Introduction
Breast cancer is the most prevalent cancer among
female patients and also the second leading cause of
cancer-associated deaths (1,2).
Invasive ductal carcinoma (IDC) and is the most common type of
invasive breast cancer, accounting for 70-80% of all cases
(3). Ductal carcinoma in
situ (DCIS) is a precursor to invasive breast cancer and is
often found in conjunction with IDC (4,5).
Studies have shown that IDCs with in situ components have
lower histological grade and tumor size, lower Ki-67 value and less
frequent local recurrence, while having a higher incidence of
estrogen receptor (ER) positivity (6-8).
Genomic studies have shown that IDC and DCIS accompanying IDC have
a similar gene expression profile (9,10).
Few studies demonstrated that IDCs with in situ components
have better prognostic features compared with IDC (11). However, whether co-existing DCIS in
IDC leads to better survival outcomes compared with IDC remains
controversial. Although the positive effects of the DCIS component
on prognosis have been demonstrated, this has not yet been
indicated in official guidelines to plan treatment and follow-up
decisions for breast cancer.
Tumor-infiltrating lymphocytes (TILs) are
mononuclear inflammatory cells with both proinflammatory and
immunosuppressive effects found within and outside the tumor.
Studies on solid tumors suggest an association between immune
system cells and clinical response (12,13).
Presence of TILs in breast cancer is significantly associated with
prolonged survival in human epidermal growth factor receptor 2
(HER2)-positive and triple-negative breast cancer (TNBC) (14,15).
Despite these data, the use of TILs as a biomarker in clinical
practice is limited and requires prospective studies.
Studies investigating the clinical,
histopathological, and prognostic significance of the DCIS
component accompanying IDC, conducted with a large number of
patients, have been based on the previous staging system American
Joint Committee on Cancer (AJCC) 7th edition (6,7).
There is a notable difference in staging and
prognosis between AJCC 7th and 8th editions. The AJCC 8th edition
includes two complementary staging systems for breast cancer:
Anatomical staging, based on the TNM classification, and prognostic
staging, which integrates tumor biology; including grade, hormone
receptor status, and HER2 expression (16). A study by Shao et al
(17) of 184,221 primary breast
cancer cases from the Surveillance, Epidemiology, and End Results
database, demonstrated that the prognostic accuracy of the 8th AJCC
staging system, which incorporates tumor grade, Estrogen receptor
(ER), Progesteron receptor (PR) and HER2 status as biological
staging factors, is superior to the 7th staging system (17).
Compared with AJCC 7, a substantial proportion of
patients (53.2%) are reassigned to a different stage under AJCC
8(17). In AJCC 7, survival rates
among patients within the same stage are notably heterogeneous.
Therefore, the prognostic significance of coexisting DCIS in IDC
cases may not have been adequately assessed. The present study
aimed to investigate the effect of the DCIS component with current
staging system and data on the tumor microenvironment when present
with IDC, the presence of TILs and their impact on the clinical and
pathological features of the tumor, disease prognosis and patient
survival.
Patients and methods
Patients
The present retrospective study was conducted on 569
patients aged between 18 and 85 years diagnosed with IDC who
presented to the outpatient clinic at Hacettepe University Oncology
Hospital (Ankara, Turkey) between January 2014 and July 2021. Data
were collected from electronic and manual medical records
retrospectively between March and September 2022. Patients with
poor treatment compliance were excluded from the study. All
patients had pre-treatment core biopsy, tru-cut biopsy or material
obtained by surgical excision of tumorous breast tissue
histopathologically examined. All specimens were fixed in 10%
neutral-buffered formalin, embedded in paraffin and stained with
hematoxylin and eosin for routine evaluation. Histological grading
was performed using the Nottingham modification of the
Bloom-Richardson system. Immunohistochemistry was conducted for ER,
PR, HER2, and Ki-67. ER and PR positivity was defined as ≥1%
nuclear staining. HER2 evaluation followed ASCO/CAP guidelines,
with 2+ cases confirmed by FISH. For TIL assessment, one
representative formalin-fixed paraffin-embedded block containing
adequate tumor stroma was selected for each case. Stromal TILs were
re-evaluated independently by two pathologists and reported as
percentages according to international guidelines. Based on TIL
density, patients were categorized into three groups: Low (0-10%),
intermediate (11-59%), and high (≥60%). In addition to the total
cohort, a matched cohort (n=328) was created by pairing patients in
a 1:1 ratio from the total group of included patients. Matching was
based on age, stage according to AJCC 8th edition TNM staging,
grade of invasive component and hormone receptor/HER2 positivity
status.
The study analyzed female patients with breast
cancer diagnosed with AJCC 8th edition stage I-III IDC.
Age at diagnosis, menopausal status, date of first report, date of
diagnosis, comorbidities such as hypertension, diabetes mellitus,
Chronic obstructive pulmonary disease and coronary artery disease,
treatment side effects, pathological diagnoses, molecular subtypes,
Ki-67 value, proportion of stromal TILs, tumor size, number of
metastatic lymph nodes, distant metastasis, type of surgery
performed, date, site and pathology of recurrence, date of death,
last follow-up and oncological treatment information were recorded.
The study was approved by Hacettepe University Non-Interventional
Clinical Research Ethics Committee (01.03.2022, approval no.
2022/04-15, registration no. KA-22219).
Statistical analysis
SPSS version 25.0 (IBM Corp.) was used for
statistical analysis. Descriptive statistics are presented as
numbers, percentages, mean ± standard deviation, median, minimum,
and maximum. The Shapiro-Wilk test was used to assess the normal
distribution of continuous variables. The χ2 test was
used to compare categorical variables between groups. Parametric
and non-parametric tests were used based on assumptions of
normality. Survival analysis was performed using the Kaplan-Meier
test to determine overall and disease-free survival. The present
study used univariate and multivariate Cox proportional hazards
models to examine the relationship between clinicopathological
factors and both overall and disease-free survival. Logistic
regression analysis was applied to identify independent predictors
of pathological complete response. The results were reported with
95% confidence intervals to show the strength and precision of the
associations. . P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of study groups
A total of 569 patients were included, of whom 165
had IDC and 404 had IDC + DCIS. For the matched cohort a total of
328 (164 IDC, 164 IDC + DCIS) patients were recruited. IDC + DCIS
group showed a significantly higher rate of ER and PR positivity
(both P<0.05; Table I).
Patients in the IDC + DCIS group were treated with hormone therapy
significantly more often compared with the IDC group (P<0.05).
