-paclitaxel (GnP) (11).
The MST was 11 months for FOLFIRINOX and 8.5 months for GnP.
However, the prognosis of PDAC with peritoneal metastases remains
poor (MST=6 weeks) (4). One of the
reported reasons for poor prognosis is that systemically
administered anticancer drugs do not necessarily reach optimal
concentrations in the peritoneal cavity (3,12-14).
Recently, chemotherapy combined with ip chemotherapy
has been introduced in patients with PDAC and peritoneal metastases
(1,3,14,15).
The efficacy of ip chemotherapy for other cancers, particularly
gastric and ovarian cancers, has been reported in phase II trials.
A single-center phase II study by Ishigami et al (12) on ipPTX and S-1 plus PTX for gastric
cancer with peritoneal metastases reported an MST of 22.5 months
and a 1-year survival rate of 78%. In a phase II study of ipPTX for
ovarian cancer with peritoneal metastases by Markman et al
(13), 61% of the patients
achieved complete response. A combination of chemotherapy with
ipPTX is considered more effective than systemic chemotherapy alone
because it exposes peritoneal lesions to high concentrations of
anticancer drugs without increasing their blood concentration
(1,14,16).
Ishigami et al (16)
reported that ip and serum PTX concentrations remained effective
for over 72 and 48 h, respectively, indicating that the ipPTX
concentration remained extremely high for a long period, and area
under the blood concentration-time curve was much higher than that
obtained by ivPTX. However, comprehensive pharmacokinetic studies
specific to ipPTX in PDAC are limited, so it is considered that
further studies are needed to confirm the above findings in a
larger cohort. Additionally, it is reported that ipPTX is
relatively safe with less adverse events when compared with
systemic chemotherapy, even for patients with massive amounts of
ascites with poor performance status (17).
Furthermore, the efficacy of ip and iv chemotherapy
and subsequent CS has been reported in PDAC with peritoneal
metastases (1,3,6). A
multicenter phase II trial of ipPTX and S-1 plus PTX for PDAC with
peritoneal metastases reported (3)
an MST of 16.3 months, 1 year survival rate of 62%, and conversion
rate of 24%. A group that underwent CS also had a significantly
higher overall survival than a group that did not (27.8 vs. 14.2
months, P=0.038). A multi-center phase II study of ipPTX and
systemic GnP therapy for PDAC with peritoneal metastases by Yamada
et al (6) reported an MST
of 14.5 months, 1 year survival rate of 61%, and conversion rate of
17%. Overall survival was significantly longer in a group that
received CS than in a group that did not (MST: not reached vs. 12.4
m, P=0.004). In a similar retrospective study, Yamamoto et
al (1) reported an MST of 27.4
months in patients who underwent CS, and 11.3 months in those who
did not (P<0.001). Considering the fact that the conversion rate
for patients with unresectable locally advanced PDAC was reported
to be 29% and MST for patients who underwent CS was 31 months
(18), ip and iv chemotherapy
promises to be an effective therapy in terms of conversion rate and
prognosis of patients who achieve CS. The criteria for CS in these
trials were as follows: no decline in performance status, size
reduction of the primary tumor, decline in tumor markers, negative
cytology, disappearance of peritoneal metastases, and a treatment
duration of at least 8 months. A treatment approach based on all
the above criteria seems to lead to a better prognosis for patients
who achieved CS.
However, at present, the number of reports on the
efficacy of CS after ip and iv chemotherapy for PDAC with
peritoneal metastases is limited. There have been failed attempts
at ipPTX therapy; for example, a study by Ishigami et al
(16) failed to show the
statistical superiority of ipPTX plus systemic chemotherapy, owing
to a crucial imbalance in the high amount of ascites in an
experimental group and a crossover use of ip therapy in a control
group (19). The 2024 National
Comprehensive Cancer Network guidelines do not recommend ip and iv
chemotherapy for the treatment of PDAC with peritoneal metastases
due to limited evidence, whereas our case offers rare insight into
a successful outcome. In our case, although the therapy was not
recommended, the decision of performing the therapy was made based
on multidisciplinary discussion and patient-specific factors,
including isolated peritoneal metastasis and observing good
response to initial therapy. A phase III trial of ip and iv
chemotherapy for PDAC with peritoneal metastases is currently in
progress (14).
In our case, the patient completed a preoperative
treatment with ip and iv chemotherapy for >8 months (1,20),
which is one of the criteria for CS. Additionally, imaging studies
did not show other distant metastases, and the CA19-9 level
decreased to <100 U/Ml (1,21),
Peritoneal washing cytology via the abdominal access device was
negative, and the disappearance of peritoneal dissemination was
confirmed by staging laparoscopy. The criteria for CS were also
met. Successful CS was the main contributing factor to the 7-year
survival without recurrence. The second reason is the favorable
effect of CT with ipPTX. The peritoneal dissemination virtually
disappeared, there were no lymph node metastases, and negative
surgical margins were achieved. The effect of preoperative
chemotherapy was grade IIb, according to the Evans classification.