The median Ki-67 for the entire cohort was 25% (range, 1-95%). In
the IDC group, the median Ki-67 was 40% (range, 5-95%) and in the
IDC + DCIS group, it was 25% (range, 1-90%). Compared with the IDC
+ DCIS group, the IDC group had a significantly higher Ki-67
percentage (P<0.05). All patients underwent surgery; 199
patients (35%) had breast conserving surgery (BCS), while 370
patients (65%) underwent a mastectomy. Patients in the IDC + DCIS
group were significantly likely to undergo mastectomy than those in
the IDC group (P<0.05). In the entire cohort, 415 patients
(72.9%) received radiotherapy. Radiotherapy administration was
significantly more frequent in the IDC + DCIS group, compared with
IDC alone group (P<0.05).
 | Table IClinical and pathological features in
patients with IDC and IDC + DCIS. |
Table I
Clinical and pathological features in
patients with IDC and IDC + DCIS.
| Variable | IDC (n=165) | IDC + DCIS
(n=404) | Matched (n=328) | Total (n=569) | P-value |
|---|
| Histological IDC
grade (%) | | | | | |
|
1 | 4 (2.4) | 18 (4.5) | 8 (2.4) | 22 (3.9) | >0.05 |
|
2 | 43 (26.1) | 124 (30.7) | 86 (26.2) | 167 (29.3) | |
|
3 | 118 (71.5) | 262 (64.9) | 234 (71.3) | 380 (66.8) | |
| ER status (%) | | | | | |
|
Positive | 91 (55.2) | 313 (77.5) | 184 (56.1) | 404(71) | <0.05 |
|
Negative | 74 (44.8) | 91 (22.5) | 144 (48.8) | 165(29) | |
| PR status (%) | | | | | |
|
Positive | 81 (49.1) | 283(70) | 168 (51.2) | 364(64) | <0.05 |
|
Negative | 84 (50.9) | 121(30) | 160 (48.8) | 205(36) | |
| HER2 status
(%) | | | | | |
|
Positive | 55 (33.3) | 123 (30.4) | 110 (33.5) | 178 (31.3) | >0.05 |
|
Negative | 110 (66.7) | 281 (69.6) | 218 (66.5) | 391 (68.7) | |
|
Median
Ki-67. % (range) | 25 (1-95) | 40 (5-95) | 30 (1-95) | 25 (1-90) | <0.05 |
| Stromal TILs
(%) | | | | | |
|
Low
(0-10%) | 88 (53.3) | 148 (36.6) | | 236 (58.1) | >0.05 |
|
Intermediate
(11-59%) | 63 (38.2) | 83 (33.7) | | 146(36) | |
|
High
(≥60%) | 9 (5.5) | 15 (3.7) | | 24 (5.9) | |
|
Unknown | 5(3) | 158 (39.1) | | 163 (28.6) | |
| Surgery (%) | | | | | |
|
Breast-conserving | 68 (41.2) | 131 (32.4) | | 199(35) | <0.05 |
|
Mastectomy | 97 (58.8) | 273 (67.6) | | 370(65) | |
| Hormone therapy
(%) | | | | | |
|
Yes | 94(57) | 313 (77.5) | | 407 (71.5) | <0.05 |
|
No | 71(43) | 91 (22.5) | | 162 (28.5) | |
| Chemotherapy
(%) | | | | | |
|
No | 26 (15.8) | 85(21) | | 111 (19.5) | <0.05 |
|
Neoadjuvant | 41 (24.8) | 58 (14.4) | | 99 (17.4) | |
|
Adjuvant | 95 (57.6) | 253 (62.6) | | 348 (61.2) | |
|
Unknown | 3 (1.8) | 8(2) | | 11 (1.9) | |
| Radiotherapy
(%) | | | | | |
|
Yes | 130 (78.8) | 285 (70.5) | | 415 (72.9) | <0.05 |
|
No | 35 (21.2) | 119 (29.5) | | 154 (27.1) | |
| Diabetes (%) | | | | | |
|
Yes | 15 (9.1) | 34 (8.4) | 36 (11.0) | 49 (8.6) | >0.05 |
|
No | 150 (90.9) | 370 (91.6) | 292 (89.0) | 520 (91.4) | |
| HT (%) | | | | | |
|
Yes | 19 (11.5) | 68 (16.8) | 53 (16.2) | 87 (15.3) | >0.05 |
|
No | 146 (88.5) | 336 (83.2) | 275 (83.8) | 482 (84.7) | |
| COPD (%) | | | | | |
|
Yes | 5 (3.0) | 6 (1.5) | 9 (2.7) | 11 (1.9) | >0.05 |
|
No | 160 (97.0) | 398 (98.5) | 307 (97.3) | 558 (98.1) | |
| CAD (%) | | | | | |
|
Yes | 5 (3.0) | 5 (1.2) | 8 (2.4) | 10 (1.8) | >0.05 |
|
No | 160 (97.0) | 399 (98.8) | 320 (97.6) | 559 (98.2) | |
| Side effects
(%) | | | | | |
|
Yes | 15 (9.1) | 21 (5.2) | 30 (9.1) | 36 (6.3) | P>0.05 |
|
No | 150 (90.9) | 383 (94.8) | 298 (90.9) | 533 (93.7) | |
There were no statistically significant differences
regarding mean age at diagnosis, menopausal status, T and N stage,
TNM classification, HER2 status, histological grade of the invasive
component, comorbidities and presence of treatment side effects
(P>0.05).