Third, the patient received long-term postoperative adjuvant
chemotherapy with ip PTX plus S-1 for 6 months, followed by ipPTX
for 6 months. The 7-year recurrence-free survival with CS after ip
and iv chemotherapy in this case may be extraordinary; however, ip
and iv chemotherapy is a promising treatment for PDAC with
peritoneal metastases, and if followed by CS, long-term survival
may be expected. In contrast, although ip and iv chemotherapy is
generally well tolerated, Satoi et al (3) reported grade 3/4 hematologic
toxicity, adverse events other than hematologic toxicity, superior
mesenteric artery thrombosis, anaphylactic reactions and abdominal
access device related infections. In this case, the patient
experienced grade 3 device-related infection and grade 3
catheter-related infection during preoperative ipPTX+FOLFIRINOX,
and grade 2 enterocolitis and grade 2 laryngitis during
postoperative ipPTX+S-1; however, there were no hematologic
toxicities, thrombosis, or anaphylactic reactions (Table I). Given the above findings,
although adverse events were rather limited and the therapy was
generally well tolerated for this case, they could not be entirely
avoided. Patients undergoing this therapy should be closely
monitored for adverse events that may, at times, become severe or
life-threatening. Another limitation of this case was that the
diagnosis of adenocarcinoma of the peritoneal dissemination
originating from pancreatic body adenocarcinoma was not
immunohistologically confirmed before preoperative chemotherapy.
Based on histological features and comparison with previously
confirmed pancreatic cancer, and the absence of any other findings
of cancer causing peritoneal dissemination, a clinical diagnosis of
peritoneal metastasis of pancreatic cancer was established, but
immunohistochemistry was not performed for the peritoneal nodules
after the primary laparotomy, and it should have been conducted to
achieve a more precise diagnosis by adding immunohistological
analysis.
 | Table IAdverse events and outcomes during
chemotherapy. |
Table I
Adverse events and outcomes during
chemotherapy.
| Adverse event | Grade | Timing | Management | Outcome |
|---|
| Device-related
infection | 3 | During cycle 3 of
ipPTX + FOLFIRINOX | Antibiotics, surgical
removal | Resolved |
| Catheter-related
infection | 3 | During cycle 3 of
ipPTX + FOLFIRINOX | Antibiotics, catheter
removal | Resolved |
| Enterocolitis | 2 | During post-operative
ipPTX + S-1 | Hydration | Improved |
| Laryngitis | 2 | During post-operative
ipPTX + S-1 | Anti-inflammatory
treatment | Resolved |
In conclusion, combination therapy with
intraperitoneal PTX plus chemotherapy is a promising treatment for
PDAC with peritoneal metastases. If followed by CS, long-term
survival may be expected. Because our findings represent a single
case, further clinical trials are required to validate the efficacy
of this approach.
Acknowledgements
The authors would like to thank Mr. David Hochman
for reviewing the language of this article.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MT is the main author of this paper. JM, YT and SH
proofread the manuscript. MT, JM and YT confirm the authenticity of
all the raw data. MT, JM, YT and SH made substantial contributions
to conception and design, and acquisition of data, and analysis and
interpretation of data, and were involved in drafting the
manuscript and revising it critically for important intellectual
content. MT, JM, YT and SH participated sufficiently in the work to
take public responsibility for appropriate portions of the content
and agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy and integrity of
any part of the work are appropriately investigated and resolved.
YO contributed by providing pathological images, offering
pathological diagnoses, and giving expert pathological advice. YS
served as the attending physician and operating surgeon and
provided comprehensive details of the clinical course and made
critical revisions to the manuscript draft. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamamoto T, Satoi S, Yamaki S, Hashimoto
D, Ishida M, Ikeura T, Hirooka S, Matsui Y, Boku S, Nakayama S, et
al: Intraperitoneal paclitaxel treatment for patients with
pancreatic ductal adenocarcinoma with peritoneal dissemination
provides a survival benefit. Cancers (Basel).