Survival analysis
The median follow-up for patients was 54 months
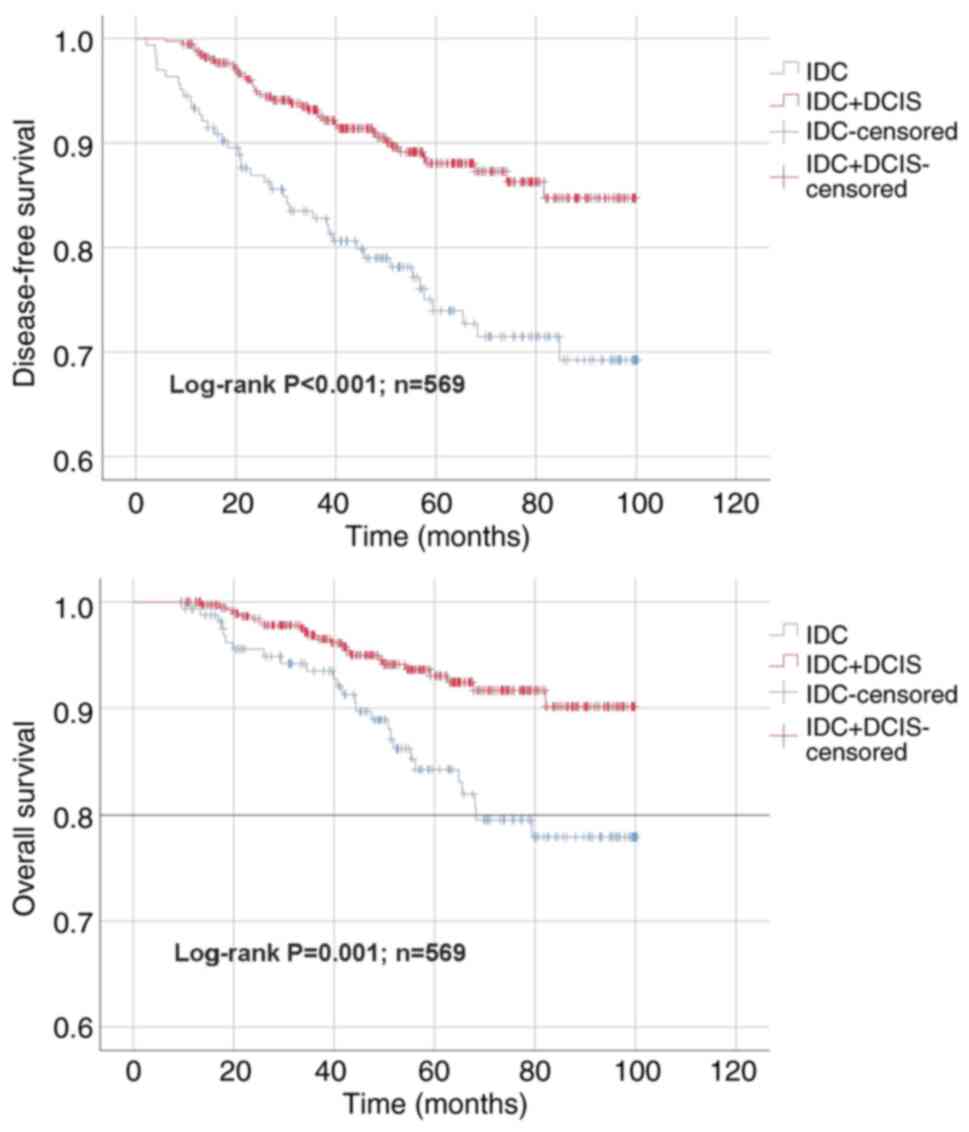
(range, 9-100). The 5-year overall survival (OS) rate was 91.4 for
the entire cohort, 84.3 for the IDC group and 93.1% for patients in
the IDC + DCIS group. The 5-year disease-free survival (DFS) rate
for the entire cohort was 83.7, compared with 73.9 for patients in
the IDC group and 88.1% for patients in the IDC + DCIS group. Mean
OS and DFS time were 88.3±2.1 and 80.1±2.7 in the IDC and 94.7±1
and 90.9±1.3 months in the IDC + DCIS group. OS and DFS were
significantly longer in the IDC + DCIS group compared with the IDC
group (both P<0.05; Fig.
1).
In the matched cohort, 5-year DFS rates for the IDC
and IDC + DCIS groups were 75.8 and 90.1%, respectively (80.6±2.7
vs. 90.4±2.2 months; Fig. S1). OS
rates for the IDC and IDC + DCIS groups were 84.8 and 90.7%,
respectively (88.8±2.1 vs. 95.7±1.5 months). Both OS and DFS were
significantly longer in the IDC + DCIS vs. the IDC group (both
P<0.05).
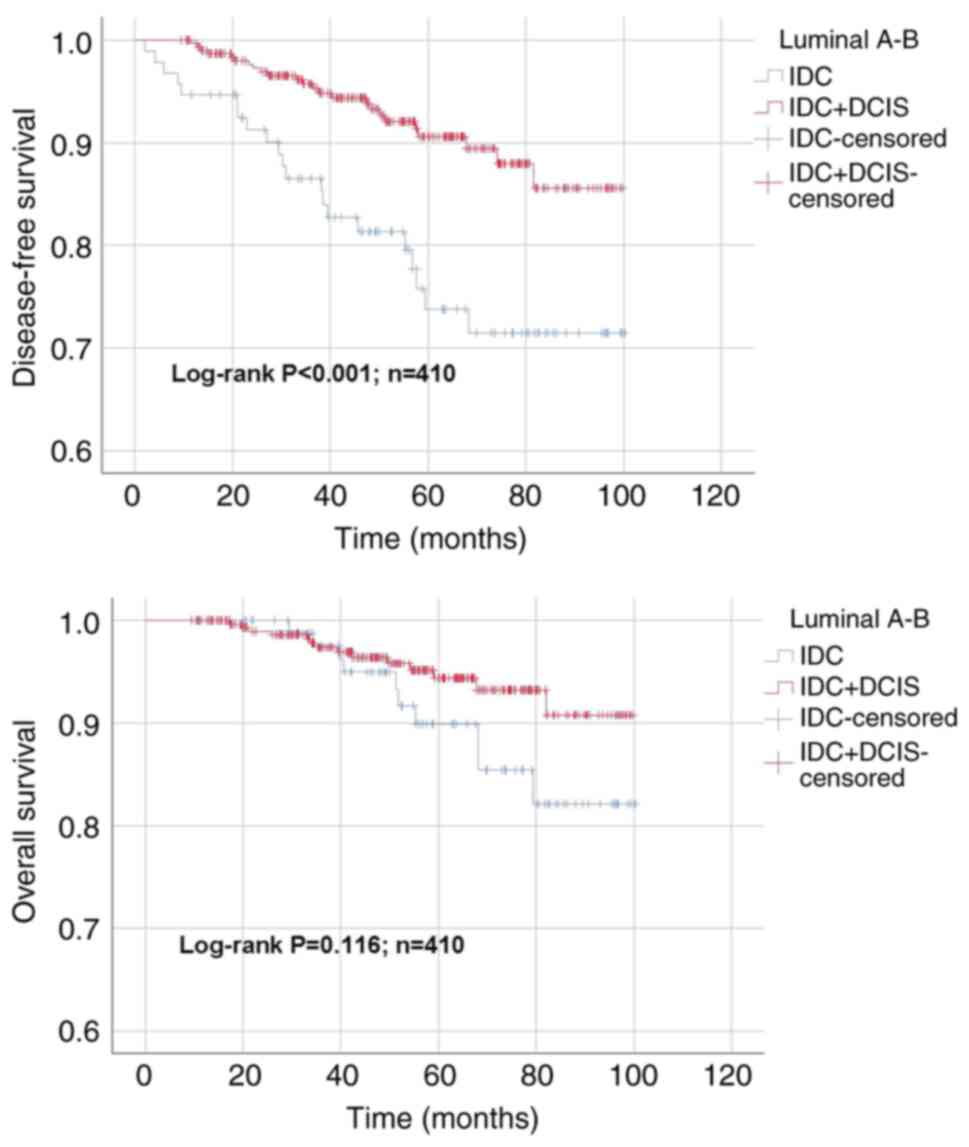
According to the survival analyses conducted in the
entire cohort for moleculer subtypes, DFS time of the IDC + DCIS
group (92.6±1.3 months) was significantly longer than that of the
IDC group (82.1±3.4 months; P<0.05; Fig. 2) in patients with luminal cancer.