14(1354)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
DeWitt J, Yu M, Al-Haddad MA, Sherman S,
McHenry L and Leblanc JK: Survival in patients with pancreatic
cancer after the diagnosis of malignant ascites or liver metastases
by EUS-FNA. Gastrointest Endosc. 71:260–265. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Satoi S, Fujii T, Yanagimoto H, Motoi F,
Kurata M, Takahara N, Yamada S, Yamamoto T, Mizuma M, Honda G, et
al: Multicenter phase II study of intravenous and intraperitoneal
paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients
with peritoneal metastasis. Ann Surg. 265:397–401. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thomassen I, Lemmens VE, Nienhuijs SW,
Luyer MD, Klaver YL and de Hingh IH: Incidence, prognosis, and
possible treatment strategies of peritoneal carcinomatosis of
pancreatic origin: a population-based study. Pancreas. 42:72–75.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Satoi S, Yanagimoto H, Yamamoto T,
Toyokawa H, Hirooka S, Yamaki S, Opendro SS, Inoue K, Michiura T,
Ryota H, et al: A clinical role of staging laparoscopy in patients
with radiographically defined locally advanced pancreatic ductal
adenocarcinoma. World J Surg Oncol. 14(14)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yamada S, Fujii T, Yamamoto T, Takami H,
Yoshioka I, Yamaki S, Sonohara F, Shibuya K, Motoi F, Hirano S, et
al: Phase I/II study of adding intraperitoneal paclitaxel in
patients with pancreatic cancer and peritoneal metastasis. Br J
Surg. 107:1811–1817. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Evans DB, Rich TA, Byrd DR, Cleary KR,
Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ and Ames FC:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339.
1992.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mackay TM, van Erning FN, van der Geest
LGM, de Groot JWB, Haj Mohammad N, Lemmens VE, van Laarhoven HW,
Besselink MG and Wilmink JW: Dutch Pancreatic Cancer Group.
Association between primary origin (head, body and tail) of
metastasised pancreatic ductal adenocarcinoma and oncologic
outcome: A population-based analysis. Eur J Cancer. 106:99–105.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takahara N, Isayama H, Nakai Y, Sasaki T,
Saito K, Hamada T, Mizuno S, Miyabayashi K, Mohri D, Kogure H, et
al: Pancreatic cancer with malignant ascites: Clinical features and
outcomes. Pancreas. 44:380–385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ishigami H, Kitayama J, Kaisaki S,
Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H
and Nagawa H: Phase II study of weekly intravenous and
intraperitoneal paclitaxel combined with S-1 for advanced gastric
cancer with peritoneal metastasis. Ann Oncol. 21:67–70.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Markman M, Brady MF, Spirtos NM, Hanjani P
and Rubin SC: Phase II trial of intraperitoneal paclitaxel in
carcinoma of the ovary, tube, and peritoneum: A Gynecologic
Oncology Group Study. J Clin Oncol. 16:2620–2624. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamamoto T, Fujii T, Hirano S, Motoi F,
Honda G, Uemura K, Kitayama J, Unno M, Kodera Y, Yamaue H, et al:
Randomized phase III trial of intravenous and intraperitoneal
paclitaxel with S-1 versus gemcitabine plus nab-paclitaxel for
pancreatic ductal adenocarcinoma with peritoneal metastasis (SP
study). Trials. 23(119)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yamada S, Fujii T, Yamamoto T, Takami H,
Yoshioka I, Yamaki S, Sonohara F, Shibuya K, Motoi F, Hirano S, et
al: Conversion surgery in patients with pancreatic cancer and
peritoneal metastasis. J Gastrointest Oncol. 12 (Suppl
1):S110–S117. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ishigami H, Kitayama J, Otani K, Kamei T,
Soma D, Miyato H, Yamashita H, Hidemura A, Kaisaki S and Nagawa H:
Phase I pharmacokinetic study of weekly intravenous and
intraperitoneal paclitaxel combined with S-1 for advanced gastric
cancer. Oncology. 76:311–314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Meguro Y, Yamaguchi H, Sasanuma H,
Shimodaira K, Aoki Y, Chinen T, Morishima K, Miyato H, Miki A, Endo
K, et al: Combined intraperitoneal paclitaxel and systemic
chemotherapy for patients with massive malignant ascites secondary
to pancreatic cancer: A report of two patients. Intern Med.
63:2015–2021. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nitsche U, Wenzel P, Siveke JT, Braren R,
Holzapfel K, Schlitter AM, Stöß C, Kong B, Esposito I, Erkan M, et
al: Resectability after first-line FOLFIRINOX in initially
unresectable locally advanced pancreatic cancer: A single-center
experience. Ann Surg Oncol. 22 (Suppl 3):S1212–S1220.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ishigami H, Fujiwara Y, Fukushima R,
Nashimoto A, Yabusaki H, Imano M, Imamoto H, Kodera Y, Uenosono Y,
Amagai K, et al: Phase Ill trial comparing intraperitoneal and
intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in
patients with gastric cancer with peritoneal metastasis: Phoenix-GC
Trial. J Clin Oncol. 36:1922–1929. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese Society of
Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Satoi S, Yamamoto T, Yamaki S, Sakaguchi T
and Sekimoto M: Surgical indication for and desirable outcomes of
conversion surgery in patients with initially unresectable
pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 4:6–13.
2020.PubMed/NCBI View Article : Google Scholar
|