However, no significant difference was found regarding OS (IDC,
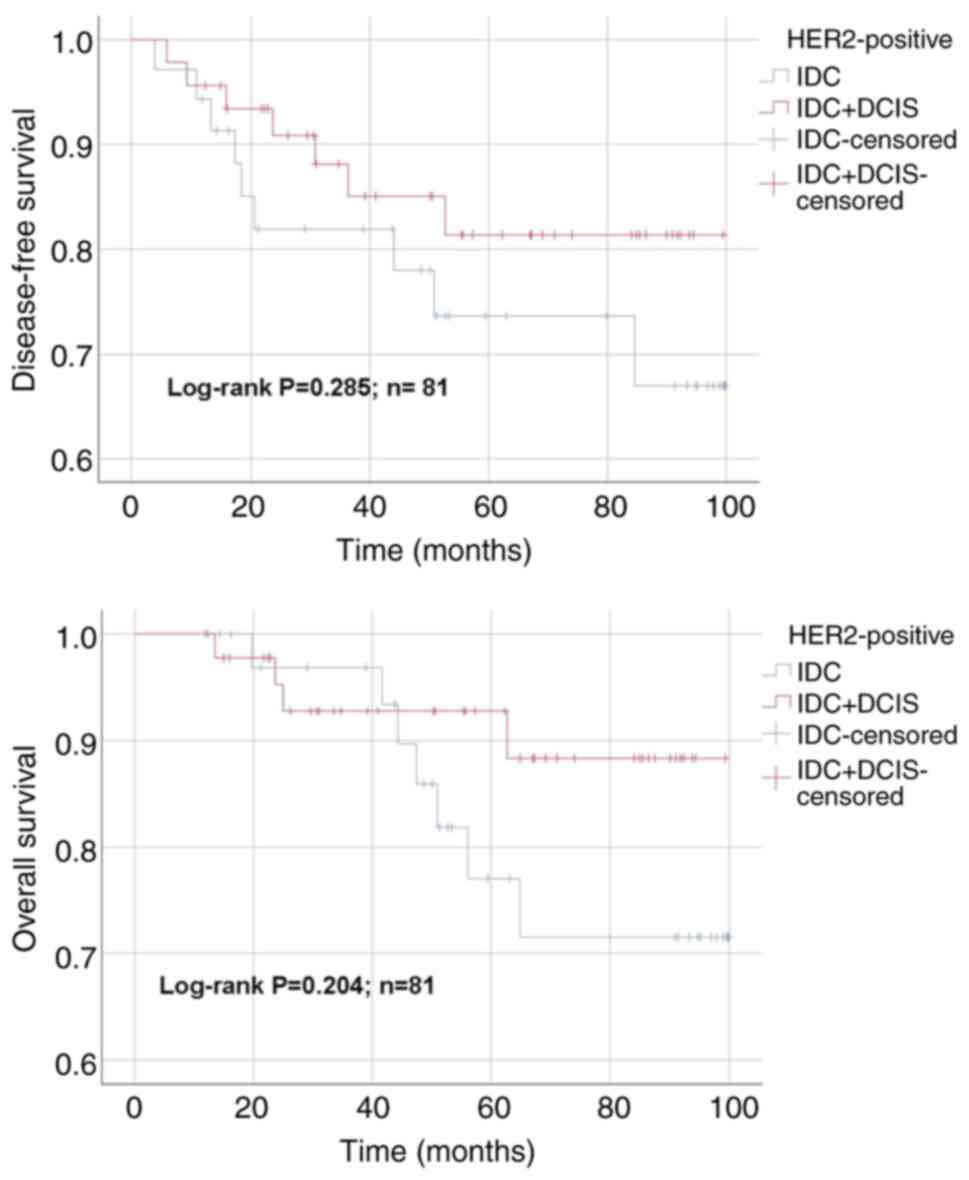
92.5±2.2; IDC + DCIS, 95.3±1 months; P>0.05). For the HER-2
positive subgroup, mean DFS time for IDC and IDC + DCIS groups were
79.0±6.0 and 86.1±4.6 months, respectively (P>0.05; Fig. 3). The OS time for IDC and IDC +
DCIS groups were 85.3±4.7 and 92.1±3.4 months in HER-2 positive
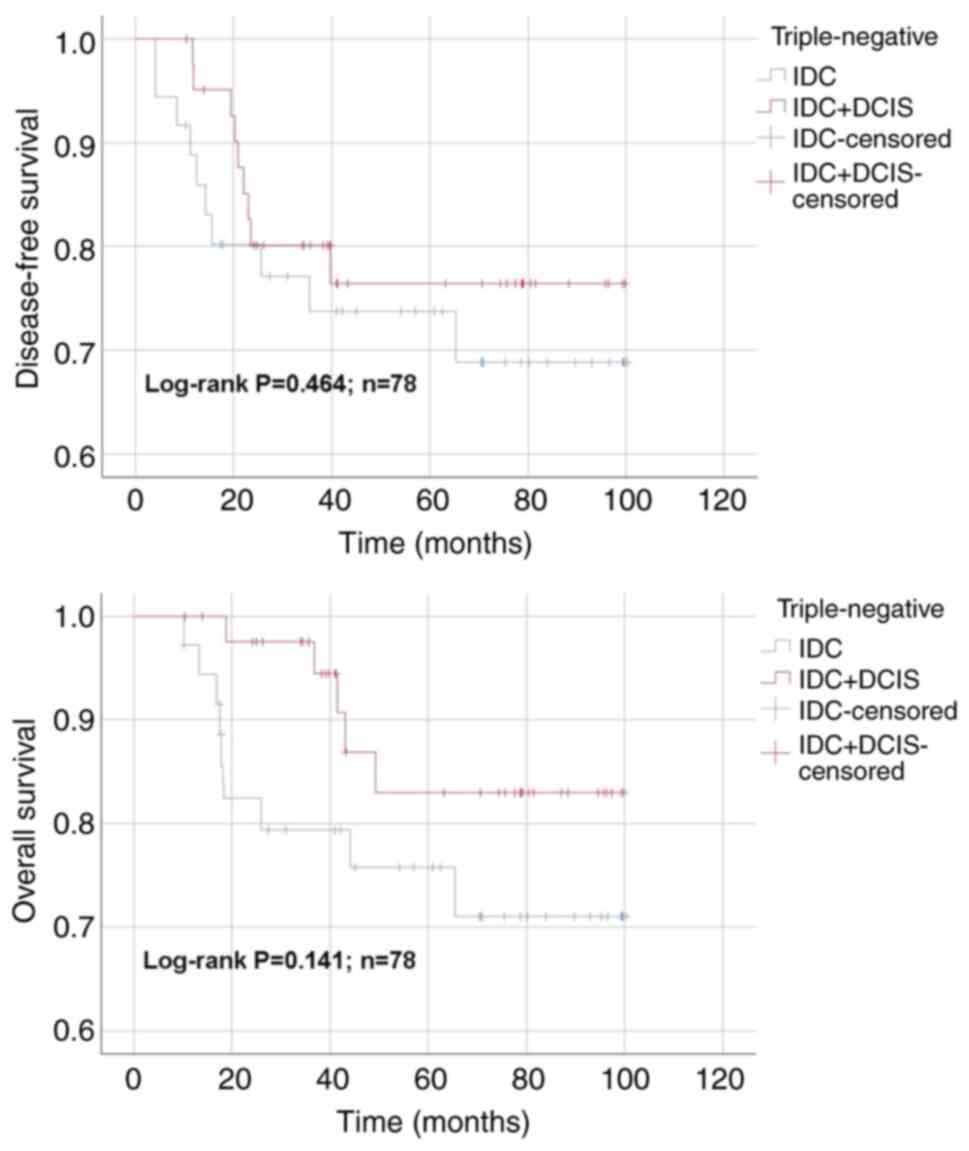
subgroup, respectively (P>0.05). In TNBC subgroup, DFS time for
the IDC and IDC + DCIS groups was 76.2±6.4 and 81.6±5.3 months,
respectively (P>0.05; Fig. 4).
The OS time for IDC and IDC + DCIS groups was 79.4±5.9 and 89.4±4.2
months in TNBC subgroup, respectively (P>0.05). No statistically
significant differences were found regarding DFS and OS between IDC
and IDC + DCIS groups in HER2-positive and TNBC cases in the entire
cohort (P>0.05).
In the matched cohort, DFS time in the luminal type
group for IDC and IDC + DCIS subgroups was 82.1±3.4 and 89.3±3.1
months, respectively (P<0.05). No significant difference was
found regarding OS between IDC and IDC + DCIS groups (92.5±2.2 and
94.3±1.8 months, respectively; P>0.05; Fig. S2). For the HER-2 positive
subgroup, mean DFS time for IDC and IDC + DCIS groups was 79.1±6
and 86.4±5.3 months, respectively (P>0.05). The OS time for IDC
and IDC + DCIS groups was 85.2±4.8 and 94.3±3.5 months in HER-2
positive subgroup, respectively (P>0.05; Fig. S3). For the TNBC subgroup, DFS time
for the IDC and IDC + DCIS groups was 78.0±6.4 and 85.3±5.6 months,
respectively (P>0.05). OS time for IDC and IDC + DCIS groups was
81.3±5.9 and 92.4±4.0 months in TNBC subgroup, respectively
(P>0.05).(Fig. S4) No
statistically significant differences were found regarding DFS and
OS between IDC and IDC + DCIS groups in HER2-positive and TNBC
cases in the matched cohort .
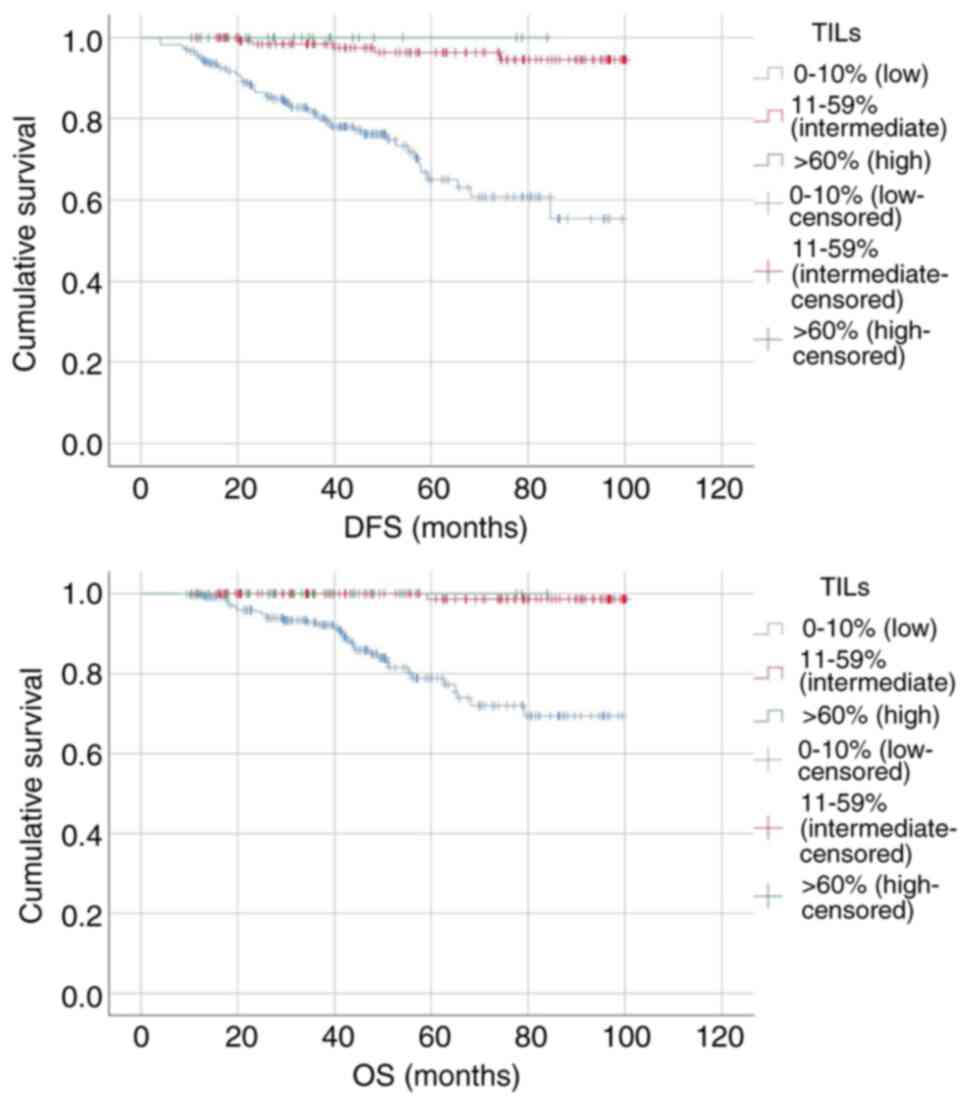
Patients were divided into three groups based on the
percentage of stromal TILs: Low (0-10%), intermediate (11-59%) and
high (≥60%). OS at 60 months for the low, intermediate and high
stromal TIL subgroups was 80.3, 99.3 and 100.0% respectively
(P<0.05; Fig. 5). DFS at 60
months for the low, intermediate and high stromal TIL subgroups was
76.6, 96.3 and 100.0%, respectively (P<0.05).
Multivariate analysis
Factors affecting DFS and OS, including age at
diagnosis, DCIS component status, ER status (positive/negative),
tumor stage (1-3),
tumor size (cm), lymph node involvement stage (N), type of surgery
(breast-conserving/mastectomy), chemotherapy status
(none/neoadjuvant/adjuvant), patient comorbidities and treatment
side effects, were investigated. Presence of DCIS component was
positively associated with both PFS and OS (OS: Hazard ratio (HR),
2.144; 95% CI, 1.178-3.901; P=0.013; PFS: HR, 2.446; 95% CI,
1.550-3.860; P<0.001). The risk of death was 2.14 times higher
in patients with IDC compared with those with IDC + DCIS in the
entire cohort. Similarly, the risk of disease progression,
including local recurrence, distant metastasis or death, was 2.44
times higher in the IDC group. OS (HR, 1.196; 95% CI, 1.048-1.363;
P=0.008) and PFS were negatively associated with tumor size (HR,
1,137; 95% CI,1.022-1.264; P=0.018) and lymph node involvement (OS:
HR, 4.819, 95% CI, 2.095-11.085, P=0.002; DFS: HR, 5.0567, 95% CI,
2.620-9.757, P<0.001) in the entire cohort. ER positivity had a
significant effect only on OS in the entire cohort (HR, 2.081; 95%
CI, 1.151-3.765; P=0.015) (Table
II).
 | Table IIMultivariate Cox regression analysis
for OS and DFS of the entire cohort. |
Table II
Multivariate Cox regression analysis
for OS and DFS of the entire cohort.
| | Univariate | Multivariate |
|---|
| | OS | DFS | OS | DFS |
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| IDC vs. IDC +
DCIS | 2.435 | 1.388-4.273 | 0.002 | 2.414 | 1.556-3.744 | <0.001 | 2.14 | 1.178-3.901 | 0.013 | 2.446 | 1.550-3.860 | <0.001 |
| ER-negative vs.
-positive | 2.627 | 1.497-4.612 | 0.001 | 2.149 | 1.383-3.340 | 0.001 | 2.081 | 1.151-3.765 | 0.015 | - | - | - |
| Tumor diameter | 1.263 | 1.140-1.400 | <0.001 | 1.232 | 1.135-1.338 | <0.001 | 1.196 | 1.048-1.363 | 0.008 | 1.137 | 1.022-1.264 | 0.018 |
| N stage | | | | | | | | | 0.002 | | | <0.001 |
|
N1 vs.
N0 | 0.161 | 0.087-0.298 | <0.001 | 0.161 | 0.087-0.298 | <0.001 | 1.531 | 0.673-3.486 | 0.31 | 1.36 | 0.725-2.552 | 0.338 |
|
N2 vs.
N0 | 0.246 | 0.129-0.468 | <0.001 | 0.246 | 0.129-0.468 | <0.001 | 1.713 | 0.708-4.143 | 0.233 | 1.839 | 0.961-3.520 | 0.066 |
|
N3 vs.
N0 | 0.422 | 0.223-0.797 | 0.008 | 0.422 | 0.223-0.797 | 0.008 | 4.819 | 2.095-11.085 | <0.001 | 5.056 | 2.620-9.757 | <0.001 |
Stromal TILs (OS: HR, 0.873, 95% CI, 0.815-0.935;
P<0.001; DFS: HR, 0.899, 95% CI, 0.861-0.939, P<0,001), tumor
diameter (OS: HR, 1.219, 95% CI, 1.037-1.434, P=0.017; DFS: HR,
1.147, 95% CI,1.010-1.302, P=0.035) and stage of lymph node
involvement (HR: 5.314, CI, 2.364-11.949, P=0.023 and <0.001,
respectively) were had a significant effect on OS and DFS in the
matched cohort (Table III).
 | Table IIIMultivariate Cox regression analysis
for OS and DFS of matched cohort. |
Table III
Multivariate Cox regression analysis
for OS and DFS of matched cohort.
| | Univariate | Multivariate |
|---|
| | OS | DFS | OS | DFS |
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Stromal TILs,
% | 0.882 | 0.827-0.940 | <0.001 | 0.908 | 0.872-0.945 | <0.001 | 0.873 | 0.815-0.935 | <0.001 | 0.899 | 0.861-0.939 | <0.001 |
| Tumor diameter | 1.234 | 1.078-1.413 | 0.002 | 1.236 | 1.119-1.366 | <0.001 | 1.219 | 1.037-1.434 | 0.017 | 1.147 | 1.010-1.302 | 0.035 |
| N stage | | | | | | | | | 0.023 | | | <0.001 |
|
N1 vs.
N0 | 0.237 | 0.096-0.584 | 0.02 | 0.161 | 0.087-0.298 | <0.001 | 1.016 | 0.347-2.978 | 0.977 | 1.339 | 0.611-2.934 | 0.466 |
|
N2 vs.
N0 | 0.239 | 0.085-0.673 | 0.07 | 0.246 | 0.129-0.468 | <0.001 | 1.119 | 0.383-3.265 | 0.383 | 1.457 | 0.665-3.190 | 0.347 |
|
N3 vs.
N0 | 0.380 | 0.141-1.021 | 0.055 | 0.422 | 0.223-0.797 | 0.008 | 3.747 | 3.747-1.434 | 0.007 | 5.314 | 2.364-11.946 | <0.001 |
Discussion
Previous studies have shown that IDC accompanied by
the DCIS component has a less aggressive course than IDC alone
(7,8,11).
In the present study, IDC + DCIS tumors were more positive for
hormone receptors (ER, PR) and had lower Ki-67 percentages. Tumors
containing a DCIS component in addition to IDC were associated with
longer OS and DFS, suggesting IDC + DCIS tumors have less
aggressive characteristics compared with IDC tumors.
Compared with IDC group, IDC + DCIS group had
significantly higher rates of hormone-positive status and
breast-conserving surgery and lower Ki-67 percentage. In a study of
1,355 patients by Wong et al (7), patients with IDC + DCIS matched for
IDC and DCIS component size had a higher prevalence of
premenopausal diagnosis and HER2 positivity than patients with IDC.
The same study found that diameter of the invasive component, ER
positivity and the Ki-67 percentage were lower in IDC + DCIS
tumors, similar to the present study (7). A study by Chen et al (11) of 98,097 patients with IDC and
149,477 with IDC + DCIS showed that patients with IDC + DCIS were
diagnosed at a younger age than those with IDC (mean age, 58.7 vs.
60.4 years), with a higher proportion of patients diagnosed before
the age of 60 years (53.4 vs. 48.3%). BCS rate was significantly
lower in patients with IDC + DCIS compared with patients with pure
IDC (60.2 vs. 62.4%) (11). A
prospective cohort study by Gordo et al, observed that DCIS
component is associated to better prognostic factors as being
positive for HR, negative for HER2, lower Ki67%, lower grade of
invasive carcinoma (18). In
another study of 3,001 patients with IDC and IDC + DCIS by Goh
et al (19), patients with
IDC + DCIS were diagnosed at an earlier age than those with IDC and
had a lower histological grade of the invasive component and fewer
regional lymph node metastases (19). Previous studies (7,11,18),
demonstrated the positive contribution of DCIS accompanying IDC to
prognosis.
In the present study, no statistically significant
difference was found between the two groups regarding age and BCS
rates were higher in IDC + DCIS group compared with IDC. This may
be due to underutilization of breast cancer screening programs in
Turkey, which may delay the detection of IDC until tumors become
more advanced. In contrast, the coexistence of DCIS may increase
the likelihood of detection at an earlier and more operable
stage.
In the present study, tumors containing a DCIS
component in addition to IDC were associated with longer OS and
DFS. Risk of death was 2.14 times higher in the IDC compared with
the IDC + DCIS group in the entire cohort and the risk of disease
(local recurrence or distant metastasis or death) was 2.44 times
higher. Tumors containing a DCIS component in addition to IDC were
associated with longer OS and DFS. When patients were grouped
according to receptor status, IDC + DCIS affected DFS in the
luminal A-B subtype. Chen et al (11) found that patients with IDC + DCIS
have significantly higher OS compared with those with pure IDC.
According to the multivariate analysis, existence of DCIS component
is an independent favorable prognostic factor for OS (HR, 0.858,
95% CI, 0.839-0.8773) (11). A
study by Zhou et al (20),
conducted with 852 patients with stage II-III IDC patients who had
neoadjuvant treatment followed by radical surgery showed that in
the TNBC population, the DFS (88.6% vs. 75.8%, P=0.032) of patients
with IDC + DCIS was significantly better than that of patients with
IDC. Multivariate analysis demonstrated that IDC + DCIS (HR: 0.502;
95% CI, 0.284-0.952; P=0.048) was an independent prognostic factor
for DFS. These findings were further supported by Liu et al
(21), who reported that among 358
patients with TNBC, the IDC + DCIS group had significantly better
DFS (87.9% vs. 82.6%) compared to pure IDC. Furthermore,
multivariate analysis identified the coexistence of DCIS as an
independent prognostic factor for DFS (HR: 0.535; 95% CI,
0.304-0.942).
The present findings suggested a significant
difference in both OS and DFS between the stromal TIL groups,
though further investigation is needed to confirm this. In the
matched cohort, there was an association between an increase in the
percentage of stromal tumor-infiltrating lymphocytes and a decrease
in the risk of death and the risk of local recurrence/distant
metastasis/death. In the matched cohort, each 1% increase in the
percentage of stromal TILs decreased risk of death by 0.87-fold and
the risk of progression by 0.89-fold. Denkert et al
suggested that an increase of 10% in the percentage of TILs may
potentially lead to a significant prolongation of DFS in patients
with TNBC and HER2-positive breast cancer (HR, 0.93; 95% CI,
0.87-0.98; P=0.011 and HR, 0.94; 95% CI, 0.89-0.99; P=0.017,
respectively). In TNBC, an increased percentage of TILs is
associated with longer OS, whereas in luminal disease, a higher
percentage of TILs is associated with shorter OS (15). In a systematic review of 14 studies
and 4,105 patients by Lam and Verill (22), increased tumor infiltrating B cells
were associated with a better disease course in invasive breast
cancer in terms of disease-free interval and survival and
recurrence-free survival and OS. A similar difference has also been
noticed in studies including TNBC and/or HER2-positive cases
(23,24). The association between stromal TIL
percentage and survival rate is consistent with the present
results.
Using the AJCC 8th edition in this study allowed us
to stage patients based not only on tumor size and nodal status,
but also on biological features such as hormone receptor and HER2
status. This provided a more clinically relevant risk
classification. This comprehensive approach makes our findings more
reflective of real world breast cancer patients and improves their
relevance to clinical practice.
The data regarding grade, stromal TILs and
socioeconomic status were incomplete due to the retrospective
nature of the study. Also, TILs were not isolated from tumor tissue
and specific surface and intracellular markers were not used to
identify TIL subtypes (such as CD4/8+T and B cells).
Analysis of these TIL subpopulations would provide valuable
insights into the immune microenvironment and its impact on
prognosis. The patient cohort had a 90.8% 5-year OS rate, which may
underestimate late recurrence or treatment-related adverse
outcomes, it would be beneficial to extend the follow-up period to
predict long-term outcomes, particularly in hormone
receptor-positive breast cancer patients.
The present study indicated that there might be a
difference in the anti-tumoral immune response between the IDC and
IDC + DCIS groups, which behave differently biologically. DCIS
component was associated with better prognostic and pathological
features. The improved PFS and OS rates associated with DCIS may
encourage consideration of more conservative surgical and systemic
treatment options. These findings may lead to more individualized
approaches in patient follow-up and risk stratification, allowing
less aggressive monitoring in low-risk patients. Additionally, the
present results may facilitate treatment de-escalation decisions by
identifying DCIS accompanying IDC group which has a less aggressive
behavior and led further investigations into the potential effect
of DCIS accompanying IDC on prognosis. Increased levels of stromal
TILs may also be associated with a favorable prognosis in invasive
breast cancer. Further studies are needed to assess the potential
of stromal TILs for the identification of patients who may benefit
from chemotherapy and immunotherapy.
Supplementary Material
Kaplan-Meier curve for disease-free
and overall survival analysis based on IDC and IDC + DCIS groups
for the matched cohort. IDC, invasive ductal carcinoma; DCIS,
ductal carcinoma in situ.
Kaplan-Meier curve for disease-free
and overall survival analysis on luminal type breast cancer in IDC
and IDC + DCIS for the matched cohort. IDC, invasive ductal
carcinoma; DCIS, ductal carcinoma in situ.
Kaplan-Meier curve for disease-free
and overall survival analysis based on HER2-positive status for the
matched cohort. IDC, invasive ductal carcinoma; DCIS, ductal
carcinoma in situ; HER2, human epidermal growth factor
receptor 2.
Kaplan-Meier curve for disease-free
and overall survival analysis based on triple-negative breast
cancer for the matched cohort. IDC, invasive ductal carcinoma;
DCIS, ductal carcinoma in situ.
Acknowledgements
Not applicable
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NKF and NK confirm the authenticity of all the raw
data. NKF, IK, GK, AU and MU collected and interpreted the data.
NKF, SA and NK analyzed data. NKF, IK, GK, SA and NK wrote the
manuscript. SA and NK edited the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study received approval from the
Hacettepe University Non-Interventional Clinical Research Ethics
Committee (approval date, 01.03.2022; approval no. 2022/04-15;
registration no. KA-22219). The requirement for informed consent
was waived due to the retrospective nature of the study.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li CI, Uribe DJ and Daling JR: Clinical
characteristics of different histologic types of breast cancer. Br
J Cancer. 93:1046–1052. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sgroi DC: Preinvasive breast cancer. Annu
Rev Pathol. 5:193–221. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Espina V and Liotta LA: What is the
malignant nature of human ductal carcinoma in situ? Nat Rev Cancer.
11:68–75. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Chagpar AB, McMasters KM, Sahoo S and
Edwards MJ: Does ductal carcinoma in situ accompanying invasive
carcinoma affect prognosis? Surgery. 146:561–567. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wong H, Lau S, Yau T, Cheung P and Epstein
RJ: Presence of an in situ component is associated with reduced
biological aggressiveness of size-matched invasive breast cancer.
Br J Cancer. 102:1391–1396. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kole AJ, Park HS, Johnson SB, Kelly JR,
Moran MS and Patel AA: Overall survival is improved when DCIS
accompanies invasive breast cancer. Sci Rep. 9(9934)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cowell CF, Weigelt B, Sakr RA, Ng CK,
Hicks J, King TA and Reis-Filho JS: Progression from ductal
carcinoma in situ to invasive breast cancer: Revisited. Mol Oncol.
7:859–869. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lesurf R, Aure MR, Mørk HH and Vitelli V:
Oslo Breast Cancer Research Consortium (OSBREAC). Lundgren S,
Børresen-Dale AL, Kristensen V, Wärnberg F, Hallett M and Sørlie T:
Molecular features of subtype-specific progression from ductal
carcinoma in situ to invasive breast cancer. Cell Rep.
16:1166–1179. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen H, Bai F, Wang M, Zhang M, Zhang P
and Wu K: The prognostic significance of co-existence ductal
carcinoma in situ in invasive ductal breast cancer: A large
population-based study and a matched case-control analysis. Ann
Transl Med. 7(484)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Galon J, Angell HK, Bedognetti D and
Marincola FM: The continuum of cancer immunosurveillance:
Prognostic, predictive, and mechanistic signatures. Immunity.
39:11–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Whiteside TL: Tumor-infiltrating
lymphocytes and their role in solid tumor progression. Exp Suppl.
113:89–106. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kashiwagi S, Asano Y, Goto W, Takada K,
Takahashi K, Hatano T, Takashima T, Tomita S, Motomura H, Ohsawa M,
et al: Using TILs to predict therapeutic effect of chemotherapy
(Pertuzumab, Trastuzumab, Docetaxel) on HER2-positive breast
cancer. Anticancer Res. 37:5623–5630. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast cancer-major changes in the American Joint
Committee on Cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shao N, Xie C, Shi Y, Ye R, Long J, Shi H,
Shan Z, Thompson AM and Lin Y: Comparison of the 7th and 8th
edition of American Joint Committee on Cancer (AJCC) staging
systems for breast cancer patients: A Surveillance, Epidemiology
and End Results (SEER) Analysis. Cancer Manag Res. 11:1433–1442.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lopez Gordo S, Blanch Falp J, Lopez-Gordo
E, Just Roig E, Encinas Mendez J and Seco Calvo J: Influence of
ductal carcinoma in situ on the outcome of invasive breast cancer.
A prospective cohort study. Int J Surg. 63:98–106. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Goh CW, Wu J, Ding S, Lin C, Chen X, Huang
O, Chen W, Li Y, Shen K and Zhu L: Invasive ductal carcinoma with
coexisting ductal carcinoma in situ (IDC/DCIS) versus pure invasive
ductal carcinoma (IDC): A comparison of clinicopathological
characteristics, molecular subtypes, and clinical outcomes. J
Cancer Res Clin Oncol. 145:1877–1886. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou S, Shi Y, Huang Z, Teng Y and Xing W:
Does the presence of ductal carcinoma in situ affect prognostic
outcomes after neoadjuvant therapy in invasive ductal carcinoma of
the breast? Clin Oncol (R Coll Radiol). 40(103781)2025.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu Y and Yu T: Clinicopathological
characteristics and prognosis of triple-negative breast cancer
invasive ductal carcinoma with ductal carcinoma in situ. J Cancer
Res Clin Oncol. 149:11181–11191. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lam BM and Verrill C: Clinical
significance of tumour-infiltrating B lymphocytes (TIL-Bs) in
breast cancer: A systematic literature review. Cancers (Basel).
15(1164)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Loi S, Michiels S, Salgado R, Sirtaine N,
Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V,
Desmedt C, et al: Tumor infiltrating lymphocytes are prognostic in
triple negative breast cancer and predictive for trastuzumab
benefit in early breast cancer: Results from the FinHER trial. Ann
Oncol. 25:1544–1550. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schüler K, Bethmann D, Kaufhold S, Hartung
C, Stückrath K, Lantzsch T, Uleer C, Hanf V, Peschel S, John J, et
al: Prognostic value of tumour-infiltrating lymphocytes in an
unselected cohort of breast cancer patients. Diagnostics (Basel).
12(2527)2022.PubMed/NCBI View Article : Google Scholar
|